Spontaneous Pseudoaneurysm of Femorofemoral Crossover Bypass: A Case Report Pseudoaneurysm of Femorofemoral Crossover Bypass
by Paulo Roberto da Silva Lima1*, Marcus Welber do Nascimento Guimarães2, Sérgio Ricardo Ferreira Vieira3
1Vascular and Endovascular Surgery, PhD, Department of General Surgery, Paraíba Federal University (UFPB), Campus I Lot. Cidade Universitaria, PB, 58051-900, Brazil
2Vascular Surgery, Department of Vascular Surgery, , Intervasc Intervenções Preventivas, PB, Brazil
3Anesthetist, UNIMED Hospital, PB, Brazil
*Corresponding author: Paulo Roberto da Silva Lima, Department of Vascular and Endovascular Surgery, Paraíba Federal University (UFPB), Campus I Lot. Cidade Universitaria, PB, 58051-900, Brazil
Received Date: 19 September 2025
Accepted Date: 25 September 2025
Published Date: 27 September 2025
Citation: Lima PRS, Guimarães MWN, Vieira SRF (2025) Spontaneous Pseudoaneurysm of Femorofemoral Crossover Bypass: A Case Report Pseudoaneurysm of Femorofemoral Crossover Bypass. J Surg 10: 11454 https://doi.org/10.29011/2575-9760.011454
Abstract
Background: Pseudoaneurysm is defined as a hematoma with pulsatile flow inside, which progresses into a pulsatile vascular mass. It subsequently forms an aneurysmal sac whose wall is composed of surrounding tissues and connective tissue adjacent to the hematoma. Unilateral axillofemoral bypass was first described by Louw in 1962 as a treatment for occlusive arterial disease. Extra-anatomic bypass routes should be reserved for patients whose poor general health or specific local conditions preclude the use of a traditional access route or aortofemoral bypass. We must remember the nutritional status of patients, as it influences surgical outcomes. Prosthetic materials, whether Dacron, PTFE, or endoprosthesis, have a useful life, which varies greatly from material to material. We must consider their lifespan and the patient’s survival time when deciding which to use.
Case Report: The patient developed two pseudoaneurysms secondary to wear of the vascular graft. The first was in the crossover femorofemoral bypass for abdominal aortic aneurysm repair, and the second was for the repair of this pseudoaneurysm itself. This resulted in infection and requiring an extra-anatomical bypass (right unilateral axillofemoral) to resolve the condition.
Conclusions: This case report highlights the importance of monitoring patients with arterial disease who undergo vascular prosthesis implantation, and the conventional surgery still plays a critical role in the vascular surgery arsenal for limb and life preservation. An extra-anatomical bypass should remain a last-resort option, as its own nature implies deviation from normal anatomy. Continuous monitoring of these patients is necessary to prevent and identify problems early.
Keywords: Axillofemoral Bypass Grafting; False Aneurysm; Femorofemoral Bypass Grafting; Polytetrafluoroethylene (PTFE); Polyethylene Terephthalates (Dacron); Ruptured aneurysm
Background
False aneurysm (pseudoaneurysm) is defined as a hematoma with pulsatile flow inside, which progresses into a pulsatile vascular mass. It subsequently forms an aneurysmal sac whose wall is composed of surrounding tissues and connective tissue adjacent to the hematoma. The walls of the aneurysm sac lack histological elements of an arterial wall. Its expansion often requires intervention. Direct thrombin injection is indicated for narrownecked aneurysmal sacs, minimizing the risk of embolization. Surgery is reserved for wide-necked pseudoaneurysm, in the absence of required materials and structure for thrombin injections, or in cases involving prosthetic grafts. The most common sites of pseudoaneurysm formation include the femoral, brachial, radial, and subclavian arteries, as well as distal anastomoses of an aortofemoral bypass. Pseudoaneurysms result from complete rupture of the arterial wall, creating direct communication between the arterial lumen and the pulsatile hematoma resulting from arterial injury [1]. Anastomotic pseudoaneurysms may occur in arterial grafts due to suture line dehiscence, often secondary to arterial wall weakening. In the presence of infection, they are also called infected pseudoaneurysms. The term pseudoaneurysm applies because the lesion is not lined by the three layers of the arterial wall, unlike true aneurysms, but rather by a fibrous capsule formed around the perianastomotic hematoma, which prevents immediate bleeding. Possible complications include pain, rupture, hypovolemic shock, distal embolization, neuropathy, and skin necrosis [1].
