Salvage Therapy Using Mesenchymal Stromal Cells “MSC-FFM” For a Severe Gastrointestinal Steroid-Refractory Acute Graft-Versus-Host Disease in a 12-Year-Old Boy with Sickle Cell Disease
by Mélanie Valpacos1*, Serge Grazioli1, Frederic Baleydier2,3, André von Bueren2,3, Fanette Bernard2,3, Nathalie Rock4, Anne-Claire Mamez5, Anne-Laure Rougemont6, Peter Bader7, Marc Ansari2,3
1Division of Neonatal and Pediatric Intensive Care, Department of Pediatrics, Gynecology, and Obstetrics, Geneva University Hospitals, University of Geneva, Switzerland
2Cansearch Research Platform for Pediatric Oncology and Hematology, Faculty of Medicine, Department of Pediatrics, Gynecology, and Obstetrics, University of Geneva, Geneva, Switzerland
3Division of Pediatric Oncology and Hematology, Department of Pediatrics, Gynecology, and Obstetrics, Geneva University Hospitals, University of Geneva, Switzerland
4Gastroenterology, Hepatology, and Nutrition Unit, Division of Pediatric Specialties, Department of Pediatrics, Gynecology, and Obstetrics, Geneva University Hospitals, University of Geneva, Switzerland.
5Division of Hematology, Department of Oncology, Geneva University Hospitals, University of Geneva, Geneva, Switzerland.
6Division of Clinical Pathology, Geneva University Hospitals, Geneva, Switzerland.
7Division of Pediatric Stem Cell Transplantation, Johann Wolfgang Goethe University Hospital, Frankfurt am Main, Germany.
*Corresponding author: Mélanie Valpacos, Division of Neonatal and Pediatric Intensive Care, Department of Pediatrics, Gynecology, and Obstetrics, Geneva University Hospitals, University of Geneva, Switzerland.
Received Date: 27 December 2023
Accepted Date: 02 January 2024
Published Date: 04 January 2024
Citation: Valpacos M, Grazioli S, Baleydier F, Bueren AV, Bernard F, et al. (2024) Salvage Therapy Using Mesenchymal Stromal Cells “MSC-FFM” For a Severe Gastrointestinal Steroid-Refractory Acute Graft-Versus-Host Disease in a 12-Year-Old Boy with Sickle Cell Disease. Ann Case Report 09: 1577. https://doi.org/10.29011/2574-7754.101577.
Abstract
Acute Graft-versus-Host Disease (aGvHD) is a frequent complication after a Hematopoietic Stem Cell Transplantation (HSCT). Its gastrointestinal subtype is associated with a high mortality, especially in steroid-refractory cases. Managing Steroid-Refractory aGvHD (SR-aGvHD) remains challenging due to the lack of validated second-line immunosuppressive strategies. Mesenchymal Stromal Cells (MSCs) are a therapeutic option for SR-aGvHD, but its efficacy and clinical response in children varies. Two recent studies using pooled bone marrow mononuclear cells from multiple donors, MSC-FFM, have shown satisfactory overall response rates but the literature is still lacking in children.
We report the case of a 12-year-old boy with severe sickle cell disease and exchange transfusion dependency, indicating HSCT. Following allogeneic related-donor HSCT, he developed grade IV, gastrointestinal SR-aGvHD with an unfavorable clinical outcome despite intensive treatment with mycophenolate mofetil, methylprednisolone, and ruxolitinib.
After nine days of methylprednisolone, a weekly MSC–FFM treatment was initiated that led to partial resolution of the gastrointestinal symptoms at D+18 after the first infusion of MSC–FFM and transient complete clinical remission of the gastrointestinal SR-aGvHD at D+79.
In summary, MSC–FFM infusion was well tolerated, leading to significant clinical improvement and transient complete clinical remission of severe gastrointestinal SR-aGvHD.
