Shiban Immune Formula 2/5 Eradicate HIV in a Positive Patient: First Case Report
by Abdulhameed Abdulridha Kodous Al-Shaibani1, Abderahim Maallah1, Abdeljabar El Andaloussi2*
1Shiban Pharma Inc., Laval, Québec, Canada
2BioImmune Solutions Inc., Laval, Quebec, Canada
*Corresponding authors: Abdeljabar El Andaloussi, BioImmune Solutions Inc., Laval, Quebec, Canada. Email: ajelandaloussi@bioimmunesolutions.com
Received Date: 31 October 2025
Accepted Date: 06 November 2025
Published Date: 10 November 2025
Citation: Al-Shaibani AAK, Maallah A, El Andaloussi A (2025) Shiban Immune Formula 2/5 Eradicate HIV in a Positive Patient: First Case Report. Infect Dis Diag Treat 9: 282. https://doi.org/10.29011/2577-1515.100282
Abstract
Background: HIV-1 cases continue to occur, especially in the developing world. The available treatment for HIV-1 patients include daily prescription antiretroviral medications and carry multidrug-resistant viral strains. So far, no therapeutic intervention has been proved effective to clear the viral load of HIV in a short lapse of time of one year which is good to avoid toxicity due to a long treatment period estimated at several years. Case presentation: Here, we report the case of an HIV positive patient with immune recovery under a natural therapy Shiban 2/5 with biological response over 12 months free of toxicity. Conclusion: Shiban 2/5 natural therapy was able to clear HIV detection by quantitative real-time RT-PCR with immune response free of side effects.
Background
Since the start of the HIV-1 pandemic in the 1980s, approximately 39.9 million people were living with HIV at the end of 2023. HIV1 cases continue to occur, especially in the developing world. The available treatment for HIV-1 patients include daily prescription antiretroviral medications and carry multidrug-resistant viral strains. So far, no therapeutic intervention has been proved effective to clear the viral load of HIV in a short lapse of time of one year which is good to avoid toxicity due to a long treatment period estimated at several years.
The human immunodeficiency virus (HIV-1) was described as a novel retrovirus responsible for acquired immunodeficiency syndrome or AIDS [1,2]. Since the start of the pandemic in the 1980s, approximately 39.9 million people were living with HIV at the end of 2023 [3,4]. HIV-1 belongs to the lentivirus genus of retroviruses. The HIV genome encodes a total of three structural proteins, two envelope proteins, three enzymes, and six accessory proteins. It is characterized by its irreversible integration into the host genome followed by long clinical incubation period, and the ability to infect non-dividing cells [5]. The virions are described by a lipid bilayer embedded with glycoproteins gp120 and gp41, termed Envelope or Env, which mediate binding to the host CD4 T cell receptors and fusion with its membrane. The viral particles contain two single-stranded RNA molecules, matrix and Capsid, viral enzymes including Reverse Transcriptase or RT, Integrase, and Protease [6,7]. In 2023, nearly 40 million people were carrying HIV-1 worldwide with a majority of two-thirds in subSaharan Africa [8]. HIV-1 cases continue to occur, especially in the developing world. The available treatment for HIV-1 patients include daily prescription antiretroviral medications and carry multidrug-resistant viral strains.
The development of prophylactic HIV-1 vaccine needs many years to be reached and there is a continued need to search for more novel and affordable HIV-1 therapies, such as natural therapy. Recently, the exploration of natural products to solve several limitations and issues of the available therapies in terms of safety and efficiency, was recommended by WHO to be systematically tested to eradicate HIV-1 [1,2]. Shiban 2/5 as a natural formulation exhibiting antiviral and anti-inflammatory properties against SARS-CoV-2 in vitro [9] and in vivo (data not published). Our results demonstrate that IF 2/5 treatment of HIV-1 patients was able to reduce the copy number of HIV-1 present in the blood and to clear the virus definitely after 5 months of treatment, using quantitative real time PCR. We are reporting for the first time a novel anti-HIV natural product therapy high-efficient and safe.
Case Presentation
Here, we report the first case of an HIV positive 43-year-old patient suffering from clinical symptoms of HIV infection mainly: fatigue, swollen lymph nodes and muscle aches. After the first diagnosis of the HIV infection, the patient was treated based on antiretroviral therapy (ART) (tenofovir. Lamivudine, dolutegravir at the dose of 300, 300 and 50 respectively) causing side effects. Shiban 2/5 is an immuno formula known under the commercial name of Shibanico 2 and Shibanico 5 (Shiban Pharma inc. Laval, Canada), are a mixture of extracts from propolis, cinnamon, acacia, tannic acid, and clove.