Unilateral axillofemoral bypass was first described by Louw in 1962 [2,3] as a treatment for occlusive arterial disease. Extraanatomic bypass routes should be reserved for patients whose poor general health or specific local conditions preclude the use of a traditional access route or aortofemoral bypass. Cardiac, renal, or pulmonary comorbidities may make abdominal surgery unacceptably risky. Axillobifemoral bypass, when performed with a ringed prosthesis, shows primary patency rates similar to those of aortofemoral grafts at five years [4]. The technique involves anastomosing the prosthesis to the subclavian artery, tunneling it beneath the pectoralis major, then subcutaneously along the midaxillary line, across the anterior superior iliac spine, and then to the femoral artery. The distal anastomosis is performed on the common femoral artery or, if it is occluded, directly on the deep femoral artery [1]. Extra-anatomical bypass is considered the revascularization method of choice for limb salvage in patients with infrarenal aortoiliac occlusion and severe comorbidities [5]. The primary objective of the report is to raise awareness among the medical community about the possibility of spontaneous pseudoaneurysm formation in synthetic materials. The secondary objectives are to demonstrate that open surgical techniques remain relevant, to identify the best management strategy for such cases, and to explore possible causes.
Case Report
This article reports on a case that was followed for fourteen months, involving pseudoaneurysm formation in a Dacron graft used for femorofemoral crossover bypass and a subsequent second pseudoaneurysm after endovascular reconstruction of the same bypass. An 81-year-old man, non-diabetic, presented to the emergency department at a private hospital in the city of João Pessoa, PB, Brazil, on November 16, 2023, with a sudden onset of a bulge in the suprapubic region and mild local pain. He denied a history of local trauma. There was no hypotension, skin lesions, signs of inflammation, or signs of lower limb ischemia. However, he reported undergoing abdominal aortic aneurysm repair approximately ten years earlier using a left-sided monoiliac Dacron graft (due to right iliac obstruction; Figure 2) and a femorofemoral crossover bypass using Dacron. Computed tomography (CT) angiography (November 16, 2023) revealed a contained rupture of the Dacron femorofemoral crossover bypass, forming a pseudoaneurysm and a suprapubic hematoma measuring 14.8 × 14.9 × 9.6 cm (Figure 1 and Table 1).
|
Nov 16, 2023 |
Results |
|
Suprapubic region |
Femorofemoral crossover bypass with a subcutaneous course through the pelvic region. Large partially thrombosed aneurysmal dilation of the femorofemoral bypass, measuring 14.8 × 14.9 × 9.6 cm, extending into the right inguinoscrotal region. The lumen of the aneurysm sac measures approximately 11.6 × 5.2 cm. Densification of the adjacent fat planes and parietal irregularities in the lower portion, which may indicate graft instabilityOcclusion of the right common, internal, and external iliac arteries. Recanalization of the common femoral artery with flow from the epigastric artery. |
|
Abdominal aorta |
Normal course and diameter, with no evidence of dissection or aneurysm.Diffuse atheromatosis of the aorta and branches, but no significant stenosis. Occlusion of the right common, internal, and external iliac arteries. |
|
Iliac arteries |
Left common, internal, and external iliac arteries with normal course and diameter, without significant stenosis. |
|
Visceral arteries |
Celiac trunk, renal and mesenteric arteries, and their main branches with normal course and diameter. |
Table 1: CT angiography of the abdominal aorta.
