Serum ZFPL1 as a Clinical Biomarker for Diagnosis, Progression Tracking, and Treatment Response in Lung Cancer
by Teng Yu1#, Honglin Liu2#, Yingxuan Chen2, Lili Xu2, Xiaoni Pang2, Haihui Yang2, Shuwen Chen2, Guanjie Lu1, Jianhua Feng1*, Biwen Mo3,4,5*
1Department of Tuberculosis, Zhongshan Second People's Hospital, China.
2Department of Clinical Laboratory, Zhongshan Second People's Hospital, China.
3Department of Respiratory and Critical Care Medicine, The Second Affiliated Hospital of Guilin Medical University, China
4Guangxi Clinical Research Center for Diabetes and Metabolic Diseases, The Second Affiliated Hospital of Guilin Medical University, China
5Education Department of Guangxi Zhuang Autonomous Region, Guilin Medical University, China
#Contributed equally to this work as co–first authors
*Corresponding authors: Biwen Mo, Department of Respiratory and Critical Care Medicine, The Second Affiliated Hospital of Guilin Medical University, 541199, Guilin, China.
Jianhua Feng, Department of Tuberculosis, Zhongshan Second People's Hospital, Zhongshan, Guangdong, 528447, China.
Received Date: 21 July, 2025
Accepted Date: 06 August, 2025
Published Date: 08 August, 2025
Citation: Yu, T, Liu H, Chen Y, Xu L, Pang X, et al. (2025) Serum ZFPL1 as a Clinical Biomarker for Diagnosis, Progression Tracking, and Treatment Response in Lung Cancer. J Med Biomed Discoveries 7: 141. https://doi.org/10.29011/2688-8718.100141.
Abstract
Objective: Zinc finger protein-like protein 1 (ZFPL1) is a key protein involved in various biological processes such as cell growth, migration, proliferation, and metabolism. It plays a significant role in the development and progression of multiple malignancies, including endometrial cancer and gastric cancer. However, research on ZFPL1 in lung cancer remains relatively limited. This study aimed to investigate the clinical application value of serum ZFPL1 in the diagnosis, disease monitoring, and therapeutic evaluation of lung cancer by detecting its expression levels in the peripheral blood of lung cancer patients using enzyme-linked immunosorbent assay (ELISA). Methods: Serum ZFPL1 levels were measured by ELISA in 75 lung cancer patients (including 32 cases of lung adenocarcinoma, 31 cases of squamous cell carcinoma, and 12 cases of small cell lung cancer) and 75 healthy controls. Differences in serum ZFPL1 expression were analyzed based on various clinical characteristics (gender, age, smoking history, pathological type, tumor location, and TNM stage). Bioinformatics analysis was performed to explore ZFPL1-related signalling pathways. Serum ZFPL1 levels were dynamically monitored before and after treatment in 57 patients receiving two chemotherapy cycles and 18 patients undergoing surgery. The diagnostic efficacy of serum ZFPL1 for different types and stages of lung cancer was evaluated using receiver operating characteristic (ROC) curve analysis. Results: Serum ZFPL1 levels showed no significant differences among lung cancer patients stratified by gender, age, smoking history, pathological type, or tumor location. Bioinformatics analysis revealed that ZFPL1 was significantly enriched in pro-oncogenic signaling pathways such as mTORC1, E2F, MYC, PI3K/Akt, and the reactive oxygen species pathway. Serum ZFPL1 levels were significantly higher in the lung cancer group than in the healthy control group (P < 0.05) and paradoxically higher in early stages (I-II) vs. advanced (III-IV). Both chemotherapy and surgical treatment significantly reduced serum ZFPL1 levels (P < 0.05). The objective response rate (ORR) and disease control rate (DCR) in the chemotherapy group were 17.54% and 89.47%, respectively, while the surgical group achieved a 100% efficacy rate. The area under the ROC curve (AUC) for serum ZFPL1 in diagnosing lung cancer was 0.921 (sensitivity 78.67%, specificity 98.67%). The AUC values for diagnosing lung adenocarcinoma, squamous cell carcinoma, and small cell lung cancer were 0.920, 0.912, and 0.951, respectively. The AUC for differentiating early-stage from mid-to-late-stage lung cancer was 0.657 (sensitivity 91.23%, specificity 44.44%). Conclusion: Serum ZFPL1 may serve as a novel serological biomarker for lung cancer diagnosis. Changes in serum ZFPL1 levels can effectively evaluate the efficacy of chemotherapy and surgical treatment, and serum ZFPL1 has potential predictive value for disease progression in lung cancer.
Keywords: Zinc finger protein-like 1 (ZFPL1); Lung cancer diagnosis; Serum biomarker; Treatment Response evaluation
Introduction
Lung cancer, recognized as the most prevalent and aggressive solid malignancy worldwide, has attracted significant attention due to its genomic complexity and high mortality rate [1],Epidemiological data indicate approximately 1.8 million new lung cancer cases are diagnosed annually, with about 75% of patients presenting at advanced stages upon initial diagnosis, the 5-year survival rate varies substantially between 4% and 17%, influenced by tumor staging and regional disparities in healthcare access [2-4]. Given this generally poor prognosis, achieving early diagnosis before distant metastasis occurs is critical for improving patient outcomes. However, current diagnostic approaches are limited by inadequate sensitivity, high costs, and invasive procedures [5], underscoring the urgent need to develop novel molecular biomarkers to optimize early screening and therapeutic evaluation systems.