The Shiban 2/5 treatment was given at the dose of six and two tablets from Shibanico 5 and Shibanico 2 respectively, daily for 12 months. The biological response was monitored over 12 months. Prior molecular analysis, we used the one step rapid test rapid detection kit (Figure 1A). The results show that the relative sensitivity of the HIV 1/2 rapid test cassette is >99.9 % and the relative specificity is >99.8 %. The cassette was loaded with a sample prior and post Sibanico 2/5 treatment with control (C) positive Under both samples. However, the detection of HIV 1/2 band (T) was confirmed only in the sample post treatment with Shibanico 2/5. In parallel the quantification of the viral load determined in cp/ml of HIV copies was done on the blood samples of the patient following the manufacturer recommendation (Cepheid-GeneXpert, USA). The quantification range of this assay in the range 40 cp/ml – 10.000.000 cp/ml. The detected quantity of HIV DNA represents the amount of viral genomic nucleic acid at withdrawal blood sample (Figure 1B). For that, we used two samples, prior December 2022 and post July 2023 Shibanico 2/5 therapy. We obtained a significant decrease in the viral load from 1 870 000 cpm/ml (Dec 2022) to 1005 cpm/ml (Jul 2023).
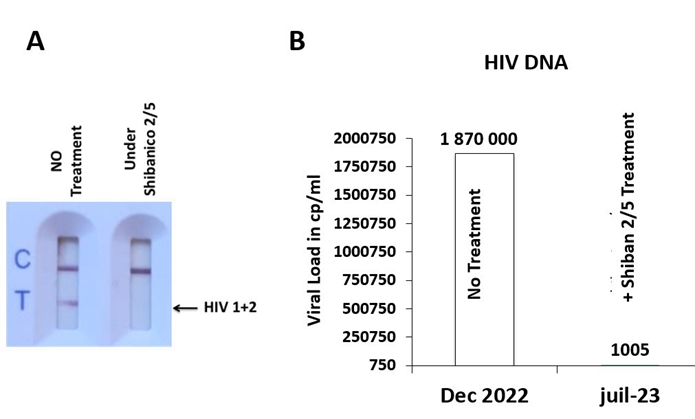
Figure 1: HIV DNA Detection and Quantification. (A) Rapide Test cassette loaded with both samples prior and post Shiban 2/5 treatment. (B) HIV DNA quantification by RT-PCR.
Laboratory work was targeting to explore the immune defense function by monitoring the blood cells and focusing on the WBC (a group of a five cell types playing different roles covering pathogens such as HIV in our case, destruction and antibodies production : neutrophils, lymphocytes, monocytes, eosinophils, and basophils) as the crucial cells of the immune system that défend the patient body against HIV infection.
The generated results from patient blood samples prior and post natural therapy, showed a significant increase in the major blood cells under the Shiban 2/5. The white blood cells count reached 7,3x103/L, with 59,4 % (50-70) neutrophils, 1,4 % eosinophils (0,5 - 5), 0,1 %basophils (0 - 1), 32,8 % lymphocytes (20 - 40), and 6,3 % monocytes (3 12). Hemoglobin was 15,7 g/dl, and platelets were 172x109/L (Table 1).
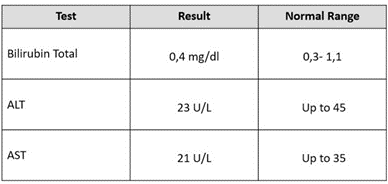
Table 1: Effect of Shiban Natural Treatment on Blood Cellularity Distribution of HIV Patients. (ALT: Alanine Transaminase; AST: Aspartate Transferase).
To evaluate the toxicity of the Shiban 2/5 treatment, further investigation were performed on the serum sample of 26/05/2023 (Table 2), including bilirubin total (0,4 mg/dl), ALT (23 U/L) and AST (21 U/L). Our results confirmed the safety of the Shiban 2/5 treatment and its ability to preserve the Hb (hemoglobin stability) (Figure 2A) and to promote the red blood cells (RBC) (Figure 2B), protecting against anemia. In addition the WBC as the main cells involved in the immune response strengthening the immune response were promoted and protected as well to keep the patient body free of HIV (Figure 3) and to prevent further infections with opportunistic contaminations.
