Rigid Autologous Bone Reconstruction after Endoscopic Endonasal Olfactory Neuroblastoma Resection
by Wen-Ching Chuang1,2, Kai‐Ping Chang2,3,4, Cheng-Chi Lee2,5,6, ShihMing Jung2,7, Yi-Wei Chen2,8, Chia-Hsiang Fu2,8,9, Chien-Chia Huang2,8,9, Tsung-You Tsai2,3, Ping-Ching Pai2,10, Ta-Jen Lee2,8,11, Chi-Cheng Chuang2,5,12*
1Department of Otolaryngology, Chang Gung Memorial Hospital, Taoyuan, Taiwan
2School of Medicine, Chang Gung University, Taoyuan, Taiwan
3Department of Otolaryngology Head and Neck Surgery, Chang Gung Memorial Hospital, Taoyuan, Taiwan
4Molecular Medicine Research Center, Chang Gung University, Taoyuan, Taiwan
5Department of Neurosurgery, Chang Gung Memorial Hospital, Taoyuan, Taiwan
6Department of Biomedical Engineering, National Taiwan University, Taipei, Taiwan
7Department of Anatomic Pathology, Chang Gung Memorial Hospital Linkou Medical Center, Taoyuan, Taiwan
8Department of Otolaryngology, Division of Rhinology, Chang Gung Memorial Hospital, Taoyuan, Taiwan
9Graduate Institute of Clinical Medical Sciences, College of Medicine, Chang Gung University, Taiwan
10Department of Radiation Oncology, Chang Gung Memorial Hospital, Taoyuan, Taiwan
11Department of Otolaryngology, Xiamen Chang Gung Hospital, Xiamen, China
12Department of Neurosurgery, Xiamen Chang Gung Hospital, Xiamen, China
*Corresponding author: Chi-Cheng Chuang, Department of Emergency Surgery, Department of Neurosurgery, Chang Gung Memorial Hospital, Taoyuan, Taiwan
Received Date: 27 July, 2023
Accepted Date: 01 August, 2023
Published Date: 03 August, 2023
Citation: Chuang WC, Chang KP, Lee CC, Jung SM, Chen YW, et al. (2023) Rigid Autologous Bone Reconstruction after Endoscopic Endonasal Olfactory Neuroblastoma Resection. J Surg 8: 1861 https://doi.org/10.29011/2575-9760.001861
Abstract
Background: For endoscopic endonasal reconstruction of skull base defects, minimizing the sequelae of Cerebrospinal Fluid (CSF) leakage, graft migration, and infection is challenging. This study aims to evaluate the outcomes of endoscopic multilayer autologous rigid buttress reconstruction in patients with olfactory neuroblastoma.
Methods: This study retrospectively reviews the records of olfactory neuroblastoma patients who underwent endoscopic endonasal surgery and reconstruction with a rigid buttress at one institution between May 2015 and December 2021. After tumor resection, reconstruction was performed using a multilayered fascia lata and autologous rigid buttress, and the defect was covered with a vascularized nasoseptal flap. Autologous bone chips or septal cartilage was placed between the dura and bone of the skull base. We reviewed demographic data, postoperative complications, body mass index, the follow-up time, and tumor Kadish stage and Hyams grade.
Results: Reconstruction was performed in 12 patients (4 males; 8 females). The median follow-up period was 24.8 months. 3 patients with Kadish stage D tumors with lymph node invasion underwent neck dissection surgery. Hyams tumor grade classification showed 5 patients with tumor grade II, 6 with grade III, and 1 with grade IV. Lumbar drainage was placed in one patient for monitoring (patient #1). 4 patients underwent radiotherapy, and 4 underwent chemoradiotherapy. No postoperative CSF leakage occurred in any patient.
Conclusions: Reconstruction of skull base defects using a multilayered anatomical approach with an autologous rigid buttress and vascularized flap is effective and can prevent complications in patients after endoscopic endonasal surgery for olfactory neuroblastoma.
