Safety of Locoregional Anesthesia in Upper Limb Tumor Resection: A Single-Center Retrospective Analysis
by Charlie Soleil,¹ Robin Evrard2, Thomas Schubert2, Emmanuelle Henry1*
1Department of Anesthesiology, Cliniques Universitaires Saint-Luc, Belgium
2Department of Orthopedics, Cliniques Universitaires Saint-Luc, Brussels, Belgium
Corresponding author : Henry Emmanuelle, Department of Anesthesiology, Cliniques Universitaires Saint-Luc, Av. Hippocrate 10, 1200 Woluwe St, Brussels, Belgium
Received Date: 22 September, 2025
Accepted Date: 29 September, 2025
Published Date: 02 October, 2025
Citation: Soleil C, Evrard R, Schubert T, Henry E (2025) Safety of Locoregional Anesthesia in Upper Limb Tumor Resection: A Single-Center Retrospective Analysis. J Orthop Res Ther 10: 1403. https://doi.org/10.29011/2575-8241.001403
Abstract
Background: Orthopedic oncologic surgeries, particularly tumor resections, are major procedures frequently associated with substantial postoperative pain. Locoregional anesthesia (LRA) is increasingly employed to enhance perioperative management. However, concerns remain regarding its oncologic safety, particularly the potential risk of recurrence or dissemination. This retrospective study evaluates the safety of LRA in upper limb tumor resections at a specialized orthopedic oncology center. Methods: We retrospectively reviewed upper limb tumor surgeries performed between 2017 and 2024 by a single expert orthopedic oncologic surgeon. Included patients had either benign or malignant bone tumors or low-grade or malignant soft tissue tumors. Patients with simple benign lesions or initially metastatic disease were excluded. Two groups were compared: patients who received LRA and those who did not. The primary outcome was tumor recurrence (local or distant); the secondary outcome was postoperative complications. Results: Ninety-five patients were included, 30 of whom received LRA. No significant difference was observed in recurrence rates (LRA: n = 5 (16.7%); non-LRA n = 9 (13.8%); χ²(1) = 0.13, p = 0.718) or complication rates (LRA: n = 8 (26.7%); non-LRA n = 13 (20%); χ²(1) = 0.53, p = 0.467). However, recurrence was more frequent when a perineural catheter was placed directly at the tumor site (p = 0.05). Conclusion: LRA appears safe in musculoskeletal tumor surgery, but caution is advised when placing perineural catheters near the tumor site, especially if neural preservation compromises surgical margins.
Key Messages
- Locoregional anesthesia offers clear benefits for perioperative management, but its oncologic safety in orthopedic tumor surgery remains under-investigated and occasionally debated.
- Our study suggests that LRA is safe in this surgical context, except when the catheter is placed directly at the tumor site.
- LRA should be more widely adopted in orthopedic oncologic procedures, provided that catheter placement within the surgical field, particularly in close proximity to the tumor site, is carefully considered in order to avoid increasing the risk of tumor recurrence.
Keywords: Humans Locoregional anesthesia; Orthopedic surgery; Tumor recurrence; Retrospective study
Intruduction
Surgical stress and various anesthetic -particularly opioids-have been shown to affect immune function [1-3] and may thereby influence postoperative tumor recurrence and dissemination in oncologic settings [4].
Locoregional anesthesia (LRA) is widely employed in orthopedic surgery, either alone or in combination with general anesthesia (GA), offering enhanced anesthetic management by reducing the need for GA and its associated adverse effects [5]. LRA is associated with reduced surgical stress, systemic inflammation, surgery-induced immunosuppression, as well as decreased opioid consumption [6,7]. It has also demonstrated strong efficacy in reducing postoperative pain in both the short and medium term [8-11].
In orthopedic oncology, however, concerns remain regarding the potential impact of LRA on local tumor recurrence, distant metastasis, and postoperative complications. While LRA has been extensively studied in various oncologic surgeries and remains an active area of investigation, data specific to orthopedic oncologic procedures are still limited. Several meta-analyses and reviews have reported potential oncologic benefits of LRA, including improved overall survival [12], a possible reduction in tumor recurrence 1, decreased risk of biochemical relapse in prostate cancer [13], and inhibition of tumor progression in vitro and in vivo in certain carcinomas and lymphomas [14].