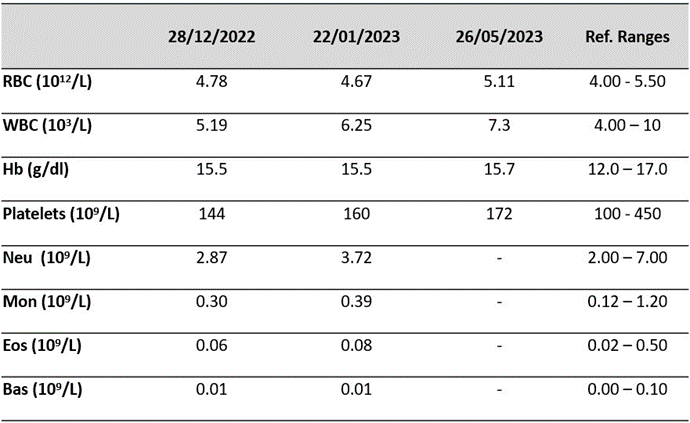
Table 2: Toxicity Biomarkers under Shiban Natural Treatment in Normal Range. (RBC: Red Blood Cells; WBC: White Blood Cells; Hb: Haemoglobine; Neu: Neutriophils; Mon: Monocytes; Eos: Eosinophils; Bas: Basophils).
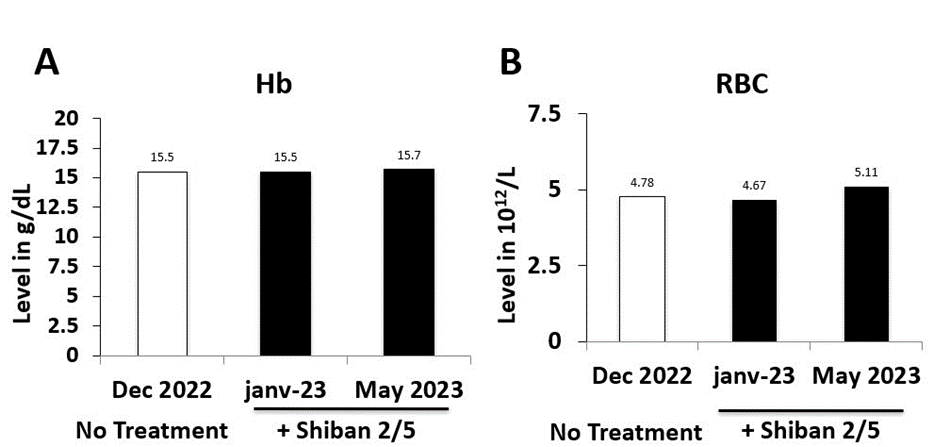
Figure 2: Blood Anemia Evaluation under HIV and/or Shiban 2/5 natural Therapy. (A) Hemoglobin (Hb) test and (B) Red blood cells (RBC).
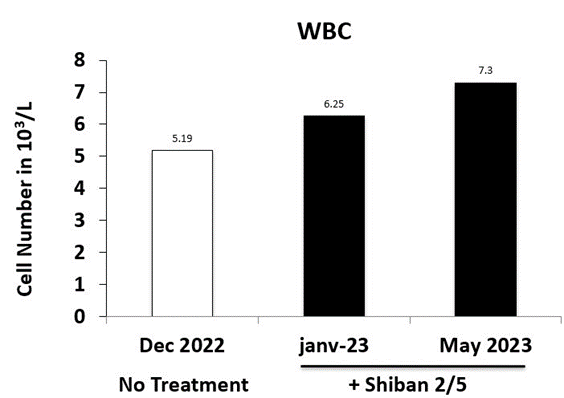
Figure 3: Biological Exploration of Immunity. The pool of white blood cells (WBC) was compared between prior and post treatment using Shiban 2/5 and at the treatment start and end for a period of 5 months.
Shiban IF 2/5 immune natural therapy has the advantage of no toxicity compared to the available antiviral therapies in the market and no health care costs. The efficiency and toxicity was evaluated based on complete lab work to monitor the biological response to the IF 2/5 therapy. Also, no risk of treatment failure or drug resistance. Our first case presents a new natural treatment regimen.
Discussion and Conclusion
HIV therapy is far from perfect and significant progress has been made in the last 20 years. The available ART does not guarantee a safe drug regimen due to the potential for ART escape mutant requiring continuous monitoring of the drug treatment. To overcome the limitation of ART, the natural products are a promising alternative to find effective suppressive compounds for complete HIV clearance [10]. In addition, the investigation of the combination of propolis and tannic acid against SARS-CoV2 was the goal of our first report published recently (ABB, 2025). Our data highlighted the importance of the synergic effect of the two compounds in the inhibition of the SARS-CoV2 proliferation and survival in the infiltrated respiratory cells. The literature documents several natural products exhibiting the potential to repress HIV activities. The Shiban 2/5 is the first natural product to be used in vivo as a formulation of propolis and tannic acid are known to exhibit anti-viral properties, as reported in previous studies [11,12]. Furthermore, tannic acid reactivates HIV-1 latency important for the eradication of the viral reservoirs. In addition, the antioxidant and antitumor activities [13,14]. Tannic acid will help to rescue the complicated cases of HIV patients developing tumors. Despite the role of tannic acid, the blood signature showed absence of hematological features such as anaemia, leukopenia or thrombocytopenia [15]. The reported level of white blood cells, red blood cells could predict good outcomes under a safe and efficient natural immune therapy. The study of Shiban 2/5 as an immune anti-viral therapy able to clear viruses was confirmed against HIV 1/2 as well for SARS-CoV2. We conclude, our first case report reveals promising improvement of HIV therapy using natural product formulation for the first time. Furthermore, it solves the whole reported limitations of precious anti-drugs therapies, as safe and effective for a defined time period used for our present case. This first case report deserves to be assessed in future clinical trials.This case highlights the importance of natural treatment in HIV- positive patients, where immunosuppression is a serious health issue and needs immune evaluation controlled by HIV infection and total blood monitoring through HIV rapid test and blood lab work respectively. The advantage of Shiban 2/5 therapy is its ability of immunity strength and prevention from further toxicity and/or opportunistic infections and cancers.
