SOFTXIL Silicone Implants in Asian Rhinoplasty: A Comprehensive Review of Technique and Outcomes
by Bakhtiyor Najmiddinov1, Taek Kyun Kim2*
1Shox International Hospital, Tashkent, Uzbekistan
2The PLUS Plastic Surgery Clinic, Seoul, South Korea
*Corresponding author: Taek Kyun Kim, The PLUS Plastic Surgery Clinic, Seoul, South Korea
Received Date: 08 August, 2023
Accepted Date: 14 August, 2023
Published Date: 16 August, 2023
Citation: Najmiddinov B, Kim TK (2023) SOFTXIL Silicone Implants in Asian Rhinoplasty: A Comprehensive Review of Technique and Outcomes. J Surg 8: 1866 https://doi.org/10.29011/2575-9760.001866
Abstract
Asian rhinoplasty has evolved significantly in recent years with the advent of innovative surgical techniques and implant materials. Among these, SOFTXIL silicone implants have emerged as a popular choice for nasal augmentation due to their biocompatibility, versatility, and natural-looking results. This manuscript aims to provide a comprehensive review of the role of SOFTXIL silicone implants in Asian rhinoplasty, discussing the surgical technique, patient selection, complications, and long-term outcomes.
Keywords: Asian Rhinoplasty; Silicone Implant; SOFTXIL
Introduction
Asian individuals often present unique nasal anatomy and aesthetic preferences, which require specialized approaches in rhinoplasty [1]. Typical reduction rhinoplasty maneuvers are not applicable in Asian nose due to the following characteristics: 1) Asian nose tend to have weak lower lateral cartilages and thick Skin Soft Tissue Envelope (SSTE), which blunts the nasal contours [2]; 2) Nasal bones are wider and lower in Asian nose compared to Caucasian one. Therefore, dorsal augmentation is a mainstay in Asian rhinoplasty to correct the typical appearance of a low profile and inadequate tip projection. For augmentation, various materials are available, such as silicone implant, Gore-tex, ADM (Acellular Dermal Matrix), costal cartilage, deep temporal fascia, dermofat graft, and diced cartilage graft. Asian patients are known to have thicker nasal skin envelopes, and they employ alloplastic implants more commonly than Caucasian patients do [3-5].
According to degree of stiffness, nasal implants are divided into highly soft, soft, medium, and hard types. Before 2000’s, most of the silicone implants were too hard, because of that, irritation and stimulation on the nasal skin and framework distortion were increased and high incidence of calcification was occurred subsequently. Therefore, surgeons prefer to use highly soft silicone implant for augmentation rhinoplasty for Asian nose. SOFTXIL silicone implants (BISTOOL, Seoul, Korea) are the highly soft in character, which reduce the above-mentioned complications. Moreover, the SOFTXIL silicone implants offer surgeons a reliable tool to achieve the desired nasal augmentation while respecting the natural facial harmony of Asian patients with various types of implants. This manuscript aims to explore the benefits, drawbacks, and future prospect of using SOFTXIL silicone implants.
SOFTXIL Silicone Implants: Composition and Characteristics
The liquid silicone rubber used in SOFTXIL uses different types of siloxane and silicone derivatives (NuSilTM MED4820), including silanamine, 1,1,1-trimethyl-N-(trimethylsilyl)-, hydrolysis products with silica (Figure 1). These siloxane and silicone derivatives when mixed under polymerization inducing conditions convert into polydimethylsiloxane or similar polymers such as polydimethylsiloxane and polymethylhydrogensiloxane copolymers. Considering structural similarity of these derivatives in the final finished form, these will be toxicologically evaluated as polysiloxanes using PDMS as surrogate analogue.
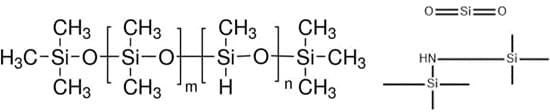
Figrure 1: Chemical structure of silanamine.
A biological safety evaluation plan of SOFTXIL, an injection molded silicone implant for use in facial surgery, has been performed. The evaluation plan identifies areas of concern to be addressed by literature review, clinical experience and testing. The evaluation of the biological safety of a medical device is a strategy planned on a caseby-case basis to identify the hazards and better estimate the risks of known hazards. Testing strategies and or waiving of tests are developed using clear, concise, logical and scientifically reasoned plans for evaluating biological safety that demonstrates that all biological hazards have been considered and relevant risks assessed and controlled. To evaluate the biological safety of the device, consideration was given to the following: type of patient contact; potential hazards of the materials of construction, the history of clinical use and testing of the materials of construction, biocompatibility testing on the device; and other information available in the literature. The reviewed available data on the materials of construction, the manufacturing process and the primary packaging suggest that SOFTXIL is designed, manufactured and packaged in such a way as to minimize the risk posed by materials, contaminants and residues to patients. This was supported by favorable results from the biocompatibility testing. Completion of the biocompatibility and chemical characterization testing recommended in this plan and subsequent evaluation of the results, combined with information from clinical experience with similar approved devices and materials, history of use, and relevant published scientific literature, will provide the basis for a biological risk assessment of the SOFTXIL.