Imaging-based screening faces particularly notable challenges. Clinical studies demonstrate that 15-52% of lung cancer cases are missed during baseline CT examinations [6]. The high prevalence of benign pulmonary nodules frequently leads to false-positive CT results, often prompting unnecessary repeat testing or even invasive biopsies [7]. Additionally, diagnostic delays may result in false-negative or indeterminate findings. From a molecular perspective, lung cancer development involves dysregulation of multiple oncogenes, tumor suppressor genes, and signalling pathways [8]. These molecular alterations drive the release of tumor DNA, cellular fragments, and abnormal proteins into bodily fluids, providing a theoretical foundation for liquid biopsy-based early diagnosis. Among potential sources, serum/plasma is considered the most clinically translatable biomarker reservoir due to its accessibility and minimal invasiveness, offering promise for risk stratification and precision management of high-risk populations undergoing CT screening.
The zinc-finger protein (ZNF) family, characterized by distinctive domain features enabling interactions with nucleic acids and proteins, plays crucial roles in key biological processes including transcriptional regulation and DNA repair [9,10]. Research has identified 61 ZNF molecules participating throughout tumorigenesis and progression. For instance, ZNF6 drives colorectal cancer advancement through Wnt/β-catenin pathway activation [11], while ZNF113A overexpression predicts poor prognosis in oesophageal squamous cell carcinoma [12]. ZNF148 demonstrates aberrant overexpression across multiple malignancies including breast cancerand ZEB1 exerts oncogenic effects by modulating E-cadherin expression [13].
As an important family member, zinc finger protein-like 1 (ZFPL1) is a conserved integral membrane protein featuring a characteristic dimeric structure comprising two zinc finger domains (C3H, C2HC) and a protein-interaction loop [14]. TCGA and KEGG data-base analyses reveal ZFPL1's significant enrichment in key cancer-related pathways including PI3K/Akt/mTOR, mTORC1, E2F, Myc, and ROS. Existing studies confirm markedly elevated ZFPL1 expression in endometrial cancer tissues, where its knockout suppresses tumor proliferation by inhibiting AKT phosphorylation and upregulating PTEN expression [15]. In gastric cancer, ZFPL1 downregulation promotes tumor cell death through autophagy rather than apoptosis pathways [16]. Secreted by prostate tumor cells of neuroendocrine (NE)/stem cell phenotype through exosomal secretion, Serum levels of ZFPL1 in cancer patients were at least 4-fold higher than those in the sera of cancer free individuals, The knockdown of endogenous ZFPL1 in (PC) cells led to a remarkable decrease in cell proliferation, and invasion while increasing their apoptosis [17]. However, ZFPL1's value in lung cancer diagnosis and therapeutic monitoring remains systematically unexplored.
Based on this background, our study employed ELISA assay to measure ZFPL1 expression levels in peripheral blood from lung cancer patients, systematically evaluating its clinical utility for diagnosis, disease monitoring, and treatment efficacy prediction. Our findings suggest that serum ZFPL1 may serve as a diagnostic biomarker, with its dynamic changes effectively reflecting chemotherapy response and surgical outcomes. Notably, its expression levels show significant correlations with disease progression. These findings underscore the diagnostic and prognostic potential of ZFPL1 in treatment-naïve lung cancer patients, providing novel molecular insights that could enhance treatment monitoring, predict disease severity, and optimize comprehensive management strategies for lung cancer
Methods
Study Population
This study enrolled 75 treatment-naïve lung cancer patients initially diagnosed at Guilin Medical University Affiliated Hospital between January 2021 and January 2022, including 32 adenocarcinoma cases, 31 squamous cell carcinoma cases, and 12 small cell lung cancer cases, along with 75 age- and sex-matched healthy controls. All cancer diagnoses were histo-pathologically confirmed. Among the cancer cohort: 57 patients received two cycles of standard chemotherapy; Squamous cell carcinoma and adenocarcinoma patients received gemcitabine, docetaxel, or pemetrexed combined with platinum-based drugs; Small cell lung cancer patients received platinum-etoposide regimens [18] patients underwent surgical resection only. All participants provided written informed consent. The study protocol was approved by the Institutional Ethics Committee of Guilin Medical University Affiliated Hospital (Approval No. YJSLL2021126) and conducted in accordance with the Declaration of Helsinki principles.
Participant Recruitment
The study subjects were selected based on the following criteria
Inclusion criteria
- Lung cancer patients confirmed by histopathological diagnosis;
- Newly diagnosed patients who had not received any treatment;
- Non-small cell lung cancer staged according to the TNM classification 18and small cell lung cancer classified as either limited or extensive stage [19];
- Complete clinical medical records available.