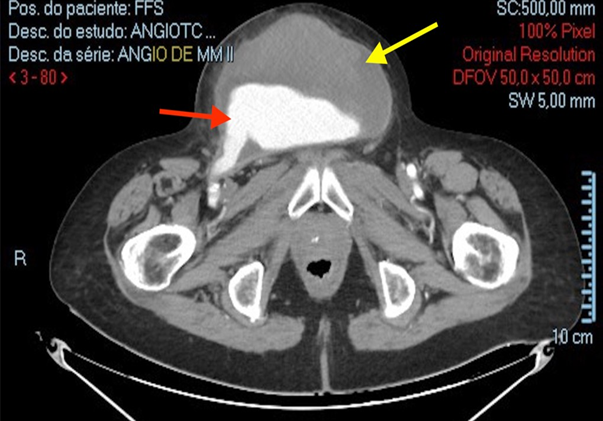
Figure 1: Contained rupture of a pseudoaneurysm in a femorofemoral crossover bypass in the suprapubic region at admission (November 16, 2023). Yellow Arrow - Large Hematoma, Red Arrow - Dacron Bypass rupture. (Personal archive)
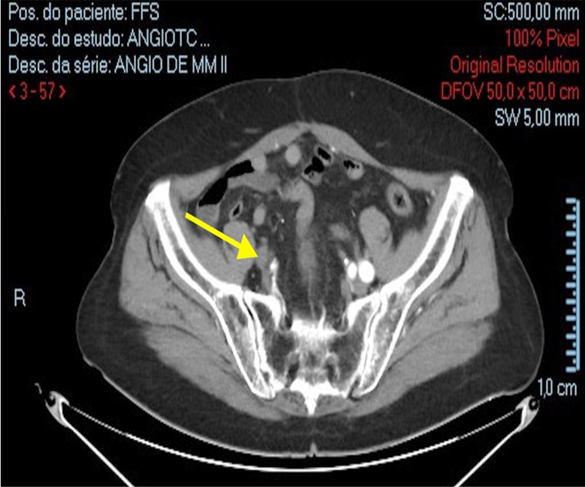
Figure 2: Occlusion of the right iliac arteries on the day of admission (November 16, 2023). Yellow Arrow – occlusion of the right iliac arteries (Personal archive)
Endovascular repair was performed using three Viabahn segments, as a single appropriately sized endoprosthesis was unavailable due to logistical limitations in the state of Paraíba. The hematoma was initially left in place for observation and potential drainage. A color Doppler ultrasound performed 24 hours postoperatively showed no abnormalities in the endoprostheses of the new femorofemoral bypass and normal perfusion of the lower limbs. On inspection, there were no signs of inflammation, and popliteal pulses were present. On November 20, 2023, percutaneous drainage was performed because the hematoma persisted. Complete drainage was not possible due to the presence of firm clots. To minimize the risk of endoprosthesis exposure, the incision was limited to 3 cm. On December 29, 2023, a repeat color Doppler ultrasound of the aorta, iliac arteries, and lower limbs revealed diffuse atheromatosis, hematoma persistence, right anterior tibial artery occlusion, and a patent bypass without endoleaks (Tables 2 and 3).