Keywords: Anterior skull base reconstruction; Autologous bone reconstruction; Endoscopic endonasal approach; Olfactory neuroblastoma; Rigid reconstruction
Abbreviations: BMI: Body Mass Index; CCRT: Concurrent Chemoradiotherapy; CSF: Cerebrospinal Fluid; EEA: Endoscopic Endonasal Approach; FL: Fascia Lata; LD: Lumbar Drainage; ND: Neck Dissection; NSF: Nasoseptal Flap; OB: Olfactory Bulb; ONB: Olfactory Neuroblastomas; RT: Radiation
Introduction
Endoscopic approaches are now widely used in the management of skull-base neoplasms, including pituitary tumors, Olfactory Neuroblastomas (ONB), chordomas, and chondrosarcomas. Surgical treatment planning for such neoplasms requires consideration of the tumor extension and the risks of subsequent skull-base defects, postoperative Cerebrospinal Fluid (CSF) leakage, meningitis, brain abscess, and pneumocephalus [1,2] Because insufficient repair is associated with postoperative complications, reliable skull-base reconstruction after endoscopic surgery is necessary [3,4]. Reconstruction of anterior skull base defects with additional rigid structural support is essential to prevent graft migration due to intracranial pulsation and gravity and to reduce the possibility of frontal lobe herniation [5-7]. To the best of our knowledge, no previous study has investigated postoperative complications following endonasal endoscopic reconstruction with an autologous rigid buttress in ONB patients. This study aims to evaluate the results of endoscopic reconstruction using an anatomical multilayered approach with a rigid buttress and vascularized flap in ONB patients.
Methods And Materials
Patient Data
In this retrospective cohort study, we reviewed the records of ONB patients who underwent endoscopic endonasal surgery with endoscopic rigid reconstruction between May 2015 and December 2021. The diagnosis of ONB was histologically confirmed in all cases. We reviewed patient data including age, sex, diagnosis, surgical procedure, repair technique, length of follow-up, and the presence of postoperative complications. The final cohort included 12 patients. This study was approved by the Institutional Ethics Committee of Chang Gung Memorial Hospital. All cases were jointly managed by the same surgical team, which consisted of otorhinolaryngology and neurosurgery services and an experienced primary surgeon. The otorhinolaryngologist resected tumors located in the nasal cavity and paranasal sinuses, and the neurosurgeon resected dural, olfactory bulb, and intracranial tumors. All patients were evaluated preoperatively using enhanced computed tomography and/or magnetic resonance imaging and positron emission tomography (Figure 1). Biopsied tumor samples were analyzed by a pathologist.
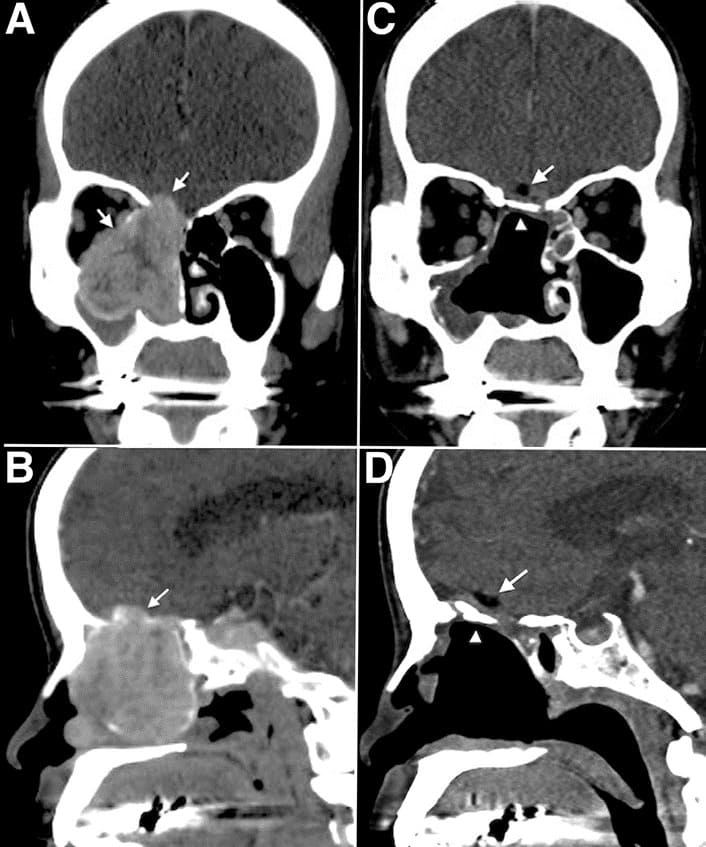
Figure 1: Preoperative and postoperative computed tomography scans. Pre-operative coronal view (A) and pre-operative sagittal view (B) identified a tumor located in the right nasal cavity with frontal base invasion. The orbital wall bone was also eroded and the periorbita laterally displaced. (C,D) Post-operative computed tomography scans showing the rigid bone reconstruction in place, in good anatomical alignment with no encephalocele or flap dislodgement, and restoration of the orbital cavity after tumor resection.