Exclusion criteria
- Patients with non-primary lung cancer;
- Patients in acute phase of any infection or active phase of immune-related diseases;
- Patients with concurrent malignancies in other sites;
- Patients who had received radiotherapy or multiple treatment modalities.
Medical Record Collection
The diagnostic criteria for lung cancer patients align with the Chinese Medical Association's 2024 guideline on clinical diagnosis and treatment of lung cancer [20]. Complete medical records of lung cancer patients were collected, including gender, age, smoking status, histopathological reports, tumor size and location, with surgical procedures recorded for operative cases. Disease staging was performed based on comprehensive evaluation of bronchoscopy, intraoperative pathology, chest computed tomography (CT) scans, abdominal CT examinations, superficial lymph node ultrasound, whole-body bone scans, brain magnetic resonance imaging (MRI), and positron emission tomography (PET) results.
Blood Sample Collection and Processing
Peripheral blood samples were collected from lung cancer patients before any treatment intervention, one week after completing two cycles of chemotherapy or after surgery, along with samples from healthy controls during the same period. Blood samples were centrifuged at 1,000×g for 15 minutes at 4°C using collection tubes, after which the supernatant was collected and stored at -80°C for subsequent analysis.
ELISA Analyses
The peripheral blood centrifuged at 4°C, 1,000×g/min for 15 min, the supernatants were stored at -80℃ for ELISA assays. The content in the supernatants was detected by referring to the instructions of the ZFPL1 ELISA kits (Wuhan Fine Biotech Co., Ltd., Wuhan, Hubei, China).
Gene Set Enrichment Analysis
Gene set enrichment analysis (GSEA) was performed using data obtained from the TCGA data-base (https://portal.gdc.cancer.gov). Data processing and transformation were conducted using R version 4.0.3 and Perl version 5.18.2. The resulting enrichment plots were generated based on KEGG pathway analysis.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism 9. Categorical clinical variables were analyzed using the chi-square test. Continuous variables were compared using the Mann-Whitney U test. Differences in ZFPL1 levels between two groups were assessed by Student's t-test, while comparisons among three groups were evaluated by one-way ANOVA. Changes in ZFPL1 levels before and after treatment in lung cancer patients were analyzed using the Wilcoxon matched-pairs signed-rank test. A P-value < 0.05 was considered statistically significant, with the following significance levels indicated: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Results
Clinical Characteristics of Enrolled Patients
This study analyzed clinical data from 75 lung cancer patients (50 males [66.7%] and 25 females [33.3%], mean age 60.17±8.19 years) and 75 healthy controls (58 males [77.3%] and 17 females [22.7%], mean age 59.83±8.05 years), with no significant differences in gender or age between groups (Table 1). Among lung cancer patients, 45 (60%) had smoking history while 30 (40%) were non-smokers. Pathological types included adenocarcinoma (32 cases, 42.67%), squamous cell carcinoma (31 cases, 41.33%), and small cell lung cancer (12 cases, 16%). Tumor locations were distributed in left lung (31 cases, 41.33%) and right lung (44 cases, 58.67%). TNM staging showed 18 cases (24%) in stage I-II, 25 (33.33%) in stage III, and 32 (42.76%) in stage IV. T-stage distribution was T1 (25 cases, 33.33%), T2 (13 cases, 17.33%), T3 (19 cases, 25.34%), and T4 (18 cases, 24%). Metastasis status included M0 (44 cases, 58.67%) and M1 (31 cases, 41.33%), with lymph node metastasis present in 30 cases (40%) and absent in 45 (60%). Diagnostic methods comprised bronchoscopic biopsy (33 cases, 44%), CT-guided biopsy (16 cases, 21.33%), postoperative pathology (18 cases, 24%), and other methods (8 cases, 10.67%) (Table 2). ELISA results indicated no statistically significant differences in serum ZFPL1 levels when stratified by gender, age, smoking status, tumor location, TNM stage, or distant metastasis status (Table 3).
|
Variable |
Lung Cancer Group (n=75) |
Healthy Control Group (n=75) |
Statistic |
P-value |
|
|
Sex |
Male |
50(66.7%) |
58(77.3%) |
χ² =2. 116 |
0. 146 |
|
Female |
25(33.3%) |
17(22.7%) |
|||
|
Age (years) |
60. 17±8. 19 |
59.83±8.05 |
Z =-0.747 |
0.455 |
|
Table 1: Clinical Characteristics of Enrolled Patients.