|
Right lower limb |
Left lower limb |
|
|
December 29, 2023 |
Chronic atherosclerotic arterial disease with sequential nonobstructive stenoses < 50% in the anterior and posterior femoral-popliteal-tibial segments. Anterior tibial artery occlusion |
Chronic atherosclerotic arterial disease with sequential nonobstructive stenoses < 50% in the anterior and posterior femoralpopliteal-tibial segments. |
Table 2: Arterial Doppler ultrasound of the lower limbs
|
Aorta |
Right iliac arteries |
Left iliac arteries |
|
|
December 29, 2023 |
Evaluated along its entire length, showing atherosclerotic plaques. Color Doppler mapping showed non-turbulent flow. Spectral curves were triphasic with normal velocities. |
Occlusion of the right common, internal, and external iliac arteries. |
Presence of a left monoiliac prosthesis. Atherosclerotic plaques along the entire length. Color Doppler mapping showed non-turbulent flow. Spectral curves were triphasic with normal velocities. Femorofemoral bypass with subcutaneous pelvic course and non-turbulent triphasic flow. Large partially thrombosed aneurysmal dilatation of the femorofemoral bypass (11.9 × 9.2 × 6.6 cm) extending into the right inguinoscrotal region. The lumen of the aneurysm sac measured approximately 9.6 × 4.2 cm. |
Table 3: Arterial color Doppler ultrasound of the aorta and iliac arteries
On February 14, 2024, pelvic CT angiography with contrast (Table 4) confirmed the absence of endoleaks, patency of the bypass, and persistence of the hematoma, resulting in a non-pulsatile suprapubic bulge. Physical examination revealed dark sanguinolent secretion from the percutaneous drain, with no signs of inflammation or fever. The drain remained in place until June 3, 2024, when a new rupture occurred in the femorofemoral bypass with endoprosthesis. Intraoperatively, there was no endoprosthesis disconnection, but an active bleeding site was identified and corrected. The tortuous segment of the endoprosthesis was resected, and end-to-end anastomosis was performed. The entire hematoma was excised, followed by thorough irrigation with 0.9% saline and primary closure of the surgical wound in deep and superficial layers. The patient was discharged seven days after the surgery. However, on July 20, 2024, he was readmitted with wound dehiscence and endoprosthesis exposure through the surgical wound (Figures 3 and 4).
|
Femorofemoral bypass |
Iliac arteries |
Femoral arteries |
|
|
February 14, 2024 |
Patent sequential endoprostheses in the femorofemoral bypass, excluding the large aneurysmal dilatation extending into the right inguinoscrotal region. No signs of endoleak. Femorofemoral bypass with a subcutaneous course through the pelvic region. Marked Z-shaped angulation of the endoprostheses to the right of the aneurysm sac, surgical correlation is needed to rule out fracture. |
Occlusion of the right common, internal, and external iliac arteries. Recanalization of the right common femoral artery with flow from the epigastric artery. |
Patent arteries with preserved course and diameter. |
Table 4: Contrast-enhanced pelvic CT.
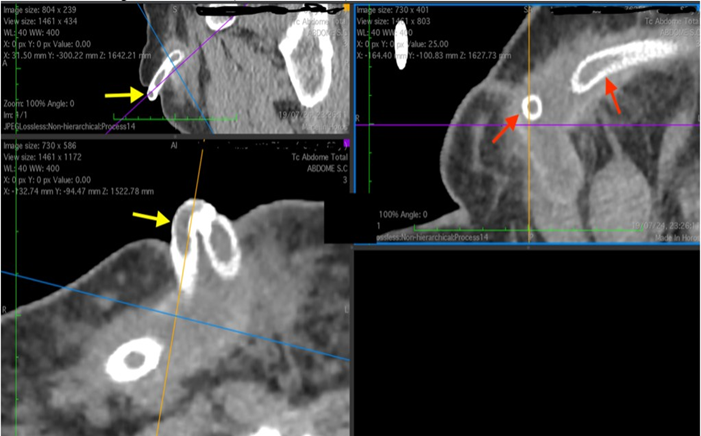
Figure 3: CT scan showing exposure of the femorofemoral crossover bypass endoprosthesis. Yellow arrows – externalization of the endoprosthesis, Red arrows – endoprosthesis in the deep subcutaneous tissue. (Personal archive)
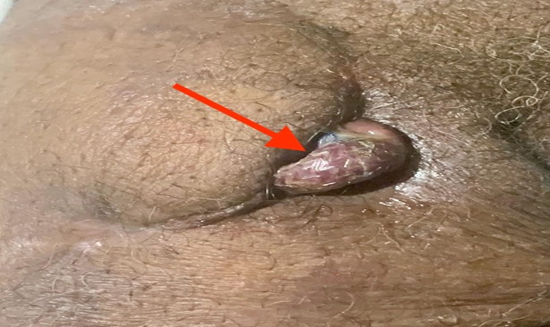
Figure 4: Externalization of the endoprosthesis through dehiscence of the surgical wound (suprapubic region). Red arrow – exteriorization of the endoprosthesis, the endoprosthesis stent mesh can be seen. (Personal archive).