The arrows indicate the fat graft. The arrowheads indicate the bone graft.
Surgical Procedures and Reconstruction Techniques
We identified and completely resected the tumor attachment. The cribriform plate, dura, olfactory bulbs, and tracts were resected depending on the extent of the tumor via navigation assistance. The skull base was then reconstructed. We harvested approximately 2.5 × 2.5 cm of the fascia lata, depending on the defect size, and subcutaneous fat from the thigh before reconstruction. An autologous fat graft was used to eliminate intracranial dead space and prevent the fascia graft from being dislodged into the space. For the first layer, a piece of autologous fascia lata was placed as the subdural inlay, and the margin was extended circumferentially about 0.5 cm beyond the edge of the dural defect. For the second layer (extradural onlay), another piece of autologous fascia lata larger than the bony defect circumferentially was placed outside the dural defect but inside the bony edge (in the epidural space). For the third layer of reconstruction, we used an autologous perpendicular plate, vomer bone chips, or septal rigid cartilage, placing the material over the fascia but inside the bony defect (Figure 2). An important step in our technique was the insertion of both the second layer of fascia and bone graft into the space between the dura and edges of the bony defect. This layer-by-layer anatomic repair ensured closure of the defect and provided rigid support for the frontal lobe. We then inspected the repair to ensure the absence of CSF leakage and brain pulsatility before proceeding to the next step of positioning the flap by Valsalva maneuver. Following bone graft placement, the defect was covered with a vascularized nasoseptal flap. Each layer was then secured with fibrin sealant (Tisseel, Baxter International Inc., Deerfield, IL, USA) to ensure closure and adjacency of the reconstruction layers. Finally, the nasal cavity was supported with a piece of Nasopores (Polyganics, Groningen, The Netherlands) to ensure the nasoseptal flap remained in place.
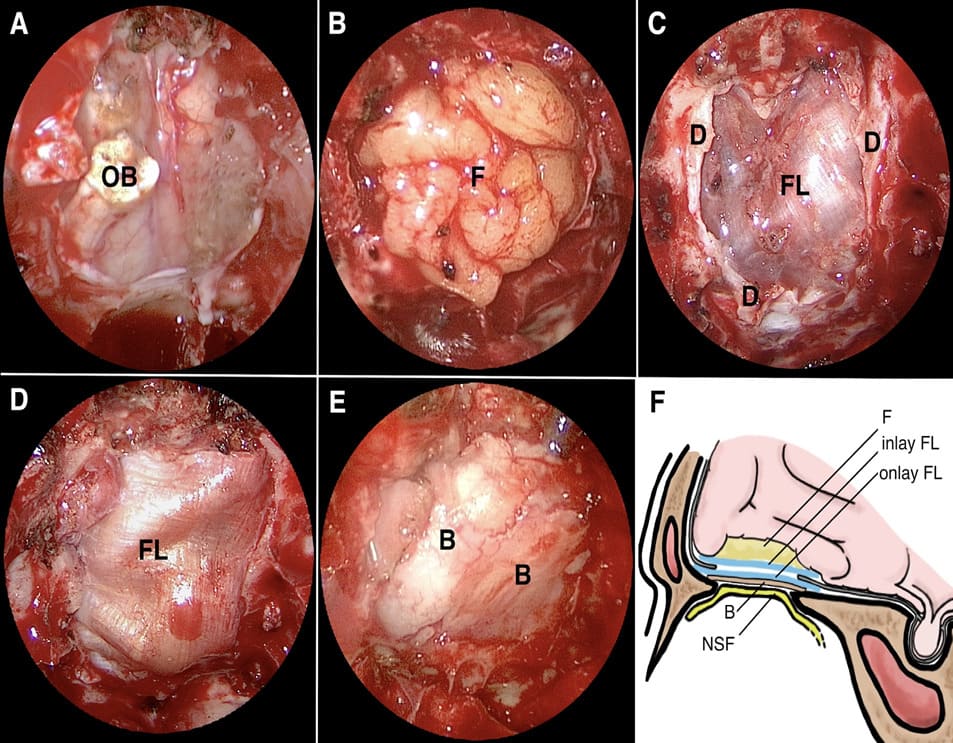
Figure 2: Intra-operative photographs. (A) The extent of skull base defect after tumor resection. (B) The fat graft was placed in the subdural resected cavity and embedded in the bony or dural border of the defect. (C) The first layer of autologous fascia lata was inserted as an inlay graft inside the dural edge. (D) The second layer of autologous fascia lata was inserted as an extradural onlay graft inside the bone edge. (E) Rigid buttress bone graft was inserted between the onlay fascia lata and bone edge. (F) Diagrammatic representation of the autologous rigid reconstruction of an anterior skull base defect layer by layer in sagittal view. (OB: olfactory bulb; FL: fascia lata; D: dura; B: bone; NSF: nasoseptal flap; F: Fat)
Postoperative Management
Lumbar drainage was initially inserted in one patient (patient 1) to prevent postoperative CSF leak (for monitoring only). We then considered that this additional procedure to prevent CSF leakage was not necessary. Therefore, midway through the study period we revised our protocol to exclude the lumbar drainage, which facilitated earlier mobilization of the patients. The patients were kept on a prophylactic antibiotic regimen consisting of a third-generation cephalosporin or a penicillin-based antibiotic (a beta-lactamase) from 1 day before until 7 days after the surgery.