Keywords: Steroid-Refractory Acute Graft-Versus-Host Disease; Pediatric; Gastrointestinal aGvHD; Mesenchymal Stromal Cells; MSC–FFM
Introduction
Allogeneic Hematopoietic Stem Cell Transplantation (HSCT) is indicated for multiple malignant and non-malignant disorders. The number of transplants worldwide is increasing annually [1]. However, despite well-conducted prophylaxis, Graft-versus-Host Disease (GvHD) remains a major complication of transplantation, affecting 20–80% of patients [2–4]. Acute Graft-versus-Host Disease (aGvHD) is graded according to the Glucksberg criteria [5].
Studies show that gastrointestinal aGvHD is usually given the most severe grade, and it is linked to very high rates of non-relapse mortality [3]. Treating gastrointestinal GvHD (GI-GvHD) is a major challenge because of the imbalances in fluids and electrolytes caused by secretory diarrhea and the risks of bleeding and infection inherent among immunosuppressed and often thrombocytopenic patients [3]. GI-GvHD is also associated with malnutrition, which represents a further significant and independent risk factor for mortality.
Systemic glucocorticoids are the first-line treatment for aGvHD [2,6]. About half of patients present with SR-aGvHD [7]. Steroid-Refractory Graft-versus-Host Disease (SR-GvHD) is defined as a progression from GvHD after 3–5 days of methylprednisolone (2 mg/kg/day) or no improvement after 5–7 days of corticosteroid therapy or an incomplete response after ³ 28 days of immunosuppression [6].
In addition to human leukocyte antigen matching, the severity of GvHD at presentation (Glucksberg grade) is the strongest predictor of resistance to corticosteroid therapy [7]. Indeed, 60–70% of cases with severe aGvHD at presentation (≥ grade III) are SR-aGvHD and associated with a high mortality rate, estimated at 80% at one year [3].
There is currently no consensus on the management of SR-GvHD. Although several immunosuppressive strategies are available for second-line therapy, including ruxolitinib and Mesenchymal Stromal Cells (MSCs), none has yet shown its superiority in a pediatric randomized trial [2,8].
Ruxolitinib has recently been approved as a second-line treatment for SR-GvHD in children over 12 years old and adults [9]; it is a selective inhibitor of Janus-Activated Kinases (JAKs), specifically JAK-1 and JAK-2 protein kinases. Inhibition disrupts cytokine and growth factor signaling cascades, which reduces circulating pro-inflammatory cytokines and the proliferation of activated T cells [10]. According to the REACH2 study, this treatment shows a better 28-day response than other therapies in SR-aGvHD; its superiority is even greater in more severe SR-aGvHD [11].
MSCs are a promising therapy for SR-aGvHD because of their potential immunomodulatory properties. MSCs are multipotent cells isolated from bone marrow. They display cell renewal, can differentiate into multiple cell lineages, and have immunosuppressive capabilities [12–15]. Because of MSC therapy’s mixed results regarding its efficacity and overall patient survival, and despite its proven tolerance, it is not commonly approved as a second-line treatment [13,16]. However, data has shown significant donor-to-donor variability, resulting in some inequality between MSC products [17]. Using a pool of MSCs from different donors—otherwise known as MSC Frankfurt am Main (MSC-FFM) therapy— however, can address this and lead to a strong allogeneic reaction. Promising preliminary results from two pediatric studies have demonstrated a significant positive response rate and better overall survival among children with aGvHD treated with MSC-FFM but literature is still lacking.
Case
We report on a 12-year-old boy who presented with a severe lower gastrointestinal SR-aGvHD 31 days (d+31) after a matched sibling donor bone marrow transplantation. He underwent an MSC-FFM treatment.
The patient had homozygous Sickle Cell Disease (SCD) and heterozygous alpha-thalassemia. Alongside multiple vaso-occlusive crises and acute chest syndromes, he was undergoing a transfusion exchange program due to abnormal transcranial Doppler ultrasound results. He also had left-carotid siphon stenosis and nocturnal oxygen dependence due to bi-basal pulmonary fibrosis resulting from SCD. The patient’s severe form of SCD, with neurological and respiratory complications, indicated the HSCT.