In contrast, data from orthopedic oncology remain conflicting. A retrospective study of 100 patients with extremity bone sarcomas by Bijan A. et al. in 2023 reported significantly improved metastasisfree survival in patients who received LRA [15]. Conversely, a retrospective study published in 2016 by A.K. Freeman et al., evaluating 90-day postoperative survival in 174 patients undergoing resection of pelvic or sacral bone tumors, found no difference in survival based on the use of epidural anesthesia [16]. Furthermore, a recent literature review by Mohd S. Ramly et al. (2024), covering studies from 1994 to 2024, found no significant impact of LRA or any other anesthetic technique on oncologic outcomes [17], except for peri-tumoral lidocaine infiltration in breast cancer, which was associated with improved overall survival [18].
This study therefore aims to evaluate the safety of LRA in upper limb orthopedic tumor surgeries. We hypothesize that LRA does not increase the risk of recurrence, metastatic dissemination, or postoperative complications.
Materials and Methods
This single-center retrospective study, conducted at Cliniques Universitaires Saint-Luc in Brussels, Belgium, aimed to evaluate tumor recurrence and complication rates in patients undergoing surgical excision of upper limb orthopedic tumor lesions, based on whether or not LRA was used.
The primary inclusion criterion was any patient who underwent orthopedic surgery for the excision of an upper limb tumor lesion at our institution between 2017 and 2024. Metastatic lesions were excluded. Tumors were classified into four categories: benign bone tumors, malignant bone tumors, low-grade soft tissue tumors, and malignant soft tissue tumors. This grouping was used due to the large number of distinct histological subtypes-26 in total such as liposarcoma, chondrosarcoma, myxofibrosarcoma, Ewing’s sarcoma, etc. -each with varying stages at the time of surgery. Regarding soft tissue tumors, simple lipomas were excluded. In contrast, atypical lipomatous tumors were included if they met at least one of the following criteria: size greater than 5 cm, proximity to neurovascular structures, intrinsic aggressiveness, or atypical depth of infiltration. We also excluded patients with initially metastatic lesions.
Data were collected and organized into four main categories:
- Patient-related data: age, sex, ASA score (excluding tumor-related pathology), diabetes status, smoking status, followup duration, and mortality.
- Anesthesia-related data: type of anesthesia (GA alone, LRA alone, or a combination of both), performance of LRA (with or without GA), type of nerve block, perineural catheter placement (by the anesthesiologist remotely, intraoperatively by the surgeon, or none), type and dose of local anesthetic, use of adjuvants to the local anesthetic, intravenous dexamethasone (dose and administration), use of a ketamine pump, and postoperative use of a morphine pump with a patient-controlled analgesia (PCA).
- Surgical data: procedure type (simple excision, reconstruction with prosthesis, flap, or graft), operative duration, postoperative complications (local recurrence, metastatic progression, soft-tissue complications, mechanical complications, infection, chronic pain, or none), time to complication onset, and requirement for revision surgery.
- Oncological data: lesion location and type, tumor stage, administration of adjuvant or neoadjuvant therapy, whether the procedure was a second-stage surgery, tumor recurrence, and any evidence of metastatic progression.
The Primary outcome was tumor recurrence in the group receiving LRA (with or without GA) compared to the GA-only group. The Secondary outcome was the occurrence of other postoperative complications.
Statistical analyses were conducted using IBM SPSS Statistics (version 27). Descriptive statistics summarized the data: quantitative variables were expressed as means ± standard deviation (SD), and qualitative variables as percentages (%). Normality of distribution was assessed using QQ plots, which indicated a deviation from normality in both groups. Furthermore, given unequal group sizes and non-normal distribution, non-parametric tests were used for inferential analysis. Statistical significance was defined as p < 0.05.
Continuous quantitative variables were analyzed using the MannWhitney U test. Discrete quantitative variables with limited degrees of freedom were treated as categorical variables and analyzed using the Pearson Chi-squared (χ²) test. This test was also applied to categorical variables with more than two categories. Associations between binary categorical variables were assessed using Cramér’s V.
For the comparison between the two groups a Wald test in multinomial logistic regression was used.
Kaplan-Meier survival curves were generated to compare time to tumor recurrence and complication onset between groups, and differences were tested using the log-rank (Mantel-Cox) test.
Results
Patient Selection and Anesthetic Techniques
Among all patients operated on by the lead orthopedic oncology surgeon between 2017 and 2024, we identified those meeting the primary inclusion criterion: surgical excision of an upper limb tumor lesion, and exclusion criteria were applied. This yielded a final cohort of 95 patients.