Results
BMI: Body Mass Index; F: Female; M: Male; EEA: Endoscopic Endonasal Approach; RT: Radiation; CCRT: Concurrent Chemoradiotherapy; ND: Neck Dissection. Table 1: Characteristics of patients who underwent endoscopic endonasal surgery for olfactory neuroblastoma. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The clinical characteristics of the patients are shown in Table 1. Of the 12 included patients, 4 (33.3%) were male and 8 (66.6%) were female. Eleven patients had complete reconstruction with a bone graft, and 1 patient had incomplete reconstruction due to inadequate bone reconstruction. The mean patient age was 50.9 years (range, 27-78 years), and the mean postoperative followup period was 24.8 months (range, 7-57 months). The mean Body Mass Index (BMI) was 22.3 kg/m2. Three patients with Kadish stage D tumors and lymph node invasion underwent neck dissection surgery. Hyams tumor grading revealed a grade II tumor in 5 patients, grade III tumor in 6 patients, and grade IV tumor in 1 patient. The mean hospital stay for the 9 patients without neck dissection was 8.8 days, compared to 17.3 days for the 3 patients with neck dissection. All patients underwent postoperative endoscopic examination upon return to the otolaryngology clinic. No cases exhibited persistent crusting at the repair site, and there were no cases of postoperative meningitis, mucocele formation, or tension pneumocephalus. Four patients underwent radiation therapy, and 4 patients underwent chemoradiation therapy. One patient (patient #9) had vomer bone erosion leading to inadequate rigid reconstruction, which was managed with complete bed rest for 5 days. The patient did not undergo a second reconstructive surgery. Endoscopic examination was performed by an otolaryngologist every month after surgery in patients treated using the Valsalva maneuver to check for CSF leakage. In a total of 26 months of follow up, no leakage was noted among these patients. In the total patient cohort, we observed no CSF leakage, encephalocele, or central nervous system infection. The mean defect width was 1.56 cm, and the mean defect length was 1.97 cm. The cancer recurred in 3 patients during the follow-up period, as follows: Patient #2, inferior right nasal septum metastasis 23 months after tumor resection; patient #3, lumbar spine bone metastasis 55 months after radiotherapy; patient #4, right lower section of the inferior turbinate metastasis 26 months after concurrent chemoradiotherapy.
Discussion
Our study had described the experience and results of endoscopic reconstruction using an anatomical multilayered approach with a rigid buttress and vascularized flap in ONB patients. ONBs are rare, anatomically complex neuroendocrine malignancies that are thought to arise from the nasal olfactory epithelium. Due to this site of origin, they have a tendency to extend directly into the central nervous system through the cribriform plate and anterior skull base. No uniform treatment algorithm has been established for patients with ONB; however, multidisciplinary management including surgery alone or surgery with radiotherapy is generally preferred [8-11]. While surgical resection can be the sole therapy for early tumors, combination treatment is the preferred method for later tumors at most institutions [12]. The 5-year overall survival rate is 86-100% in patients with Kadish stage A and B tumors and 70.8-76% in patients with Kadish stage C and D tumors [13-15].