The femorofemoral crossover bypass was explanted and replaced with a right axillofemoral bypass using a 6 mm × 70 cm ringed Polytetrafluoroethylene (PTFE) prosthesis. Intraoperatively, we identified the complete wear of the Dacron graft used in the first femorofemoral bypass. A segment of the exposed endoprosthesis was sent for culture and antibiogram (July 20, 2024), which isolated Morganella morganii subsp. morganii, a Gram-negative bacterium. On December 11, 2024, arterial color Doppler ultrasound of the right lower and upper limbs confirmed patent right axillofemoral bypass, diffuse atheromatosis in both limbs, and the right lower limb, and monophasic flow in the right anterior tibial and dorsalis pedis arteries. No endoleaks or pseudoaneurysms were detected (Table 5). At follow-up on January 9, 2025, the patient was afebrile, conscious, alert, and oriented, with good perfusion of the lower limbs and no evidence of flow steal from the right upper limb. No hematomas were observed.
|
Right upper limb |
Right lower limb |
|
|
December 11, 2024 |
Peripheral atherosclerosis No hemodynamically significant stenosis or aneurysmal dilatation. |
Peripheral atherosclerosis. Adequate flow in femoral, popliteal, posterior tibial, and fibular arteries. Sequential hemodynamically significant stenoses affecting the anterior tibial and dorsalis pedis arteries. |
Table 5: Arterial Doppler ultrasound of the right upper and lower limbs and right axillofemoral bypass.
Discussion
Although some studies support the use of Dacron vascular prosthesis [6-8], its rupture in this patient was attributed to prosthesis wear, as there was no local trauma and the device had been implanted approximately ten years prior. In general, Dacron grafts are designed to last for many years, but their durability ranges from 5.5 to 30 years, after which the mesh wears and dilatation or rupture can occur. [9-12]. We chose an endovascular approach to repair the pseudoaneurysm due to the absence of signs of inflammation, infection, or skin lesions in the suprapubic region. This allowed for a clean minimally invasive surgery associated with lower morbidity and mortality for older patients [13,14]. According to the literature, endovascular treatment has been used for penetrating, iatrogenic, and surgical injuries, with stent implantation achieving success in 96.9% of cases. Clinical and radiological follow-up showed 84.4% patency, although long-term follow-up was limited. We found no long-term follow-up data in the literature, but current outcomes are encouraging [1]. The new pseudoaneurysm, which occurred in June 2024, was attributed to endoprosthesis fracture. In February 2024, a contrast-enhanced pelvic CT scan revealed a Z-shaped bypass configuration (Table 4).
At that time, there was no evidence of disconnection or endoleak; otherwise, active arterial bleeding and rapid hematoma expansion would have been expected. The “Z” configuration suggested endoprosthesis displacement, likely due to the necessity of using three separate units, as a single appropriately sized endoprosthesis was unavailable due to logistical limitations in the state of Paraíba. This configuration predisposed the system to disconnection. Intraoperatively, there was no endoprosthesis disconnection, but an active bleeding site (fracture) was identified and corrected. The tortuous segment of the endoprosthesis was resected, and end-to-end anastomosis was performed. The entire hematoma was excised, followed by thorough irrigation with 0.9% saline and primary closure of the surgical wound in deep and superficial layers. This approach was chosen because there was no evidence of local infection and the endoprosthesis was amenable to suturing. As noted in the systematic review by Duffy (2015), endoprosthesis fractures can occur, in addition to other possible complications associated with endovascular therapy [15].