The patient underwent a conditioning regimen consisting of doses adjusted to pharmacokinetics of busulfan (4 x 0.8 mg/kg body weight (BWt), target concentration at steady state of 800 ng/ml, area under the curve 80 mg/L.h), cyclophosphamide (200 mg/kg BWt), and in vivo T-depletion with anti-thymocyte globulin (Thymoglobuline®, 20 mg/kg BWt). He was transplanted with hematopoietic stem cells from bone marrow containing 2.0x 108 total nucleated cells/kg and 2.88x 106 CD34+ non-depleted cells/kg BWt from a 10/10 matched sibling donor. As GvHD prophylaxis, he received ciclosporin (3 mg/kg BWt/day). Due to posterior reversible encephalopathy syndrome on day 1 after HSCT (d+1), ciclosporin was replaced by mycophenolate mofetil, methotrexate was not administered, and prednisone was added (1 mg/kg/d BWt until d+15, then decreasing doses until d+28). Hematological recovery was observed on d+22. Bone marrow engraftment was confirmed by full donor chimerism on d+21. The patient was discharged on d+29.
However, he was readmitted on d+31 due to a BK virus hemorrhagic cystitis. Two days later, he presented with high-volume watery diarrhea and hematochezia complicated by major electrolyte imbalances. This required admission to the pediatric intensive care unit on d+36. A grade IV gastrointestinal aGvHD was confirmed by upper gastrointestinal and rectal histology on d+38 (Figure 1, A and B; Figure 2) and was accompanied by a grade II cutaneous aGvHD (on d+37). A bronchoalveolar lavage performed at the same time revealed the presence of rare epithelial cell atypia compatible with a diagnosis of pulmonary GvHD. The patient was thus diagnosed with an overall grade IV aGvHD. Its management and evolution are summarized in Figure 3. Although a concomitant increase in bilirubin was observed, values remained below the threshold for hepatic aGvHD.
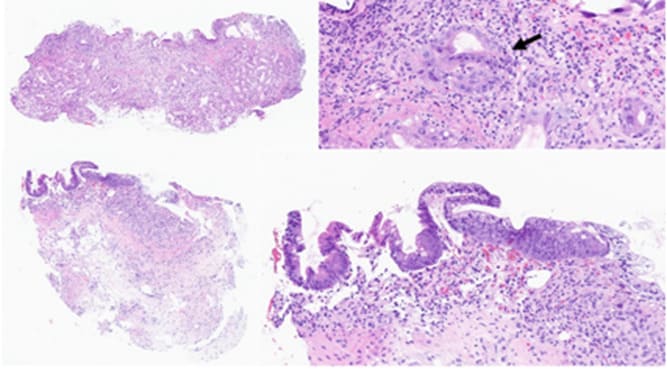
Figure 1: Histological findings. A and B (Hematoxylin&Eosin, H&E, x75 and x400), gastric biopsy at d+38. The gastric biopsy shows focal denudation, with major gland dystrophy and partial loss. Clustered apoptotic bodies are seen (arrow). C and D (H&E, x60 and x200), duodenal biopsy at D+23 (d+70). Partial re-epithelialization and regeneration of the duodenal mucosa is seen, with persisting gland loss
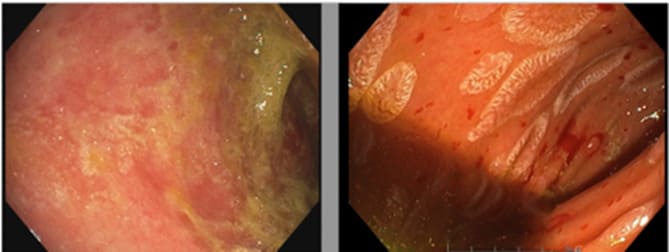
Figure 2: Endoscopic images of the duodenum. On the left (d+38) before mesenchymal cell administration, showing mucosal atrophy, absence of villi and fibrin deposits. On the right (d+70) and D+23 after the first injection of MSCs, revealing islands of re-epithelialization despite mucosal hemorrhagic fragility.