The Era of Endoscopic Surgery for Patients with ONB
En-bloc resection through a craniofacial approach has been the traditional technique used in the management of ONB. However, this procedure results in a large facial wound and requires a long hospitalization and intense postoperative care [2,16,17]. The endonasal endoscopic approach avoids the facial incision, extensive osteotomies, and frontal lobe retraction and is therefore less invasive with better preservation of function. The endonasal endoscopic approach is also reported to achieve better survival and local control rates [16,18,19]. A meta-analysis of 36 studies with 609 ONB patients suggests higher disease-specific survival and lower rates of CSF leak and intracranial complications in patients undergoing endoscopy compared to those undergoing craniofacial surgery [16]. The origin of the olfactory neuroblastoma is the olfactory neuroepithelium located in superior nasal vault and cribriform plate. Given this presumed origin, the appropriate surgical margin is considered to be resection of the dura and the olfactory apparatus. Our present retrospective study reveals no association between the recurrence site and reconstructed tissue. We had also reviewed the literature regarding tissue reconstruction and tumor recurrence. According to Florent Carsuzaa et al.,[20] the rate of local recurrence after surgery for malignant sinonasal tumors was about 30-50%. To date, few studies have addressed tumor recurrence in reconstructed tissue in ONB patients.
Common Complications Of Endonasal Endoscopic Surgery
The complications of endonasal endoscopic surgery, including nasal obstruction, CSF leak, meningitis, pneumocephalus, and intracranial hypotension, may be troublesome and are often related to inadequate reconstruction [6,21,22]. Surgical site mobility may require further CSF diversion techniques or revision surgery, resulting in delayed treatment. Postoperative CSF leakage has been reported in 1-8% of anterior skull base reconstructions [5,23,24].
Differences in Endonasal Endoscopic Reconstruction Techniques
Multilayer reconstruction to establish watertight closure is the gold standard for skull base defects [4,25,26] A number of reconstruction materials have been proposed, including abdominal fat, nasal turbinate mucosa, turbinate bone, septal cartilage, temporalis fascia, a pedicled nasoseptal flap, and an alloplastic plate [27-32] Most case series of skull base reconstruction techniques include pituitary adenomas, which are generally extra-arachnoidal tumors with smaller defects and lower rates of postoperative CSF leak. A summary of anterior skull base reconstruction techniques excluding pituitary adenomas is shown in Table 2. The bilayer button technique for skull base reconstruction has been proposed, however the alignment has been shown to be unsuitable for firm repair. Luginbuhl et al. applied the bilayer button technique in smaller defects by sandwiching the dural edge.[33] The gasket technique, which uses a rigid buttress, is one of the most common cranial base reconstruction methods. Garcia-Navarro et al. described use of the gasket technique with vomer bone or MEDPOR (Porex Corp, Newnan, Georgia, USA) in 46 patients, reporting a postoperative CSF leakage rate of 5.2%. [34] Most of their enrolled patients had craniopharyngioma, and lumbar drainage was placed in 32 patients. However, the surgical defect in patients with ONB may be wider, thus requiring multiple layers of reconstruction. The development of new reconstruction techniques and materials should improve the complication rates.