Ethics approval and consent to participate: The treatment for the patient is performed under the tenets of the Declaration of Helsinki. The patient reported in the study provided vidéo recorded informed consent for this publication.
Declaration of Interest: Shiban Pharma has 3 patents related to IF2/5 immunoformulations (1-Canada # 3.151.164, 2- European Union # 21890438.1, 3- PCT: WO 2022/099414 A1).
Acknowledgments: The authors would like to thank Bioimmune Solutions (www.bioimmunesolutions.com) for their wonderful scientific work in the manuscript conceptualisation including the formal analysis, writing, review and editing.
Conflict of interest: The authors declared no conflict of interest.
References
- Kim JG, Shan L (2022) Beyond Inhibition: A Novel Strategy of Targeting HIV-1 Protease to Eliminate Viral Reservoirs. Viruses 14:1179.
- Gallo RC, Salahuddin SZ, Popovic M, Shearer GM, Kaplan M, et al. (1984) Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science 224:500-503.
- Stevens O, Sabin K, Anderson RL, Garcia SA, Willis K, et al. (2024) Population size, HIV prevalence, and antiretroviral therapy coverage among key populations in sub-Saharan Africa: collation and synthesis of survey data, 2010-23. Lancet Glob Health 12:e1400-e1412.
- Gallay P, Hope T, Chin D, Trono D (1997) HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc Natl Acad Sci U S A 94:9825-9830.
- Anderson DW, Virmani R, Reilly JM, O’Leary T, Cunnion RE, et al. (1988) Prevalent myocarditis at necropsy in the acquired immunodeficiency syndrome. J Am Coll Cardiol 11:792-799.
- Engelman A, Cherepanov P (2012) The structural biology of HIV-1: mechanistic and therapeutic insights. Nat Rev Microbiol 10:279-290.
- Merk A, Subramaniam S (2013) HIV-1 envelope glycoprotein structure. Curr Opin Struct Biol 23:268-276.
- Haeuser E, Serfes AL, Cork MA, Yang M, Abbastabar H, el al., (2022) Mapping age- and sex-specific HIV prevalence in adults in subSaharan Africa, 2000-2018. BMC Med 20:488.
- Al Shaibani AA, Maallah MT, Maallah A (2025) Antiviral Activity of Natural Compounds Immuno Formula Shiban2/5 against SARSCoV-2. Advances in Bioscience and Biotechnology 16:3.
- Richard K, Poli ANR, Andrae-Marobela K, Tietjen I (2024) Medicinal Plant and Traditional Knowledge-guided Strategies to Combat HIV Persistence. Curr HIV/AIDS Rep 22:5.
- Gekker G, Hu S, Spivak M, Lokensgard JR, Peterson PK (2005) AntiHIV-1 activity of propolis in CD4(+) lymphocyte and microglial cell cultures. J Ethnopharmacol 102:158-163.
- Harish Z, Rubinstein A, Golodner M, Elmaliah M, Mizrachi Y (1997) Suppression of HIV-1 replication by propolis and its immunoregulatory effect. Drugs Exp Clin Res 23:89-96.
- Chen C, Zhong Z, Zhang W, Xia B, Wu L, et al. (2025) Tannic acid reactivates HIV-1 latency by mediating CBX4 degradation. J Virol 99:e0117324.
- Wu Y, Zhong L, Yu Z, Qi J (2019) Anti-neuroinflammatory effects of tannic acid against lipopolysaccharide-induced BV2 microglial cells via inhibition of NF-kappaB activation. Drug Dev Res 80:262-268.
- Zhou Y, Lu T, Li Y, Qin Y, Lu Y, et al. (2023) Severe anemia, severe leukopenia, and severe thrombocytopenia of amphotericin B deoxycholate-based induction therapy in patients with HIV-associated talaromycosis: a subgroup analysis of a prospective multicenter cohort study. BMC Infect Dis 23:707.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.