On d+38, budesonide (9 mg/d) and methylprednisolone (2 mg/kg BWt/d) were introduced, without any improvement after 5 days of corticosteroids, suggesting a severe SR-aGvHD. Ruxolitinib was added on d+43 (10 mg/d because of a severe transfusion-dependent thrombocytopenia). Despite receiving this triple immunosuppression treatment, his gastrointestinal-aGvHD continued to worsen, so an MSC-FFM therapy (Obnitix®, Medac) was initiated on d+47 (after 9 days of methylprednisolone) with the administration of a weekly infusion of 1.5 x 106 cells/kg BWt, which continued for a total of 6 weeks.
Eighteen days after the first MSC-FFM infusion (D+18), we observed a partial resolution with a decrease of gastrointestinal-aGvHD from grade IV to grade III, which was reflected in a decrease in blood transfusions and endoscopic clot removal. Endoscopic evaluation and histology performed on D+23 showed focal duodenal re-epithelialization, co-existing, however, with areas of ulceration on D+23 (Figure 1, C and B; Figure 2). The evolution continued to be favorable with a transient complete clinical remission on D+79 (Figure 3). The diarrhea and bleeding resolved completely. A persistent abdominal discomfort prevented a complete resumption of oral feeding and induced persistent need for parenteral nutrition. From D+97, during the attempt to start enteral nutrition, the patient’s digestive losses slightly increased, with values mostly consistent with a grade I GI-GvHD until the end of his life (Figure 3).
Furthermore, the patient’s cutaneous aGvHD was considered in complete remission on D+8 of his MSC-FFM infusion.
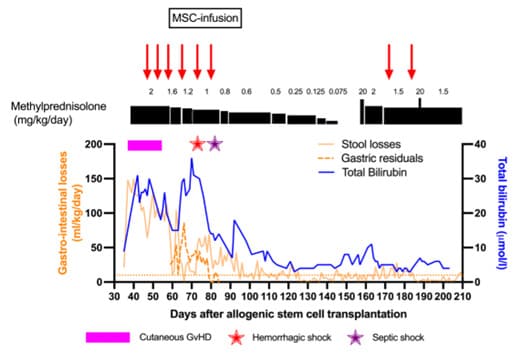
Figure 3: Evolution of liver and gastrointestinal SR-aGVHD with steroids and after MSC–FFM infusions
The horizontal dotted orange line shows the ≥ 10 ml/kg/d limit indicating grade I GvHD.
Lymphocyte subpopulations after MSC-FFM infusions showed a decrease in T cells and B cells and an increase in natural killer (NK) cells between D+18 and D+36 (Table 1). These data show a decrease in cellular cytotoxicity, particularly through a drop in the CD4/CD8 ratio, which was closely related to the patient’s clinical improvement.
|
D+18 after first MSC–FFM treatment (d+67) |
D+36 after first MSC–FFM treatment (d+85) |
D+70 after first MSC–FFM treatment (d+119) |
|
|
Units: /ml; % |
|||
|
CD3+ (T cells) Normal reference range: 800–3500; 52–78% |
29/ml; 84% |
75/ml; 59% |
150/ml; 66% |
|
CD4+CD3+ Normal reference range: 400–2100; 25–48% |
18/ml; 53% |
53/ml; 59% |
50/ml; 22% |
|
CD8+CD3+ Normal reference range: 200–1200; 9–35% |
10/ml; 29% |
19/ml; 22% |
97/ml; 43% |
|
CD19+ (B cells) Normal reference range: 200–600; 8–24% |
2/ml; 7% |
2/ml; 2% |
7/ml; 3% |
|
CD56+CD16+ (NK) Normal reference range: 70–1200; 6–27% |
3/ml; 9% |
12/ml; 14% |
68/ml; 30% |
Table 1: Lymphocyte subsets (measured using flow cytometry)
Slow weaning off methylprednisolone was able to start on D+12, with complete discontinuation on D+100 (d+147). Budesonide was stopped on D+58 (d+105), and ruxolitinib was stopped on D+104 (d+151). The patient was transferred from the pediatric intensive care unit to the pediatric oncology unit on d+120.