|
Author, year |
No. of cases |
Case distribution (%); Cases of ONB (%) |
Reconstruction |
No. CSF leak (%) |
Other complications |
No. adjuvant therapy (%) |
||||
|
Layers |
Rigid |
Rigid Material |
LD and others |
|||||||
|
(+/-) |
||||||||||
|
1 |
Nicolai et al., 2008 [32] |
134 |
adenocarcinoma (37%); ONB (12%) |
fascia lata + fascia lata+ fat + nasal mucoperiosteum |
- |
- |
0 |
4 (2.9%) |
2/134 mucocele, 1/134 meningitis |
8/134 (5.9%) RT; 1/134 (0.7%) CCRT |
|
2 |
Hanna et al., 2009 [24] |
93 |
ONB (17%) |
NR |
NR |
NR |
0 |
3 (33.2%) |
1 meningitis |
37 % RT; 13% CCRT |
|
3 |
Eloy et al., 2012 [3] |
9 |
olfactory groove meningiomas (22%); ONB (22%) |
autologous fascia lata + acellular dermal allograft + NSF |
- |
- |
0 |
0 |
0 |
NR |
|
4 |
Eloy et al., 2013 [35] |
10 |
olfactory groove meningiomas (20%); ONB (20%) |
autologous fascia lata+ acellular dermal allograft + NSF |
- |
- |
0 |
0 |
0 |
NR |
|
5 |
Al-Asousi et al., 2017 [1] |
7 |
ONB (14.2%) |
polydioxanone flexible plate + mucosa graft + middle meatal spacer (Merocel) |
+ |
polydioxanone flexible plate |
0 |
0 |
1 transient headache and facial pressure |
2/7 (28.5%) RT; 1/7 (14.2%) Chemotherapy |
|
6 |
Gallia et al., 2018 [36] |
20 |
ONB (100%) |
± acellular dermal graft ± synthetic materials ± NSF |
- |
- |
Yes +/- 14 Fr. Foley catheter |
1 (5%) |
2 pneumocephalus and 1 CSF leak |
19 (95%) RT; 5 (25%) CCRT |
|
7 |
Gabriel et al., 2019 [32] |
9 |
meningioma (33%); ONB (11%) |
fascia lata + pericranial flap + NSF |
- |
- |
0 |
0 |
0 |
7/9 (77.7%) RT |
|
8 |
Abdelmeguid et al., 2019 [1] |
167 |
ONB (22.6%) |
synthetic autografts ± fascia lata ± NSF |
- |
- |
NR |
14/167 (8.3%) |
NR |
96/167 (40.2%) RT; 41/167 (17.2%) CCRT; 1/167 (0.5%) preoperative RT |
|
9 |
Fiacchini et al., 2020 [21] |
20 |
intestinal type adenocarcinoma (60%); ONB (10%) |
Iliotibial tract/fascia lata + fascia lata + NSF / pericranial flap |
- |
- |
0 |
0 |
0 |
Yes, no detail |
|
10 |
Luginbuhl et al., 2020 [15] |
39 |
craniopharyngioma (30%); ONB (2%) |
bilayer button ± mucoperichondrial septal flap /NSF |
- |
- |
7 |
11 (27.5%) |
NR |
NR |
|
11 |
Seaman et al., 2021 [37] |
24 |
spontaneous CSF rhinorrhea (50%); ONB (4%) |
resorbable poly (D,L) lactic acid plates + sponges (Nasopore) ± LD ± NSF |
+ |
resorbable plates |
20/25 |
0 |
0 |
NR |
|
12 |
Cai et al., 2022 [31] |
42 |
ONB (100%) |
fascia lata ± mucosal flap |
- |
- |
NR |
1 (2.3%) |
1/42: cervical hematoma 2/42: seizure |
27/42 (64.2%) RT; 14/42 (33.3%) CCRT |
|
13 |
Our study |
12 |
ONB (100%) |
fat + fascia lata + fascia lata + bone + NSF |
+ |
Autologous bone graft |
1 (for monitoring) |
0 |
0 |
4/12 (33%) RT; 4/12 (33%) CCRT |
ONB: Olfactory Neuroblastoma; LD: Lumbar Drainage; CSF: Cerebrospinal Fluid; NR: Not Reported; RT: Radiation Therapy; CCRT: Concurrent Chemoradiotherapy; NSF: Nasoseptal Flap
Table 2: Literature review of cases of anterior skull base reconstruction excluding pituitary lesions.