Due to infection of the endoprosthesis and the surrounding area, an extra-anatomic right-sided unilateral axillofemoral bypass [5,16] was performed on July 20, 2024 to restore perfusion to the right lower limb. In this patient, previous treatment for an infrarenal aneurysm had involved a left-sided monoiliac aortic bypass due to right-sided iliofemoral occlusion. The axillofemoral bypass was chosen for its easy execution, safety, and favorable patency outcomes [5]. A ringed PTFE prosthesis was selected for the right axillofemoral bypass. Although no significant difference has been reported between PTFE and Dacron for this type of bypass [16,17], we opted to switch materials, avoiding Dacron based on the study by Johnson (2000), which recommends PTFE in the absence of a suitable autologous vein [18]. Laboratory tests performed on July 23, 2024 (Tables 6 and 7) revealed hypoalbuminemia, which contributed to surgical wound dehiscence and endoprosthesis exposure.
Nov 25, 2023 | Nov 26, 2023 | Nov 27, 2023 | Jun 3, 2024 | Jun 6, 2024 | Jul 20, 2024 | Jul 23, 2024 | |
Hemoglobin (g/dL) | 9.4 | 11.2 | 9.3 | 11 | 12.7 | 9.5 | |
Hematocrit (%) | 30.6 | 37.1 | 28.2 | 33 | 38.7 | 28.5 | |
Leukocytes | 6,500 | 7,320 | 12,530 | 6,370 | 5,080 | 6,310 | |
Platelets 1,000/mm3 | 338 | 357 | 229 | 229 | 175 | 119 | |
High-sensitivity Troponin (ng/dL) | 7.92 | 7.67 | 5.51 | ||||
Urea (mg/dL) | 62 | 68 | 56 | 39 | |||
Creatinine (mg/dl) | 1.3 | 1.3 | 1.3 | 1.4 | |||
Prothrombin time | 11.6” | 18.4” | |||||
Enzyme activity | 96% | 44% | |||||
International normalized ratio (INR) | 1.03 | 1.69 | |||||
Na+ (mmol/L) | 141 | 142 | 144 | ||||
K+ (mmol/L) | 4.1 | 4.9 | 4.8 | ||||
Ca++ (mmol/L) | 4.8 | 4.6 | 4.8 | ||||
Mg++ (mg/dL) | 2 | 2 | 2.1 | ||||
Direct bilirubin (mg/dL) | 0.39 | 0.52 | 0.24 | 0.09 | |||
Indirect bilirubin (mg/dL) | 0.29 | 0.2 | 0.76 | 0.21 | |||
Total bilirubin (mg/dL) | 0.68 | 0.72 | 1 | 0.3 | |||
C-reactive protein (CRP) (mg/dL) | 38 | 34 | 28 | 54.6 | |||
Albumin | 2.7 | ||||||
Glutamic-oxaloacetic transaminase (GOT) | 24 | ||||||
Glutamic-pyruvic transaminase (GPT) | 22 |
Table 6: Laboratory.
|
Date |
Results |
|
Nov 17, 2023 |
*Transthoracic echocardiogram within normal limits |
Table 7: Transthoracic echocardiogram.
Hypoalbuminemia was corrected using the ImpactÒ nutritional supplement. The patient showed favorable progress, with resolution of the vascular condition, right lower limb salvage, and survival. Patients who have undergone treatment for aneurysms, whether true or false, must be subjected to constant surveillance; according to the Brazilian guidelines for the treatment of abdominal aortic aneurysms, treated patients must undergo CT angiography 30 days after the procedure and, in the absence of complications, the patient must undergo CT angiography annually; angiotomography can also be replaced by Vascular Color Doppler Ultrasonography [19].
Conclusion
This case report highlights the importance of monitoring patients with arterial disease who undergo vascular prosthesis implantation, whether conventional or endovascular, considering the variable durability of graft materials. Another key point is that conventional surgery still plays a critical role in the vascular surgery arsenal for limb and life preservation. This case was particularly challenging due to the rarity of endoprosthesis fractures given the current advanced device technology. An extra-anatomical bypass should remain a last-resort option, as its own nature implies deviation from normal anatomy. Continuous monitoring of these patients is necessary to prevent and identify problems early.