On d+122, the patient developed a new oxygen dependence that was initially attributed to fluid overload. Despite fluid restriction and diuretics, his respiratory condition worsened, and a thoracic computerized tomography scan performed on d+148 was suggestive of pulmonary GvHD with areas of cryptogenic organizing pneumonia and interstitial fibrosis. Due to a differential diagnosis of an infection, antibiotics and antifungal therapy were resumed on d+149. A bronchoalveolar lavage performed on d+151 revealed a predominantly histiocytic infiltrate with rare epithelial cell atypia of unknown significance, making it difficult to either confirm or exclude a diagnosis of lung GvHD.
His respiratory condition continued to worsen, with the development of hypercapnic and hypoxemic respiratory failure on d+156, requiring a transfer to the pediatric intensive care unit for respiratory support. At this point, the differential diagnosis remained wide between an idiopathic pneumonia syndromes, a cryptogenic-organizing pneumonia, and a bronchiolitis obliterans related to a lung GvHD. Methylprednisolone (2 mg/kg BWt) and ruxolitinib were resumed on d+156; steroids are the first-line treatment for all these conditions, and ruxolitinib is also used for bronchiolitis obliterans [18-22]. Unfortunately, due to the patient’s clinical instability, a pulmonary biopsy was infeasible. The suspicion of bronchiolitis obliterans as a manifestation of a chronic lung GvHD was high, so the patient received a pulmonary targeted anti-inflammatory treatment (fluticasone, azithromycin, and montelukast), as well as a new course of MSC-FFM therapy, with two infusions. On d+186, he needed to be intubated and supported by mechanical ventilation. Due to the refractory presentation of lung GvHD, extracorporeal photopheresis was initiated, with two cycles performed weekly. Despite maximum support involving pharmacological and extracorporeal immunomodulatory treatments, the patient’s condition continued to deteriorate, leading to multi-organ failure and his death on d+213.
Discussion
We have presented the case of an aggressive, SR-aGvHD that responded to MSC-FFM therapy.
aGvHD is a much-dreaded complication following HSCT. A GI-GvHD represents an even greater therapeutic challenge with a very guarded prognosis [3,4,23-27].
MSCs are of interest as a second-line treatment in SR–GvHD. Factors associated with a better response to MSCs include their early administration, a pediatric population, and cutaneous or gastrointestinal involvement [13,16,28,29]. The severity of GvHD is also a prognostic factor: patients with refractory severe (grade III–IV) GvHD require multiple infusions, and despite their responsiveness to MSCs, two-year mortality remains high in this population [30].
However, when using a pool of MSCs from different donors (MSC-FFM), the survival of patients presenting with severe aGvHD is significantly improved (64% at 6 months, according to the study by Bonig et al.), and the response within 28 days serves as a reliable predictor of survival [19]. Furthermore, infusions of MSC-FFM are not associated with major side effects.
For this patient, MSC-FFM therapy was added after a short, 9-day course of corticosteroids, as earlier administration appears to be more effective [28,29]. MSC-FFM therapy led to a transient complete clinical remission of gastrointestinal involvement by D+79, supported by an intestinal biopsy performed at D+31 showing partial duodenal re-epithelialization, and complete resolution of skin involvement by D+8 (Figure 3). MSC-FFM therapy’s effects on our patient were in line with recent studies showing that responses to MSC correlate with the severity of SR–aGvHD and with cutaneous or gastrointestinal involvement [13,16]. This suggests that a highly pro-inflammatory environment is important for an optimal effect of MSCs [14].
However, the absence of a sustained complete response to MSC-FFM, with the recurrence of abnormal stool output (> 10 cc/kg/d of diarrhea) from D+97 (d+144) may be attributable to either a relapse of GI-GvHD (with a concurrent respiratory deterioration) or to the lengthy process of intestinal repair, which can also manifest as loose stools [29].
We wonder whether ruxolitinib had a synergistic effect with the MSCs received by the patient. MSC-FFM therapy was added 4 days after ruxolitinib administration began, which did not fulfil the usual criteria for use in cases of ruxolitinib-resistant SR–aGvHD. The latter for using MSC-FFM are: (i) progressing aGvHD even after 5–10 days of ruxolitinib; or (ii) a failure to improve after 14 days; or (iii) losing response after initial improvement under treatment [7]. However, given the case’s clinical severity, adding an additional line of treatment seemed judicious.