|
Variable |
Parameter |
Cases (n=75) |
Percentage (%) |
|
Age |
<60 years |
37 |
49.33 |
|
≥60 years |
38 |
50.67 |
|
|
Smoking Status |
Yes |
45 |
60 |
|
No |
30 |
40 |
|
|
Pathology |
Adenocarcinoma |
32 |
42.67 |
|
Squamous cell carcinoma |
31 |
41.33 |
|
|
Small cell carcinoma |
12 |
16 |
|
|
Tumor Location |
Left lung |
31 |
41.33 |
|
Right lung |
44 |
58.67 |
|
|
TNM Stage |
I-II |
18 |
24 |
|
III |
25 |
33.33 |
|
|
IV |
32 |
42.67 |
|
|
T Stage |
T1 |
25 |
33.33 |
|
T2 |
13 |
17.33 |
|
|
T3 |
19 |
25.34 |
|
|
T4 |
18 |
24 |
|
|
M Stage |
M0 |
44 |
58.67 |
|
M1 |
31 |
41.33 |
|
|
Lymph Node Metastasis |
Yes |
30 |
40 |
|
No |
45 |
60 |
|
|
Diagnostic Method |
Bronchoscopy |
33 |
44 |
|
CT-guided biopsy |
16 |
21.33 |
|
|
Surgery |
18 |
24 |
|
|
Other (pleural effusion, lymph node biopsy) |
8 |
10.67 |
Table 2: Clinical Characteristics of Lung Cancer Patients.
|
Variable |
Parameter |
Cases (n=75) |
Percentage (%) |
|
Age |
<60 years |
37 |
49.33 |
|
≥60 years |
38 |
50.67 |
|
|
Smoking Status |
Yes |
45 |
60 |
|
No |
30 |
40 |
|
|
Pathology |
Adenocarcinoma |
32 |
42.67 |
|
Squamous cell carcinoma |
31 |
41.33 |
|
|
Small cell carcinoma |
12 |
16 |
|
|
Tumor Location |
Left lung |
31 |
41.33 |
|
Right lung |
44 |
58.67 |
|
|
TNM Stage |
I-II |
18 |
24 |
|
III |
25 |
33.33 |
|
|
IV |
32 |
42.67 |
|
|
T Stage |
T1 |
25 |
33.33 |
|
T2 |
13 |
17.33 |
|
|
T3 |
19 |
25.34 |
|
|
T4 |
18 |
24 |
|
|
M Stage |
M0 |
44 |
58.67 |
|
M1 |
31 |
41.33 |
|
|
Lymph Node Metastasis |
Yes |
30 |
40 |
|
No |
45 |
60 |
|
|
Diagnostic Method |
Bronchoscopy |
33 |
44 |
|
CT-guided biopsy |
16 |
21.33 |
|
|
Surgery |
18 |
24 |
|
|
Other (pleural effusion, lymph node biopsy) |
8 |
10.67 |
Table 3: Association Between Serum ZFPL1 Levels and Clinical Characteristics in Lung Cancer Patients
Pathway Enrichment Analysis of ZFPL1
To elucidate the potential role of ZFPL1 in tumorigenesis and progression, we performed KEGG pathway enrichment analysis to identify cancer-related signalling pathways associated with ZFPL1. The results demonstrated significant enrichment of ZFPL1 in several critical oncogenic pathways, including: the autophagy-related mTORC1 signalling pathway, breast cancer-associated E2F signalling pathway, proto-oncogene MYC signalling pathway, PI3K/Akt oncogenic pathway, and reactive oxygen species (ROS) signalling pathway which promotes tumor cell proliferation, enhances treatment resistance, and facilitates metastasis (Figure 1A). These findings collectively suggest that ZFPL1 may contribute to tumor development through multifaceted regulation of various biological processes including cellular autophagy, proliferation, metabolism, and metastasis. Notably, its prominent enrichment in both mTORC1 and PI3K-Akt pathways strongly implies that ZFPL1 might exert its pro-tumorigenic effects by modulating cellular energy metabolism and proliferation signal transduction.
Serum ZFPL1 Level Alterations in Lung Cancer
To evaluate the potential of ZFPL1 as a diagnostic biomarker for lung cancer, we first compared serum ZFPL1 levels between treatment-naïve lung cancer patients (Pre-treatment Baseline, PTB) and healthy controls (HC). ELISA analysis revealed significantly elevated ZFPL1 expression in PTB patients compared to HC (Figure 1B). Among the 75 lung cancer cases, we observed stage-dependent expression patterns, with early-stage (I-II) patients (n=18) showing significantly higher ZFPL1 levels than advanced-stage (III-IV) patients (n=57) (Figure 1C), suggesting ZFPL1's potential role not only in diagnosis but also in disease progression monitoring.