Of the 95 patients included, 30 (31.6%) received LRA. Regarding the anesthetic technique, 90.5% (n = 86) underwent GA with sevoflurane, 2.1% (n = 2) received total intravenous anesthesia (TIVA) with propofol, and 7.4% (n = 7) were operated on under LRA alone.
Table 1 presents the comparison between the two groups. It is noteworthy that the LRA group had a statistically higher proportion of malignant bone tumors, and the non-LRA group had statistically more low-grade soft tissue tumors.
LRA | Non LRA | p-value a,b | |
Sample size, n | 30 | 65 | |
Patient related data | |||
Age, mean (years +/- SD) | 48 +/- 22.4 | 55 +/- 20.2 | 0.85 |
Female sex, n (%) | 13 (43.4) | 27 (41.5) | 0.1 |
Male sex, n (%) | 17 (56.6) | 38 (58.5) | |
Diabetes, n (%) | 3 (10) | 8 (12.3) | 0.74 |
Tobacco, n (%) | 5 (16.7) | 11 (16.9) | 0.36 |
ASA classification, n (%) | |||
I | 8 (26.7) | 12 (18.5) | 0.93 |
II | 18 (60) | 44 (67.7) | 0.5 |
III, IV or V | 4 (13.3) | 9 (13.8) | 0.17 |
Follow up time, mean (days +/-SD) | 784.8 +/- 741.4 | 622.6 +/- 822.5 | |
Anesthesiological data | |||
PCA use , n (%) | 1 (3.3) | 16 (24.6) | 0.01 |
Surgical data | |||
Tumoral type, n (%) | |||
Benign bone tumor | 3 (10) | 2 (3) | 0.68 |
Low grade soft tissue tumor | 5 (16.7) | 23 (35.4) | < 0.01 |
Malignant bone tumor | 14 (46.7) | 13 (20) | < 0.01 |
Malignant soft tissue tumor | 8 (26.6) | 27 (41.6) | 0.12 |
Abbreviations : LRA, locoregional anesthesia; ASA, American Society of Anesthesiologists; PCA, Patient controlled analgesia; SD, standard deviation a : p-value compares 2 groups : LRA vs Non LRA b : t-test used to compare means, chi-square test used to compare proportions and Wald test used to compare means in multinominal variables | |||
Table 1: Patient characteristics comparison between groups LRA (n = 30) and Non LRA ( n = 65).
Tumor Types and Anesthesia Details
In the LRA group, four types of nerve blocks were performed by the anesthesia team: interscalene (36.7%, n = 11), supraclavicular (6.7%, n = 2), infraclavicular (13.3%, n = 4), and axillary (23.3%, n = 7). Additionally, 20% of patients (n = 6) received a perineural catheter placed in situ by the surgeon. 36.7% (n = 11) had a catheter placed preoperatively by anesthesiologists, and 43,3% (n = 13) received a single-shot LRA.
The local anesthetics used were ropivacaine (62.5%, n = 16), levobupivacaine (12.5%, n = 3), mepivacaine (16.7%, n = 4 ), or a combination of ropivacaine and mepivacaine (8.3%, n = 2).
Recurrence and Complications
No statistically significant difference was observed in tumor recurrence rates between the two groups (LRA: n = 5 (16.7%); Non-LRA: n = 9 (13.8%); χ²(1) = 0.13, p = 0.718). Similarly, no statistically significant difference was found in overall postoperative complication rates (LRA: n = 8 (26.7%); Non-LRA n = 13 (20%); χ²(1) = 0.53, p = 0.467).
These results include all types of complications. The breakdown by complication subtype is presented in Table 2; no individual subtype showed a significant difference between groups.
|
Soft tissue failure |
Mechanical failure |
Infection |
Chronic pain |
No complication |
Total |
||
|
LRA |
Yes |
3 |
2 |
3 |
0 |
22 |
30 |
|
No |
3 |
5 |
1 |
4 |
52 |
65 |
|
|
Total |
6 |
7 |
4 |
4 |
74 |
95 |
|
Table 2: Distribution of Surgical Complications: Absolute Numbers in the locoregional anesthesia (LRA) vs. Non-LRA Groups.
Conversely, Figure 1 highlights a marginally statistically significant association between tumor recurrence and the placement of perineural catheters by the surgeon within the surgical field.