The Importance Of A Rigid Buttress In Reconstruction
Radical tumor excision has been shown to be beneficial for overall survival. [35,37] However, oncological resection may result in large bony defects that require rigid reconstruction. The use of bony grafts to repair large defects in high-pressure areas or in cases of intracranial hypertension provides resistance to pulsation and pressure while allowing for healing and mucosalization.[38,39] Some studies recommend rigid reconstruction in patients at higher risk of postoperative CSF leak and meningocele. Such risk factors include elevated BMI, higher CSF pressure, larger bony and arachnoid defects, previous radiation, and future adjuvant therapy. From our point of view, for patients requiring further adjuvant treatment, better mucosal lining and healing are also considerations for rigid repair.[25] A rigid buttress is more effective and facilitates mucosal flap ingrowth and epithelization, which also contribute to wound closure after reconstruction. Moreover, a rigid buttress also avoids the need for a Foley balloon, which has inherent risks of overinflation, local infection, and postoperative distress to the patient. [28,40,41] Whether bony reconstruction is necessary in all patients undergoing anterior skull base reconstruction is debatable. [36] A rigid buttress is believed to prevent dislodgement. [42,43] Furthermore, spontaneous bony regrowth and osseous closure have been reported in rats, rabbits, and dogs. [30] In addition, ElBanhawy et al. reported that after repair with free bone grafts in 13 patients, the grafts were incorporated into the bony skull base in 84.6% of the patients. [44] These studies highlight the importance of rigid reconstruction and suggest that it is preferable to nonrigid reconstruction to avoid complications and graft migration.
Radiation As A Risk Factor for Reconstruction Failure
Comprehensive therapy is recommended for patients with ONB, including radiation for high-risk patients. In general, radiation therapy is recommended for postoperative treatment in patients who undergo non-radical surgery for T3 and T4, Kadish C and D, and high Hyams grade tumors.[45] The long-term effect of radiation on autologous flaps in ONB patients is unclear. The detrimental effects of radiation therapy are reported to be caused by vascular damage and endothelial cell injury. Postoperative radiation toxicity also causes the tissue to become fibrotic, leading to radiation-induced chronic nonhealing wounds. Accordingly, Herle et al. suggest that the defect should be reconstructed using a rigid technique before radiotherapy.[46] From our point of view, a rigid buttress plays a vital role in reconstruction for ONB patients, since they are likely to undergo radiotherapy.
Reconstruction Materials
The desired features of reconstruction materials are strength, the ability to revascularize, and biotechnical compatibility. Alloplastic implants such as titanium, porous polyethylene (MEDPOR), and silicone are foreign buttresses that may be susceptible to implantrelated infection, hemorrhage, distortion of magnetic resonance images, and the formation of granulation tissue.[43] Bioresorbable alloplastic implants have adequate strength and flexibility to fit the defect while having a lower potential risk compared to permanent alloplastic buttresses, therefore offering an alternative choice for reconstruction. Disadvantages including longer resorption time, high cost, and image disruption have been reported.[28,40,38] Seaman et al. reported on 24 patients with extrasellar skull base tumors who underwent reconstruction with bioabsorbable plates. [40] In 19 patients who received postoperative lumbar drainage, the postoperative CSF leak rate was 0%; however, this study did not report on the need for further radiation treatment or long-term infection rates.[40] Autologous grafts remain a popular choice, and the use of harvested bone or septal cartilage for rigid buttresses in skull base reconstruction has several advantages, including fewer adverse immunologic reactions, greater biocompatibility, lower cost, lower risk of infection, and lower impact on adjuvant therapy and image interpretation. [43,44] In particular, the production of fibroblasts and angiogenic factors in the acellular dermis can be faster in autografts than to allografts, therefore resulting in a shorter period of healing and wound crusting.[43] In addition, Jin et al. reported that bone in situ reconstruction can prevent adhesion of the nasoseptal flap to intracranial nerves or vessels, providing safe access for patients who require reoperation.[7] In this study, we introduce a technique for repairing skull base defects with an autologous rigid buttress and vascularized nasoseptal flap using a multilayered approach. This method achieved remarkable results in our ONB patients.
Limitations
This study has several limitations. First, selection bias is possible with regards to the selection of suitable candidates for endoscopic surgery. Thus, direct comparison between the results of our study and other reconstruction techniques is difficult. Second, given the relative rarity of the disease and approach, it is important to evaluate both intraoperative and postoperative complications. Long-term follow-up of surgical site mobility and the effect of post-radiation therapy should also be taken into consideration. Third, a larger sample size is needed to evaluate the oncological outcomes.
Conclusions
The use of a multilayered approach to autologous rigid graft reconstruction preserves anatomical structures. In addition, this technique avoids the intraoperative complications of endoscopic surgery, provides longstanding safety and adequate watertight closure, facilitates defect healing, and enables an earlier return to daily activities and adjuvant therapy.