Combining MSC and ruxolitinib appears to be an interesting solution, particularly in gastrointestinal aGvHD: associating the anti-inflammatory effects of ruxolitinib with MSCs’ regenerative effects on the intestinal epithelium has the potential to improve outcomes [3].
Intestinal stem cells are naturally present at the base of intestinal crypts and are essential to intestinal regeneration [31]. They are likely fewer in number in severe gastrointestinal aGvHD due to intestinal destruction. Studies exposing mice to radiation have demonstrated that MSCs can enhance the proliferation of intestinal stem cells [32]. If this were to be proven in humans, MSCs would not only be valuable for their immunomodulatory effects but also for their direct impact on intestinal regeneration. In addition to current studies on the effect of MSCs on inflammatory bowel diseases [33], further investigations on MSCs in GI-GvHD will help to determine whether this therapy should be combined with other treatments, such as ruxolitinib, or whether it should be promptly added in case of gastrointestinal involvement.
The analysis of lymphocyte subpopulations after MSC-FFM infusions showed a decrease in T cells and a better CD4/CD8 ratio after multiple infusions (2.8 at D+36 versus 1.8 at D+18), as well as a decrease in B cells (Table 1). These findings support other data suggesting that MSCs inhibit the proliferation of T and B cells and facilitate the induction of Tregs and Bregs from T and B cells, respectively [14]. However, the present case report does not clearly demonstrate the latter point as it lacks specific data on cellular subtypes.
Interestingly, a 1.5-fold increase in NK cells was observed between D+18 and D+36. Due to the patient’s clinical improvement, two potential mechanisms could explain this: an increase in the number of donor NK cells (these cells are the first to recover after HSCT) [5] or an increase in the regulatory role of those NK cells, thereby inhibiting T cell proliferation [34]. The role played by MSCs in modulating NK cells remains controversial as some studies have shown inhibition of NK cells, whereas others have shown a stimulation of cellular function. Modulation appears to depend on the co-culture conditions, such as the MSC/NK cell ratio [34,35].
At D+70, the increase in T cells (CD4/CD8 ratio: 0.5) and NK cells was likely related to the patient’s clinical respiratory deterioration, whether it was initially related to pulmonary GvHD or to an infection. Due to this clinical deterioration and ultimately death, this case provides no information on MSCs’ medium-to-long-term effects on lymphocyte differentiation.
The patient’s tolerance to MSC-FFM treatment was very good, with no observed side effects related to the infusions received. However, the patient experienced two infections and a cytomegalovirus reactivation. Using triple immunosuppression carries a significant risk of infection, highlighting the importance of closely monitoring the patient using clinical and laboratory assessments and the necessity of reducing immunosuppression whenever feasible.
Conclusion
In conclusion, MSC-FFM infusion in this patient with severe gastrointestinal SR–aGvHD was well tolerated and resulted in substantial clinical improvements, including the transient complete clinical remission of his severe gastrointestinal SR-aGvHD and the complete remission of his cutaneous aGvHD. This clinical response to MSC-FFM adds further evidence to the utility of this treatment and highlights the importance of further studies examining the role of MSC-FFM therapy in children with SR–aGvHD.
Informed consent: Written informed consent was obtained from the patient’s parents.
Acknowledgements:
M. Rodolfo Lo Piccolo, clinical research assistant of the onco-hematology unit, HUG.
Mrs Carole Dantin of the Laboratories of Transplantation and Cellular Therapy in Haemato-Oncology, HUG, Geneva.
The CANSEARCH foundation.
Author contributions: MV contributed to the first draft, approved the final version, and take responsibility for the accuracy or reported findings. SG, FB, AB, FB, NR, AM, AR, PB, and MA contributed to the draft and proved the final version.
Conflict of interest: The authors declare that they have no competing interests related to this manuscript. Peter Bader declares patent and royalties from Medac.
References
- Niederwieser D, Baldomero H, Bazuaye N, Bupp C, Chaudhri N, et al. (2022) One and a half million hematopoietic stem cell transplants: continuous and differential improvement in worldwide access with the use of non-identical family donors. haematol. 107: 1045-53.
- Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, et al. (2012) First- and Second-Line Systemic Treatment of Acute Graft-versus-Host Disease: Recommendations of the American Society of Blood and Marrow Transplantation. Biology of Blood and Marrow Transplantation. 18: 1150-63.
- Biavasco F, Ihorst G, Wäsch R, Wehr C, Bertz H, et al. (2022) Therapy response of glucocorticoid-refractory acute GVHD of the lower intestinal tract. Bone Marrow Transplant. 57: 1500-6.
- Ferrara JL, Levine JE, Reddy P, Holler E (2009) Graft-versus-host disease. 373: 1550-61.
- Carreras E, Dufour C, Mohty M, Kröger N, editors (2019) The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies [Internet]. Cham: Springer International Publishing.
- Schoemans HM, Lee SJ, Ferrara JL, Wolff D, Levine JE, et al. (2018) EBMT (European Society for Blood and Marrow Transplantation) Transplant Complications Working Party and the “EBMT−NIH (National Institutes of Health)−CIBMTR (Center for International Blood and Marrow Transplant Research) GvHD Task Force,” EBMT−NIH−CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. 53: 1401-15.
- Mohty M, Holler E, Jagasia M, Jenq R, Malard F, et al. (2020) Refractory acute graft-versus-host disease: a new working definition beyond corticosteroid refractoriness. Blood. 136: 1903-6.
- Verbeek AB, Jansen SA, von Asmuth EGJ, Lankester AC, Bresters D, et al. (2022) Clinical Features, Treatment, and Outcome of Pediatric Steroid Refractory Acute Graft-Versus-Host Disease: A Multicenter Study. Transplantation and Cellular Therapy. 28: 600.e1-600.e9.
- Przepiorka D, Luo L, Subramaniam S, Qiu J, Gudi R, et al. (2020) FDA Approval Summary: Ruxolitinib for Treatment of Steroid-Refractory Acute Graft-Versus-Host Disease. The Oncologist. 25: e328-34.
- Abedin SM, Hamadani M (2020) Ruxolitinib: a potential treatment for corticosteroid refractory acute graft-versus-host disease. Expert Opinion on Investigational Drugs. 29: 423-7.
- Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, et al. (2020) Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N Engl J Med. 382: 1800-10.
- Bogensperger C, Hofmann J, Messner F, Resch T, Meszaros A, et al. (2021) Ex Vivo Mesenchymal Stem Cell Therapy to Regenerate Machine Perfused Organs. IJMS. 22: 5233.
- Elgaz S, Kuçi Z, Kuçi S, Bönig H, Bader P (2019) Clinical Use of Mesenchymal Stromal Cells in the Treatment of Acute Graft-versus-Host Disease. Transfus Med Hemother. 46: 27-34.
- Li N, Hua J (2017) Interactions between mesenchymal stem cells and the immune system. Cell Mol Life Sci. 74: 2345-60.
- Dunavin N, Dias A, Li M, McGuirk J (2017) Mesenchymal Stromal Cells: What Is the Mechanism in Acute Graft-Versus-Host Disease? Biomedicines. 5: 39.
- Kebriaei P, Hayes J, Daly A, Uberti J, Marks DI, et al. (2020) A Phase 3 Randomized Study of Remestemcel-L versus Placebo Added to Second-Line Therapy in Patients with Steroid-Refractory Acute Graft-versus-Host Disease. Biology of Blood and Marrow Transplantation. 26: 835-44.
- Trento C, Bernardo ME, Nagler A, Kuçi S, Bornhäuser M, et al. (2018) Manufacturing Mesenchymal Stromal Cells for the Treatment of Graft-versus-Host Disease: A Survey among Centers Affiliated with the European Society for Blood and Marrow Transplantation. Biology of Blood and Marrow Transplantation. 24: 2365-70.
- Bonig H, Kuçi Z, Kuçi S, Bakhtiar S, Basu O, et al. (2019) Children and Adults with Refractory Acute Graft-versus-Host Disease Respond to Treatment with the Mesenchymal Stromal Cell Preparation “MSC-FFM”—Outcome Report of 92 Patients. Cells. 8: 1577.