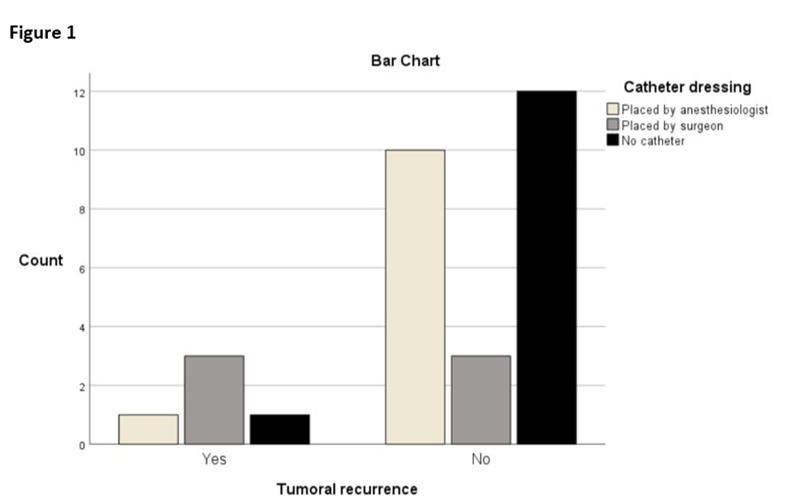
Figure 1: Perineural catheter and recurrence placement bar charts. Among the six catheters placed by the surgeon, 50% (n = 3) had tumoral recurrence, among the eleven catheter placed by anesthesiologist, 9% (n = 1) had tumoral recurrence, (χ²(2) = 6.008, p = 0.05).
Finally, Figure 2 indicates that there is no difference in recurrence-free survival or complication-free survival between the two groups.
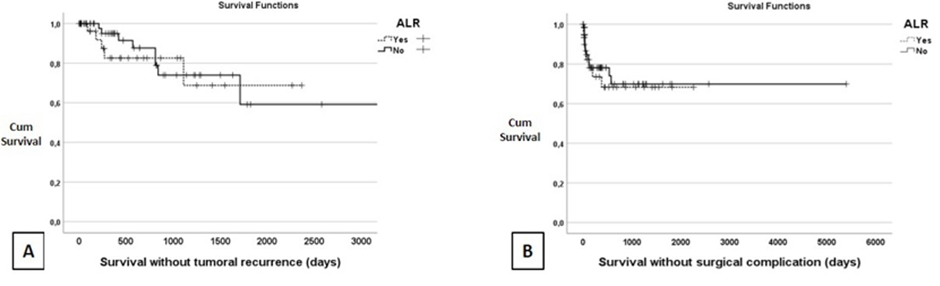
Figure 2: Kaplan-Meyer graph. (A) Survival without recurrence, LRA 1815.3 +/- 221.7 days, CI [1380.7 - 2250.6] vs No LRA 2330.5 +/- 302.9 days, CI [1736.7 - 2924.2], p > 0.05. (B) Survival without surgical complication, LRA 1584.5 +/- 202.6 days, CI [1187.3 - 1981.7] vs No LRA 3827.5 +/- 388.9, CI [3065.1 - 4589.8], p > 0.05. Abbreviation: Cum., cumulative; CI, Confidence Interval.
Discussion
This retrospective, single-center study demonstrates that LRA is not associated with an increased risk of postoperative complications or tumor recurrence when compared to general anesthesia (GA) alone in upper limb orthopedic oncologic surgery. These findings suggest that LRA is a safe anesthetic modality in this clinical setting. However, a notable observation emerged: a statistically significant association was identified between tumor recurrence and the placement of perineural catheters directly into the surgical field by the operating surgeon. Although this finding should be interpreted with caution due to the small sample size (six patients, representing 20% of the LRA group and 6.3% of the total cohort), it nonetheless raises an important clinical concern. In our view, this association can be explained by the proximity to the tumor site and the attempt to preserve as many critical neural structures as possible, even at the risk of potentially compromising the resection margins. To date, limited literature has addressed outcomes related to surgically placed perineural catheters, particularly in the context of oncologic or upper limb surgery.
All surgeries were performed by a single experienced orthopedic oncologic surgeon from our reference center, thereby minimizing inter-operator variability and reducing technical bias. This is particularly relevant, as previous literature has identified surgeon expertise as a significant prognostic factor in oncologic outcomes [19].