Literature review on the raw materials used to construct the SOFTXIL is part of a comprehensive risk analysis approach. Published literature is retrieved from different sources [6-9]. ToxPlanet, TSCATS (which catalogs toxicity studies submitted to the EPA under TSCA), and ChemFinder were consulted. These databases typically consider studies peer-reviewed by authorities, or studies conducted following the requirements of recognized standards. The search terms include elements such as CAS numbers (when available), chemical names, safety, chemistry, toxicology, toxicity, or biocompatibility. ToxPlanet is a comprehensive database that indexes dozens of relevant toxicity databases comprising, for example: • Registry of Toxic Effects of Chemical Substances (RTECS), • FDA’s Select Committee on GRAS Substances (SCOGS) Reports database, • European Chemicals Agency (ECHA) database; • Agency for Toxic Substances and
Disease Registry (ATSDR) reports database); • ChemIDplus (which indexes databases such as HSDB, DART, EMIC, CCRIS, IRIS, Medline, and Toxline), • PubMed, • Various other on-line sources/databases (i.e., FDA.gov)]. The literature review on each individual raw material is not intended to be exhaustive as presented in the guidelines of the informative Annex C of ISO 10993-1 but to provide information on actual hazards related to raw materials. To assess the overall toxicological risks, other parameters must also be taken into account such as the manufacturing process (including implementation, cleaning, packaging and sterilization where applicable) and its potential residues. For this reason, the intent of this subsection is not to review and triage all the existing published data on each raw material, but to identify any documented known toxicological risks.
Consideration on Selecting width and Height of Implant [10]
The width of the implant is as important as the height of the implant. Even an expert surgeon may focus on the height of the implant, but not consider the width of the implant unintentionally. Incorrect selection of an implant may cause the visible edge of the implant or an awkward and unnatural-looking nasal shape. Nasal width is evaluated in frontal view, similarly to inter-alar distance. It is assumed ideal if it is one fifth of the total facial width. a quarter of facial width, about 3.5cm, is generally considered beautiful in Korean females. Nasal width is subdivided into dorsal width and bony base width (Figure 2). Dorsal width is considered as the width between the dorsal aesthetic lines and is similar to the width of tip defining points, which is 6-8mm in Caucasian females, and 8-10mm in Caucasian males. The width of nasal bones seems to be wide if a bony base width is wider than the intercanthal distance and, therefore, osteotomy should be considered before selecting implant.
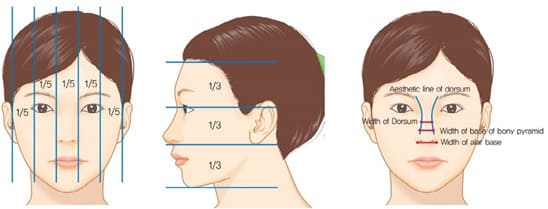
Figure 2: Nasal proportion in both frontal and lateral view & Aesthetic dorsal lines and indices in frontal view.
There are several issues to be considered when selecting the width and height of the implant
- Original nasal height of patient. Higher and wider implant is suitable for patient with extremely low nasal dorsum (Figure 3).
- Facial width in frontal view. An implant with narrow width is not suitable for patient with wide or long face (Figure 4).
- Thickness of nasal envelope. An implant with narrow and short width is suitable for patient with thick nasal envelope (Figure 5).
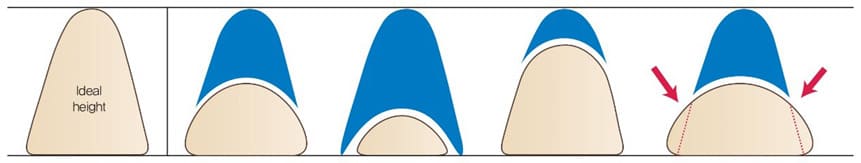
Figure 3: Implant selection according to height and width of nasal bone.
Figure 4: A selection of implant considering facial width, size and vertical height in frontal view.