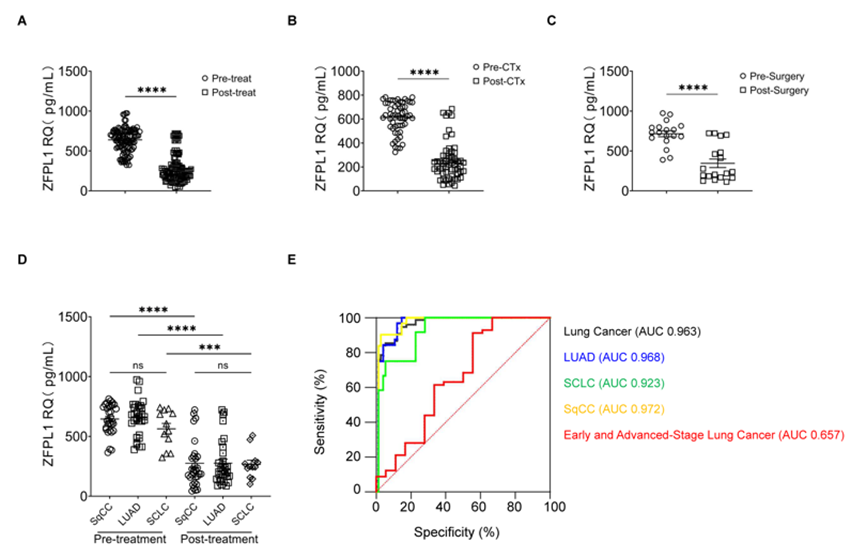
Figure 1: Dysregulated Serum ZFPL1 Levels in Lung Cancer Pathogenesis. (A) Cancer-related signaling pathways enrichment analysis of ZFPL1; (B) Serum ZFPL1 level alterations in PTB patients compared to HC; (C) Serum ZFPL1 levels in early-stage (I-II; n=18) versus advanced-stage (III-IV) lung cancer patients. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. PTB, Pre-treatment Baseline; HC, healthy controls; SEM, Standard Error of the Mean.
Treatment-Induced Modulation of Serum ZFPL1
Longitudinal analysis demonstrated consistent decreases in serum ZFPL1 levels following treatment (Figure 2A), with similar trends observed in both chemotherapy-treated (after 2 cycles, Figure 2B) and surgically-treated (1week post-operation, Figure 2C) patients. When stratified by histological subtypes - squamous cell carcinoma (SqCC, n=31), adenocarcinoma (LUAD, n=32), and small cell lung cancer (SCLC, n=12) - baseline ZFPL1 levels showed no significant differences (Figure 2D). Notably, all histological subtypes demonstrated significant ZFPL1 downregulation following either chemotherapy or surgical intervention (Figure 2D), confirming its utility as a robust treatment response marker regardless of histological classification.
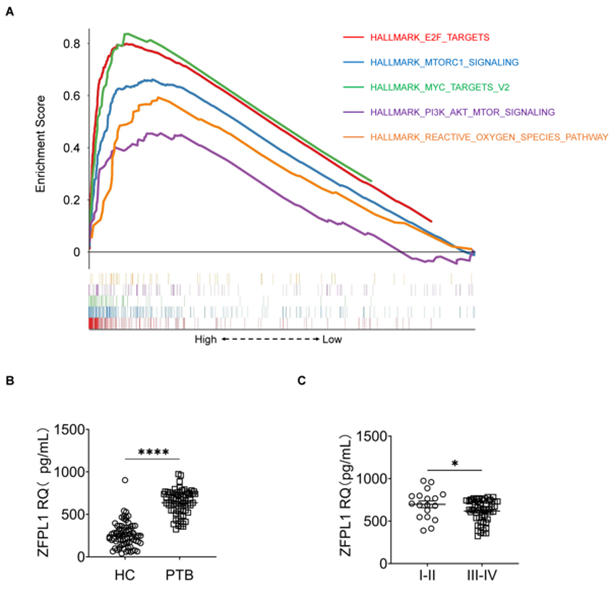
Figure 2: Serum ZFPL1 for Lung Cancer Diagnosis and Treatment Response Assessment. (A) Treatment-induced modulation of serum ZFPL1; (B-C) Serum ZFPL1 level alterations in chemotherapy-treated (B) and surgically-treated (C) patients; (D) Pre- versus post-treatment serum ZFPL1 levels in SqCC, LUAD and SCLC patients; (E) Diagnostic Value of Serum ZFPL1 Levels in Lung Cancer. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. CTx, chemotherapy; SqCC, squamous cell carcinoma; LUAD, adenocarcinoma; SCLC, small cell lung cancer; SEM, Standard Error of the Mean.
RECIST-Based Treatment Response
Treatment response was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1: complete response (CR, disappearance of all target lesions), partial response (PR, ≥30% decrease in sum of longest diameters of target lesions), stable disease (SD, neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD), and progressive disease (PD, ≥20% increase in sum of longest diameters or appearance of new lesions). Objective response rate (ORR) was calculated as (CR+PR)/(CR+PR+SD+PD) ×100%, while disease control rate (DCR) was calculated as (CR+PR+SD)/(CR+PR+SD+PD) ×100%. Treatment efficacy assessment by RECIST 1.1 criteria revealed: in the chemotherapy cohort (n=57), 0% complete response (CR), 17.54% partial response (PR), 71.93% stable disease (SD), and 10.53% progressive disease (PD), yielding an objective response rate (ORR) of 17.54% and disease control rate (DCR) of 89.47%. The surgical cohort (n=18) achieved 100% CR (Table 4).