In contrast to the findings of Bijan A. et al.15, who reported improved metastasis-free survival with combined LRA and GA, our data do not support a survival benefit associated with LRA. On the other hand, our results are consistent with several studies suggesting no significant association between LRA and postoperative recurrence or dissemination [16-22].
We chose to include low-grade tumors meeting the previously mentioned criteria because such tumors present analgesic challenges and also share several risk factors with other sarcomas like local recurrence. It should be noted that the tumors in this group were well-differentiated liposarcomas, which are considered as low-grade malignant lesions. Consequently these tumors have been assigned to a distinct category.
Several variables examined in our study proved difficult to interpret due to potential bias and limited data. These include the type of local anesthetic used, adjuvant drugs, dexamethasone administration, use of continuous ketamine infusion, and the initial tumor stage. Their impact on outcomes remains uncertain in this context.
Nevertheless, these results must be interpreted with caution. The small sample size limits statistical power, and catheter placement by the surgeon may have been more frequently used in cases involving more extensive or aggressive tumors-potentially introducing confounding bias. Furthermore, in these specific cases, the surgeon may have been tempted to salvage critical neurological structures and subsequently place the catheter in the area. The neural sheath is a commonly known and accepted margin for several sarcoma subtypes. Thus, the surgical margins were probably less extensive around significant neurological structures. Additionally, the imbalance between group sizes rendered multivariable analysis unreliable.
Given the heterogeneity of tumor types in our cohort, pathologies were grouped to enable meaningful analysis. Despite this limitation, our sample-comprising nearly 100 patients undergoing upper limb tumor surgery with or without LRA-offers valuable insights in a rare and under-researched clinical area. Future research should prioritize the development of prospective, randomized controlled trials (RCTs), whether single- or multi-center, to validate and expand upon these findings.
Although all procedures were performed by the same surgeon, anesthesiologists varied between patients, and the decision to use LRA was made individually and left to the discretion of the anesthesiologist. The diversity of tumor presentations necessitated the use of various regional anesthesia techniques at different anatomical levels, which may have influenced outcomes. These procedural variabilities represent potential sources of bias that should be considered when interpreting the results.
It should also be emphasized that the two groups differed in patient numbers, and the tumor distribution was not homogeneous. Specifically, the LRA group had statistically fewer low-grade soft tissue tumors and more malignant bone tumors. This is likely because such tumors are often more painful, and LRA techniques were therefore preferred. However, this imbalance could potentially introduce a bias that favors the detection of a harmful effect of LRA, due to the greater aggressiveness of malignant bone tumors.
Another potential confounder is the use of patient-controlled analgesia (PCA) with morphine in some patients. The immunosuppressive effects of opioids are well documented 2, and PCA was more commonly used in the non-LRA group. This may have influenced recurrence or complication rates and should be explored in future studies.
That said, the rarity and heterogeneity of tumors in this setting present substantial challenges to rigorous research and likely explain the scarcity of high-quality data. While our results are encouraging, prospective studies with larger and more homogeneous patient populations are essential to confirm the non-inferiority of LRA and encourage its broader adoption. Given the major nature of many oncologic procedures, LRA offers substantial benefits for eligible patients, and demonstrating its safety could significantly improve perioperative management in this population.
Conclusion
Our study corroborates existing evidence regarding the safety of locoregional anesthesia techniques, including in the management of musculoskeletal tumors. However, our results suggest that particular caution should be exercised when positioning equipment in close proximity to the tumor site. Maintaining a safe distance when placing a peripheral nerve catheter may be advisable to minimize the risk of tumor recurrence. These findings, however, require confirmation through larger, prospective studies.
Acknowledgements
The authors declare no conflict of interest or financial disclosure. An AI tool was used to enhance the quality of the English scientific writing of this work. AI tools were never used as generative data tools.
This study was approved by the ethics committee of the Cliniques Universitaires Saint-Luc in Brussels, Belgium, under the identification number: NCT02355301.
Conflicts of Interest: The authors declare no conflicts of interest.
Funding: The authors have no sources of funding to declare for this manuscript.
References
- Cata JP, Gottumukkala V, Sessler DI (2011) How regional analgesia might reduce postoperative cancer recurrence. Eur J Pain Suppl 5(2): 345-55.
- Kim R (2018) Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J Transl Med 16(1): 8.