References
- de Brito RM, Carlos José, EL (2020) Cirurgia vascular: cirurgia endovascular angiologia. Rio de Janeiro: Thieme Revinter Publicações.
- L JH (1963) Splenic-to-femoral and axillary-to-femoral bypass grafts in diffuse atherosclerotic occlusive disease. Lancet 2:1401.
- L JH (1964) The treatment of combined aortoiliac and femoropopliteal occlusive disease by splenofemoral and axillofemoral bypass grafts. Surgery 56: 387-395.
- Passman MA, Taylor LM Jr, Moneta GL, Edwards JM, Yeager RA, et al (1996) Comparison of axillofemoral and aortofemoral bypass for aortoiliac occlusive disease. J Vasc Surg 23: 263-269; discussion 269271.
- Yasa KP, Ryalino C (2020) Extra-anatomic axillofemoral bypass after failed stenting for aortoiliac-occlusive disease in a patient with severe comorbidities. Am J Case Rep 21: e925009.
- Rychlik IJ, Davies RS, Phillips MJ, Naylor AR, Nasim A, et al (2014) A meta-analysis to compare Dacron versus polytetrafluoroethylene grafts for above-knee femoropopliteal artery bypass. J Vasc Surg 60: 506-515.
- Takagi H, Goto SN, Matsui M, Manabe H, Umemoto T A (2010) contemporary meta-analysis of Dacron versus polytetrafluoroethylene grafts for femoropopliteal bypass grafting. J Vasc Surg 52: 232-236.
- van Det RJ, Vriens PW, van der Palen J, Goossens RH, Rouwet EV, et al (2009) Dacron or ePTFE for femoropopliteal above-knee bypass grafting: short- and long-term results of a multicentre randomised trial. Eur J Vasc Endovasc Surg 37: 457-463.
- Han I, Shim SH, Nam M, Min HK, In KH, et al (1994) Nonanastomotic aneurysm formation in a Dacron arterial graft: report of a case. Surg Today 24:1007-1010.
- Magee HR, Thomson CA (1988) Late rupture of knitted velour Dacron arterial prostheses. ANZ J Surg 58:549-553.
- Wilson SE, Krug R, Magee G, Williams L (1997) Late disruption of Dacron aortic grafts. Ann Vasc Surg 11:383-386.
- Cooke PA, Neville P, Skinner R (1974) Dacron aortic graft failure. Arch Surg 108:101-103.
- Tisi P, Callam M (2013) Treatment for femoral pseudoaneurysms. Cochrane Database Syst Rev 2013: CD004981.
- Rodrigues VMA, Silva C, Dias N (2024) Prostatic fossa pseudoaneurysm after robot-assisted radical prostatectomy (RARP): A case report. Am J Case Rep 25: e942746.
- Duffy JM, Rowlands RR, Waugh M (2015) Stent graft types for endovascular repair of abdominal aortic aneurysms. Cochrane Database Syst Rev 9: CD008447.
- Liedenbaum MH, Vahl AC, van der Graaf Y, Spithoven JH, de Groot HG, et al (2009) The outcome of the axillofemoral bypass: a retrospective analysis of 45 patients. World J Surg 33: 2490-2496.
- Burrell MJ, Williams JR, Green RT, Smith SO, Graham RG, et al (1982) Axillofemoral bypass. Ann Surg195:796-799.
- Johnson WC, Lee KK (2000) A comparative evaluation of polytetrafluoroethylene, umbilical vein, and saphenous vein bypass grafts for femoral-popliteal above-knee revascularization: a prospective randomized Department of Veterans Affairs cooperative study. J Vasc Surg 32: 268-727.
- Mulatti GC, Joviliano EE, Pereira AH (2023) Projeto Diretrizes, Sociedade Brasileira de Angiologia e Cirurgia Vascular: aneurisma da aorta abdominal. J Vasc Bras 22: e20230040.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.