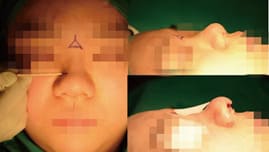
Figure 5: An implant with narrow and short width is suitable for patient with thick nasal envelope.
Implant Pocket Dissection
Once local anesthesia is given, 15 minutes is given to its action is started. Columellar and marginal incisions are made and pocket dissection is started. A cephalic margin of alar cartilage is a frequent site where many surgeons make a mistake during the pocket dissection. It is difficult to dissect within supraperichondrial dissection level cephalically because this site is the transition area into nasal SMAS, which happens more frequently with the endonasal approach. These previous mistakes can be found secondary operations. A traditional concept of augmentation rhinoplasty is simply making pocket and just inserting implant. Therefore, a proper size of pocket dissection was recommended by the majority of surgeons. However, the tip plasty technique has been well developed using an open approach and dramatic changes of the tip are possible using tip projection and rotation methods. These changes affect length and tension of the nasal envelope. Therefore, it requires a transition of the concept of pocket making because the nasal envelope should be changed as well, depending on underlying structure, without tension. The author dissects a pocket wider than dissection by the traditional tight pocket, as shown in (Figure 6). It tends to be wider caudally because of the following reasons:
- A nasal lengthening procedure is common in Asian rhinoplasty. It requires wider dissection caudally for tension-free redraping of nasal envelope.
- A tension-free closure of nasal envelope is necessary for stable dorsal implant onlay graft.
- A cephalic dissection of pocket should be minimal to prevent malposition of implant.
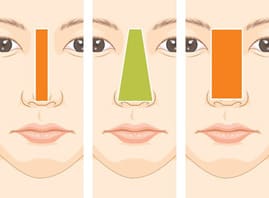
Figure 6: Ideal pocket dissection area for implant onlaying graft is narrow superiorly and wide inferiorly.
These explanations are related to the surgical manipulation of Transverse Nasalis Aponeurotic Fiber (TNAF) [1]. A strong ligament-like structure, which is the lateral wall of the pocket for the implant inset, is firmly attached to the nasal envelope, nasal bone and upper lateral cartilage. The TNAF is hard to elevate with Joseph elevator, thus sharp dissecting scissors are useful. If TNAF is elevated incorrectly with blind dissection trough the endonasal approach, problems such as asymmetry or reflecting of implant might occur due to hindering the effect of TNAF between the nasal envelope and implant. Accurate release of the aponeurotic attachment allows accurate pocket dissection and prevents implant malposition. Release of the TNAF also allows extension of the nasal skin which is essential for managing patients with a short nose or secondary contracted nose.
Implant Design and Carving
Although it is possible to prefabricate the SOFTXIL implants (Figure 7), it is often impossible to adjust the patient`s
nasal dorsum in this way for the following three reasons: First, the thickness and texture of the nasal envelope are different according to the location. Second, the exact shapes of nasal bone and upper lateral cartilage vault are not reflected through nasal envelope. Last, tip plasty alters the length and height of the implant. Therefore, it is preferred to design the implant after tip plasty is accomplished during the surgery. However, the flexibility of the operative plan and diversity of surgeon`s approaches should be respected as well. The authors prefer a nasal implant with a tapered tail shape. A tapered tail shape means that the thickness of the implant gets thinner caudally. It helps long-term stability, prevention of complications and ease of revisional operation, although implant carving itself is difficult and tip plasty has to be completive (Figure 8).
Figure 7: A various spectrum of SOFTXIL with consultation box for fitting on patient’s nose.
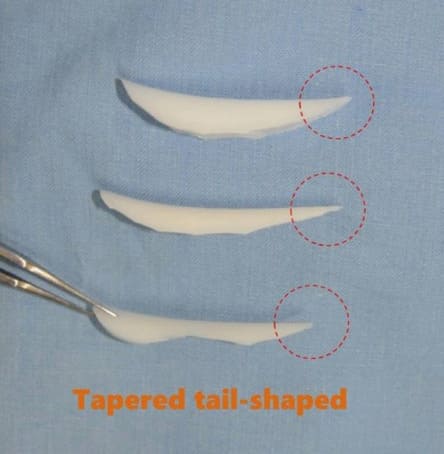
Figure 8: Tapered tail-shaped implant.