- Fodale V, Arrigo MG, Triolo S, Mondello S, Torre DL (2014) Anesthetic techniques and cancer recurrence after surgery. ScientificWorldJournal 14: 328513.
- Gottschalk A, Sharma S, Ford J, Durieux ME, Tiouririne M (2010) Review article: the role of the perioperative period in recurrence after cancer surgery. Anesth Analg 110(6): 1636-43.
- Saied NN, Helwani MA, Weavind LM, Shi Y, Shotwell MS, (2017) Effect of anaesthesia type on postoperative mortality and morbidities: a matched analysis of the NSQIP database. Br J Anaesth 118(1): 105111.
- Page GG, Blakely WP, Ben-Eliyahu S (2001) Evidence that postoperative pain is a mediator of the tumor-promoting effects of surgery in rats. Pain 90(1-2): 191-199.
- Sessler DI (2008) Does regional analgesia reduce the risk of cancer recurrence? A hypothesis. Eur J Cancer Prev 17(3): 269-272.
- Argun G, Çayırlı G, Toğral G, Arıkan M, Ünver S (2007) Are peripheral nerve blocks effective in pain control of pediatric orthopedic tumor surgery? Eklem Hast Ve Cerrahisi Jt Dis Relat Surg 30(1): 46-52.
- Rawal N (2007) Postoperative pain relief using regional anaesthesia. Curr Anaesth Crit Care 18(3):140-148.
- Weinstein EJ, Levene JL, Cohen MS, Andreae DA, Chao JY,et al. (2018) Local anaesthetics and regional anaesthesia versus conventional analgesia for preventing persistent postoperative pain in adults and children. Cochrane Database Syst Rev 6(6): CD007105.
- Martineau J, Guillier D, Maruccia M, Guiotto M, Borens O, et al. (2022) Locoregional anesthesia for post-operative pain management in microsurgical reconstruction of the lower extremities: A retrospective study. J Plast Reconstr Aesthetic Surg JPRAS. 75(9): 3190-6.
- Chen WK, Miao CH (2013) The effect of anesthetic technique on survival in human cancers: a meta-analysis of retrospective and prospective studies. Plos one 8(2): e56540.
- Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, et al. (2008) Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology 109(2): 180-7.
- Zhang Y, Jing Y, Pan R, Ding K, Chen R, et al. (2021) Mechanisms of Cancer Inhibition by Local Anesthetics. Front Pharmacol 12: 770694.
- Abar B, Gao J, Fletcher AN, Sachs E, Wong AH, et al. (2023) Regional anesthesia is associated with improved metastasis free survival after surgical resection of bone sarcomas. J Orthop Res 41(12): 2721-9.
- Freeman AK, Thorne CJ, Gaston CL, Shellard R, Neal T, et al. (2017) Hypotensive Epidural Anesthesia Reduces Blood Loss in Pelvic and Sacral Bone Tumor Resections. Clin Orthop 475(3): 634-40.
- Ramly MS, Buggy DJ (2025) Anesthetic Techniques and Cancer Outcomes: What Is the Current Evidence? Anesth Analg 140(4): 76877.
- Badwe RA, Parmar V, Nair N, Joshi S, Hawaldar R, et al. (2023) Effect of Peritumoral Infiltration of Local Anesthetic Before Surgery on Survival in Early Breast Cancer. J Clin Oncol. 41(18): 3318-28.
- Hong AM, Sundaram A, Perianayagam G, Lo H, Lawless A, et al. (2023) Surgery at specialised sarcoma centres improves patient outcomes - A systematic review by the Australia and New Zealand sarcoma association clinical practice guidelines working party. Eur J Surg Oncol 49(9): 106951.
- Sessler DI, Pei L, Huang Y, Fleischmann E, Marhofer P, et al. (2019) Recurrence of breast cancer after regional or general anaesthesia: a randomised controlled trial. Lancet 394(10211): 1807-15.
- Myles PS, Peyton P, Silbert B, Hunt J, Rigg JRA, et al. (2011) Perioperative epidural analgesia for major abdominal surgery for cancer and recurrence-free survival: randomised trial. BMJ 342: d1491.
- Sansone P, Giaccari LG, Faenza M, Di Costanzo P, Izzo S, et al. (2020) What is the role of locoregional anesthesia in breast surgery? A systematic literature review focused on pain intensity, opioid consumption, adverse events, and patient satisfaction. BMC Anesthesiol 20(1): 290.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.