|
Variable |
Chemotherapy Group (%) (n=57) |
Surgery Group (%) (n=18) |
|
Complete Response (CR) |
0 (0) |
18 (100) |
|
Partial Response (PR) |
10 (17.54) |
0 (0) |
|
Stable Disease (SD) |
41 (71.93) |
0 (0) |
|
Progressive Disease (PD) |
6 (10.53) |
0 (0) |
|
ORR |
17.54% |
100% |
|
DCR |
89.47% |
100% |
Table 4: RECIST-Based Treatment Response
Diagnostic Value of Serum ZFPL1 Levels in Lung Cancer
To evaluate the diagnostic potential of serum ZFPL1 as a biomarker for lung cancer, we performed ROC curve analysis to determine the AUC, optimal cut-off values, sensitivity, and specificity. Serum ZFPL1 demonstrated excellent diagnostic performance for overall lung cancer detection (AUC= 0.921, 95% CI: 0.876-0.966; cut-off= 356.2 pg/mL; sensitivity= 78.67%; specificity= 98.67%) (Table 5, Figure 2E). When stratified by histological subtypes, ZFPL1 showed similarly strong diagnostic value for LUAD (AUC= 0.920, cut-off= 411.9 pg/mL, sensitivity= 81.25%, specificity= 96.88%), SqCC (AUC= 0.912, cut-off= 360.3 pg/mL, sensitivity= 80.65%, specificity= 90.00%), and SCLC (AUC= 0.951, cut-off= 309.2 pg/mL, sensitivity= 83.33%, specificity= 95.88%) (Table 5, Figure 2E). However, its performance in distinguishing early-stage (I-II) from advanced-stage (III-IV) disease was more modest (AUC= 0.657, cut-off= 760.7pg/mL, sensitivity= 91.23%, specificity= 44.44%) (Table 5, Figure 2E). These findings demonstrate the superior diagnostic value of circulating ZFPL1 as a blood-based biomarker for lung cancer detection, while simultaneously offering referential value for evaluating disease progression.
|
Variable |
AUC (95% CI) |
Cut-off (pg/mL) |
Sensitivity (%) |
Specificity (%) |
P-value |
|
Adenocarcinoa vs. Controls |
0.921 (0.8764-0.9654) |
356.2 |
78.67 |
98.67 |
<0.0001 |
|
Squamous cell carcinoma vs. Controls |
0.920 (0.8494-0.9904) |
411.9 |
81.25 |
96.88 |
<0.0001 |
|
Small cell carcinoma vs. Controls |
0.912 (0.8364-0.9867) |
360.3 |
80.65 |
90 |
<0.0001 |
|
I-II stage vs. III-IV stage |
0.657 (0.4937-0.8201) |
760.7 |
91.23 |
44.44 |
0.0458 |
Table 5: Diagnostic Value of Serum ZFPL1 Levels in Lung Cancer.
Discussion
Lung cancer remains the most lethal malignant tumor worldwide [21]. Despite recent advancements in diagnosis, treatment, and care for lung cancer patients, the prognosis for most remains poor, as initial diagnoses often occur at advanced stages, with a 5-year survival rate of only 18.1% [22]. Therefore, early detection and timely diagnosis are critical for improving overall survival rates. Currently, the primary diagnostic methods for lung cancer include imaging screening, tumor biomarkers, and histopathology. However, early-stage lesions are often inconspicuous, and the low sensitivity of imaging techniques makes baseline-level detection challenging. Histopathology, the gold standard for tumor diagnosis, is invasive and unsuitable for real-time dynamic monitoring. In contrast, tumor biomarker testing offers advantages such as high efficiency, convenience, minimal invasiveness, and ease of acquisition, playing a significant role in early diagnosis, pathological classification, tumor staging, recurrence and metastasis monitoring, treatment efficacy evaluation, and prognosis prediction for lung cancer. Nevertheless, serum tumor markers like carcinoembryonic antigen (CEA), cytokeratin 19 fragment (CYFRA21-1), and neuron-specific enolase (NSE) often yield false-positive results due to infections, benign tumors, pregnancy, or other factors. Thus, developing highly specific biomarkers holds substantial clinical value for early diagnosis, dynamic disease monitoring, and precise treatment evaluation of lung cancer.
No prior studies have explored the correlation between ZFPL1 and lung cancer. Through TCGA and KEGG data-bases, we found that ZFPL1 is enriched in tumor-related signalling pathways such as PI3K/Akt/mTOR, mTORC1, E2F, MYC, and reactive oxygen species. Existing research indicates that ZFPL1 is highly expressed in endometrial cancer and mediates glycosylation and autophagy. We hypothesize that ZFPL1 may serve as a serum tumor marker for diagnosis and treatment evaluation. Comparing age- and gender-matched lung cancer patients and healthy controls, we observed significantly higher serum ZFPL1 levels in lung cancer patients, suggesting its potential association with the disease. Further analysis of clinical data, including gender, age, smoking status, tumor location, tumor size, and distant metastasis, revealed no statistically significant differences in serum ZFPL1 levels. Interestingly, ZFPL1 levels varied across disease stages, with significantly lower expression in advanced-stage (III-IV) patients.