- Bader P, Kuçi Z, Bakhtiar S, Basu O, Bug G, et al. (2018) Effective treatment of steroid and therapy-refractory acute graft-versus-host disease with a novel mesenchymal stromal cell product (MSC-FFM). Bone Marrow Transplant. 53: 852-62.
- Gossel LD, Grau J, Jarisch A, Sörensen J, Rettinge E, et al. (2022) Mesenchymal Stromal Cell Product “MSC-FFM” for Treatment of Severe Multi-Organ Steroid Refractory Acute Graft-Versus-Host Disease in an Infant with Congenital Dyserythropoietic Anemia. 7: 2310.
- Kuci Z, Bonig H, Kreyenberg H, Bunos M, Jauch A, et al. (2016) Mesenchymal stromal cells from pooled mononuclear cells of multiple bone marrow donors as rescue therapy in pediatric severe steroid-refractory graft-versus-host disease: a multicenter survey. Haematologica.101: 985–94.
- Fitch T, Myers KC, Dewan M, Towe C, Dandoy C (2021) Pulmonary Complications After Pediatric Stem Cell Transplant. Front Oncol. 11: 755878.
- Kanter J, Liem RI, Bernaudin F, Bolaños-Meade J, Fitzhugh CD, et al. (2021) American Society of Hematology 2021 guidelines for sickle cell disease: stem cell transplantation. Blood Advances. 5: 3668–89.
- Shah N, Krishnamurti L (2021) Evidence-Based Minireview: In young children with severe sickle cell disease, do the benefits of HLA-identical sibling donor HCT outweigh the risks? Hematology. 2021: 190–5.
- Brazauskas R, Scigliuolo GM, Wang HL, Cappelli B, Ruggeri A, et al. (2020) Risk score to predict event-free survival after hematopoietic cell transplant for sickle cell disease. Blood. 136: 623–6.
- Hulbert ML, Shenoy S (2018) Hematopoietic stem cell transplantation for sickle cell disease: Progress and challenges. Pediatr Blood Cancer. 65: e27263.
- Martin PJ (2020) How I treat steroid-refractory acute graft-versus-host disease. Blood. 135: 1630–8.
- von Dalowski F, Kramer M, Wermke M, Wehner R, Röllig C, et al. (2016) Mesenchymal Stromal Cells for Treatment of Acute Steroid-Refractory Graft Versus Host Disease: Clinical Responses and Long-Term Outcome. Stem Cells. 34: 357–66.
- Ball LM, Bernardo ME, Roelofs H, Van Tol MJD, Contoli B, et al. (2013) Multiple infusions of mesenchymal stromal cells induce sustained remission in children with steroid-refractory, grade III-IV acute graft-versus-host disease. Br J Haematol. 163: 501–9.
- Dotoli GM, De Santis GC, Orellana MD, de Lima Prata K, Caruso SR, et al. (2017) Mesenchymal stromal cell infusion to treat steroid-refractory acute GvHD III/IV after hematopoietic stem cell transplantation. Bone Marrow Transplant. 52: 859–62.
- Andersson-Rolf A, Zilbauer M, Koo BK, Clevers H (2017) Stem Cells in Repair of Gastrointestinal Epithelia. Physiology. 32: 278–89.
- Gong W, Guo M, Han Z, Wang Y, Yang P, et al. (2016) Mesenchymal stem cells stimulate intestinal stem cells to repair radiation-induced intestinal injury. Cell Death Dis. 7: e2387–e2387.
- Saadh MJ, Mikhailova MV, Rasoolzadegan S, Falaki M, Akhavanfar R, et al. (2023) Therapeutic potential of mesenchymal stem/stromal cells (MSCs)-based cell therapy for inflammatory bowel diseases (IBD) therapy. Eur J Med Res. 28: 47.
- Abel AM, Yang C, Thakar MS, Malarkannan S (2018) Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front Immunol. 9: 1869.
- Abbasi B, Shamsasenjan K, Ahmadi M, Beheshti SA, Saleh M (2022) Mesenchymal stem cells and natural killer cells interaction mechanisms and potential clinical applications. Stem Cell Res Ther. 13: 97.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.