A nasal tip plasty is followed by a final carving of the implant after slightly higher and longer implant is chosen and designed because an over-carved implant is useless. It is better to prepare a new implant to carve if the previous implant is over-carved. An implant insertion time should be limited as it may be one of the reasons causing problems. It is also recommended for surgeons to change the surgical gloves after completion of implant carving because an occasional hole may be created on the gloves during the carving. An aggressive irrigation of the pocket helps to remove the small silicone fragments or rasped bony segments, which helps in reducing the postoperative infection rate. Carving the anterior surface of the implant should be avoided because irregular anterior surface of silicone results in the irregular capsule formation prone to calcification. Therefore, it is preferred to carve posterior surface to control shape and height. A precise coaptation or perfect fitting between the underlying framework and the implant is vital. Inappropriate fitting is main cause of implant deviation, movability, erosion or deformity of the internal structures.
Considerations on Implant Location
The implant dissection should be symmetrical on both sides. Excessively wide or tight space for implant might cause visibility or deviation of implant due to postoperative edema and hematoma. Three-point palpation test should be done for final judgement of dorsal line. The connection between the caudal end of the implant and tip graft should be checked carefully, as this point may cause a supratip breakage. In addition, it is required to carve new implant or to fill space with cartilage or ADM if there is a space or discrepancy under the implant at supratip area. A fixation suture on caudal end of implant is needed if it is worrisome about implant migration. However, it might cause supratip depression if it is too excessive.
Comparison with Other Implant Materials
In Asian rhinoplasty, different materials are commonly used, including SOFTXIL, other silicone implants, autologous cartilage, Gore-Tex, and Medpor implants. SOFTXIL silicone implants offer a natural look and come in various shapes and sizes with lower risk of extrusion and capsular contracture over other silicone implant due to its softness. Autologous cartilage, harvested from the patient’s own body, provides natural results and integration with surrounding tissues, but the procedure is more complex and may result in additional scarring and absorption with following changes in shape. Gore-Tex implants are lightweight and biocompatible, but they can become visible and thinner under the skin over time. Moreover, stealth inflammation rate is higher than silicone implants since surrounding tissue grows into pores of the Gore-Tex implant. Therefore, it is usually severe condition once the inflammation signs are visible externally. Medpor implants also allow tissue ingrowth and come in various shapes, but they may also lead to visibility and extrusion issues due to their hardness. The choice of material depends on individual factors, and it’s essential for patients to discuss their options with a qualified surgeon to achieve the best possible outcomes. Ultimately, the surgeon’s expertise and experience play a crucial role in determining the success of the rhinoplasty procedure, regardless of the material used. However, it is better to choose better option if the surgeon does not change.
Conclusion
The SOFTXIL silicone implants have emerged as a popular choice for nasal augmentation due to their biocompatibility, versatility, and natural-looking results in Asian rhinoplasty. Proper surgical techniques can achieve the best outcome based on a comprehensive review of the SOFTXIL silicone implants in Asian rhinoplasty.
Reference
- Kim TK, Jeong JY (2019) Surgical anatomy for Asian rhinoplasty. Archives of Craniofacial Surgery 20: 147.
- Toriumi DM, Pero CD (2010) Asian rhinoplasty. Clinics in Plastic Surgery 37: 335-352.
- Shirakabe Y, Suzuki Y, Lam SM (2003) A systematic approach to rhinoplasty of the Japanese nose: a thirty-year experience. Aesthetic Plast Surg 27: 221-231.
- McCurdy JA Jr (2002) The Asian nose: augmentation rhinoplasty with Lshaped silicone implants. Facial Plast Surg 18: 245-252.
- Jang YJ, Yu MS (2010) Rhinoplasty for the Asian nose. Facial Plast Surg 26: 93-101.
- Kawabi M, Ichihara T, Sano M, Hagiwara A, Tamano S, et al. (2005) Lack of carcinogenicity of silicone resin (KS66) in F344 rats, Food and Chemical Toxicology 43: 1065-1071.
- Swanson AB, Nalbandian RM, Zmugg TJ, Williams D, Jaeger S, et al. (1984) Silicone implants in dogs, Clin Orthop Relat Res 184: 293-301.
- Bradley SG (1994) Subchronic 10 day immunotoxicity of polydimethylsiloxane (silicone) fluid, gel and elastomer and polyurethane disks in female B6C3F1 mice. Drug Chemistry and Toxicology 17: 175.
- Woutersen RA, Jonker D, Stevenson H, te Biesebeek JD, Slob W (2001) The Benchmark approach applied to a 28-day toxicity study with Rhodorsil Silane in rats, the impact of increasing the number of dose groups, Food Chem Toxicol 39: 697-707.
- Jeong JY, Kim TK (2018) Rebuilding nose: rhinoplasty for Asians. Medic Medicine 2018.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.