For patients receiving only a single chemotherapy cycle, treatment efficacy was often limited. Since most patients require efficacy evaluation before the third cycle-with progression prompting changes in chemotherapy drugs or the addition of immune inhibitors or anti-angiogenic agents-such cases were excluded from our study. Among patients who underwent surgery and completed two chemotherapy cycles, post-treatment ZFPL1 levels were significantly lower than pre-treatment levels, accompanied by favourable disease control rates. This suggests that ZFPL1 levels may serve as an indicator for evaluating chemotherapy and surgical outcomes. However, since nearly all small cell lung cancer (SCLC) cases are diagnosed at advanced stages (making surgery infeasible) and squamous cell carcinoma (SqCC) cases were underrepresented, subgroup analysis by pathological type (including chemotherapy-only and surgery-only groups) revealed no statistically significant differences in ZFPL1 levels among lung adenocarcinoma (LUAD), SqCC, and SCLC patients. This may be attributed to differing chemotherapy regimens or small sample sizes. Nonetheless, post-treatment ZFPL1 levels were consistently lower across all three groups, indicating its utility in assessing treatment efficacy regardless of pathological type, though it may not differentiate among them.
To evaluate the clinical utility of serum ZFPL1 in lung cancer diagnosis, we conducted receiver operating characteristic (ROC) curve analysis. The area under the curve (AUC) for ZFPL1 in diagnosing lung cancer was 0.921, with a sensitivity of 78.67% and specificity of 98.67%, demonstrating its effectiveness in distinguishing lung cancer patients from healthy individuals. For specific pathological types, ZFPL1 achieved an AUC of 0.920 for LUAD (sensitivity: 81.25%, specificity: 96.88%), 0.912 for SqCC (sensitivity: 80.65%, specificity: 90%), and 0.951 for SCLC (sensitivity: 83.33%, specificity: 95.88%), indicating its diagnostic value across subtypes. Additionally, for disease progression assessment, ZFPL1 yielded an AUC of 0.657 in distinguishing early- from advanced-stage lung cancer (sensitivity: 91.23%, specificity: 44.44%), suggesting its potential as a prognostic biomarker, albeit with limited specificity for staging.
Our findings reveal that serum ZFPL1 levels are significantly elevated in lung cancer patients compared to healthy controls, yet higher in early-stage than advanced-stage patients, indicating a non-canonical correlation with disease progression. We speculate that ZFPL1 may participate in undiscovered mechanisms related to tumor progression, where its early-stage levels surpass those in advanced stages. TCGA and KEGG data show ZFPL1 enrichment in autophagy-related pathways. Autophagy exhibits dual roles in cancer: it suppresses tumorigenesis by inhibiting necrosis-induced inflammation, maintaining genomic stability, and inducing autophagic cell death and senescence, while also promoting tumor growth by sustaining the metabolism of high-demand cancer cells [23]. Autophagy inhibition may occur via blocking autophagosome formation or lysosomal fusion [24]. Given autophagy's context-dependent roles in cancer, we hypothesize that the aggregate ZFPL1-mediated autophagy suppression in early-stage lung cancer outweighs that in advanced stages.
However, our study has limitations: a) Small sample sizes for both lung cancer patients and healthy controls; b) Limited follow-up duration, precluding survival analysis and detailed statistical assessments; c) Lack of younger lung cancer cases despite the rising incidence in younger populations; d) Exclusion of patients receiving multimodal therapies (e.g., surgery combined with chemotherapy, radiotherapy, immunotherapy, or interventional therapy). In conclusion, our preliminary research identifies ZFPL1 as a promising biomarker for lung cancer diagnosis, progression monitoring, and treatment evaluation, providing a foundation for early screening and precision intervention. Further studies are warranted to validate these findings.
Data availability
All data generated or analyzed during this study are included in this published article. The data that support the findings of this study are available on request from the corresponding authors.
Author contributions
Teng Yu: conceptualization, data curation, formal analysis, writing–original draft; Honlin Liu: conceptualization, formal analysis, writing–original draft; Yingxuan Chen: conceptualization, data curation, formal analysis; Lili Xu: formal analysis, investigation, methodology; Xiaoni Pang, Haihui Yang, Shuwen Chen, Guanjie Lu: formal analysis, investigation.; Jianhua Feng: conceptualization, writing–review and editing;Biwen Mo: conceptualization, data curation, writing–review and editing.
Conflict-of-interest Statement
The authors have declared that no conflict of interest exists.
Acknowledgement
This study was supported by Zhongshan Science and Technology Bureau Project (No. 2024B1096); Guangxi Key Research and Development Plan (GuiKe AB24010096); Specific Research Project of Guangxi for Research Bases and Talents (GuiKe AD24999032); National Natural Science Foundation of China (No. 82060006, 82460008, No. 82160002, No. 82160014).
References
- Carlisle JW, Steuer CE, Owonikoko TK, Saba NF (2020) An update on the immune landscape in lung and head and neck cancers. CA Cancer J Clin 70(6): 505-517.
- Wadowska K, Bil-Lula I, Trembecki L, Sliwinska-Mosson M (2020) Genetic Markers in Lung Cancer Diagnosis. Int J Mol Sci 21(13): 4569
- Calabrese F, Lunardi F, Pezzuto F, Vuljan SE, Marquette C, et al. (2019) Are There New Biomarkers in Tissue and Liquid Biopsies for the Early Detection of Non-Small Cell Lung Cancer? J Clin Med 8(3): 414.
- Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ, et al. (2017) Lung cancer: current therapies and new targeted treatments. Lancet 389(10066): 299-311.
- Yang G, Xiao Z, Tang C, Deng Y, Huang H, et al. (2019) Recent advances in biosensor for detection of lung cancer biomarkers. Biosens Bioelectron 141: 111416.
- Broodman I, Lindemans J, Sten JV, Bischoff R, Luider T (2017) Serum Protein Markers for the Early Detection of Lung Cancer: A Focus on Autoantibodies. J Proteome Res 16(1): 3-13.
- Field JK, Duffy SW, Baldwin DR, Yadegarfar D, Hansellet DM, et al. (2016) UK Lung Cancer RCT Pilot Screening Trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 71(2): 161-70.
- Wang J, Shivakumar S, Barker K, Tanget Y,Wallstromal G, et al. (2016) Comparative Study of Autoantibody Responses between Lung Adenocarcinoma and Benign Pulmonary Nodules. J Thorac Oncol 11(3): 334-45.
- Eom KS, Cheong JS, Lee SJ (2016) Structural Analyses of Zinc Finger Domains for Specific Interactions with DNA. J Microbiol Biotechnol 26(12): 2019-2029.
- Cassandri M, Smirnov A, Novelli F, Pitolliet C, Agostinial M, et al. (2017) Zinc-finger proteins in health and disease. Cell Death Discov 3: 17071.
- Ashraf W, Ibrahim A, Alhosin M, Mousliet M, Bronner C, et al. (2017) The epigenetic integrator UHRF1: on the road to become a universal biomarker for cancer. Oncotarget 8(31): 51946-51962.
- Wang S, Zhao Y, Aguilar A, Bernard D, Yang CY (2017) Targeting the MDM2-p53 Protein-Protein Interaction for New Cancer Therapy: Progress and Challenges. Cold Spring Harb Perspect Med 7(5): a026245.
- Hazawa M, Lin DC, Handral H, Xu L, Chene Y, et al. (2017) ZNF750 is a lineage-specific tumour suppressor in squamous cell carcinoma. Oncogene 36(16): 2243-2254.
- Cai MJ, Zhan FX, Kong XN, Zhu SZ, Cui Y, et al. (2018) RING domain of zinc finger protein like 1 is essential for cell proliferation in endometrial cancer cell line RL95-2. Gene 677: 17-23.
- Cai MJ, Zhan FX, Kong XN, Zhu SZ, Cui Y, et al. (2018) RING domain of zinc finger protein like 1 is essential for cell proliferation in endometrial cancer cell line RL95-2. Gene 677: 17-23.
- Xie YZ, Ma WL, Meng JM, Ren XQ (2017) Knockdown of ZFPL1 results in increased autophagy and autophagy‑related cell death in NCI‑N87 and BGC‑823 human gastric carcinoma cell lines. Mol Med Rep 15(5): 2633-2642.
- Masud N, Aldahish A, Iczkowski KA, Kale A, Shah GV (2023) Zinc finger protein‑like 1 is a novel neuroendocrine biomarker for prostate cancer. Int J Oncol 62(3): 38.
- Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT (2017) The Eighth Edition Lung Cancer Stage Classification. Chest 151(1): 193-203.
- Micke P, Faldum A, Metz T, Hengstler JG, Buhl R, et al. (2002) Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer--what limits limited disease? Lung Cancer 37(3): 271-6.
- (2024) Chinese Medical Association guideline for clinical diagnosis and treatment of lung cancer. Zhonghua Zhong Liu Za Zhi 46(9):805-843
- Zhang S, Sun K, Zheng R, Zenget H, Wang S, et al. (2021) Cancer incidence and mortality in China, 2015. J Natl Cancer Cent 1(1): 2-11.
- Lundberg R, Dahlen J, Lundeberg T (2025) Considerations regarding the selection, sampling, extraction, analysis, and modelling of biomarkers in exhaled breath for early lung cancer screening. J Pharm Biomed Anal 260: 116787.
- Losmanova T, Zens P, Scherz A, Berezowskaet S, Tschan MP, et al. (2021) Chaperone-Mediated Autophagy Markers LAMP2A and HSPA8 in Advanced Non-Small Cell Lung Cancer after Neoadjuvant Therapy. Cells 10(10): 2731.
- Altinoz MA, Ozpinar A, Alturfan EE, Elmaci I (2018) Vinorelbine's anti-tumor actions may depend on the mitotic apoptosis, autophagy and inflammation: hypotheses with implications for chemo-immunotherapy of advanced cancers and pediatric gliomas. J Chemother 30(4): 203-212.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.