References
- Rutland JW, Gill CM, Ladner T, Goldrich D, Villavisanis DF, et al. (2022) Surgical outcomes in patients with endoscopic versus transcranial approach for skull base malignancies: a 10-year institutional experience. British journal of neurosurgery 36: 79-85.
- Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH (2013) Endoscopic endonasal compared with anterior craniofacial and combined cranionasal resection of esthesioneuroblastomas. World neurosurgery 80: 148-159.
- Snyderman CH, Wang EW, Zenonos GA, Gardner PA (2020) Reconstruction after endoscopic surgery for skull base malignancies. Journal of neuro-oncology 150: 463-468.
- Lee TJ, Chang PH, Huang CC, Chuang CC (2008) Endoscopic treatment of traumatic basal encephaloceles: a report of 8 cases. Journal of neurosurgery 108: 729-735.
- Hannan CJ, Kelleher E, Javadpour M (2020) Methods of Skull Base Repair Following Endoscopic Endonasal Tumor Resection: A Review. Frontiers in oncology 10: 1614.
- Abiri A, Abiri P, Goshtasbi K, Lehrich BM, Sahyouni R, et al. (2020) Endoscopic Anterior Skull Base Reconstruction: A Meta-Analysis and Systematic Review of Graft Type. World neurosurgery 139: 460-470.
- Jin B, Wang XS, Huo G, Mou JM, Yang G (2020) Reconstruction of skull base bone defects using an in situ bone flap after endoscopic endonasal transplanum-transtuberculum approaches. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery 277: 2071-2080.
- Fiani B, Quadri SA, Cathel A, Farooqui M, Ramachandran A, et al. (2019) Esthesioneuroblastoma: A Comprehensive Review of Diagnosis, Management, and Current Treatment Options. World neurosurgery 126: 194-211.
- Safi C, Spielman D, Otten M, Bruce JN, Feldstein N, et al. (2020) Treatment Strategies and Outcomes of PediatricEsthesioneuroblastoma: A Systematic Review. Frontiers in oncology 10: 1247.
- Song X, Wang J, Wang S, Yan L, Li Y (2020) Prognostic factors and outcomes of multimodality treatment in olfactory neuroblastoma. Oral oncology 103: 104618.
- Gallia GL, Asemota AO, Blitz AM, Lane AP, Koch W, et al. (2018) Endonasal endoscopic resection of olfactory neuroblastoma: an 11year experience. Journal of neurosurgery 131: 238-244.
- Cai X, Peng Z, Zhang H, Fan R, Fang Y, et al. (2021) Olfactory Neuroblastoma: Surgical Treatment Experience of 42 Cases. Frontiers in surgery 8: 799405.
- Nakamura N, Zenda S, Tahara M, Okano S, Hayashi R, et al. (2017) Proton beam therapy for olfactory neuroblastoma. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 122: 368-372.
- Dulguerov P, Allal AS, Calcaterra TC (2001) Esthesioneuroblastoma: a meta-analysis and review. The Lancet Oncology 2: 683-690.
- Nicolai P, Battaglia P, Bignami M, Bolzoni Villaret A, Delù G, Khrais T, et al. (2008) Endoscopic surgery for malignant tumors of the sinonasal tract and adjacent skull base: a 10-year experience. American journal of rhinology 22: 308-316.
- Fu TS, Monteiro E, Muhanna N, Goldstein DP, de Almeida JR (2016) Comparison of outcomes for open versus endoscopic approaches for olfactory neuroblastoma: A systematic review and individual participant data meta-analysis. Head & neck 38 Suppl 1: E2306-2316.
- Hanna E, DeMonte F, Ibrahim S, Roberts D, Levine N, et al. (2009) Endoscopic resection of sinonasal cancers with and without craniotomy: oncologic results. Archives of otolaryngology--head & neck surgery 135: 1219-1224.
- Song CM, Won TB, Lee CH, Kim DY, Rhee CS (2012) Treatment modalities and outcomes of olfactory neuroblastoma. TheLaryngoscope 122: 2389-2395.
- Abdelmeguid AS, Raza SM, Su SY, Kupferman M, Roberts D, et al. (2020) Endoscopic resection of sinonasal malignancies. Head & neck 42: 645-652.
- Carsuzaa F, Verillaud B, Marcy PY, Herman P, Dufour X, et al. (2022) Interdisciplinary challenges and aims of flap or graft reconstruction surgery of sinonasal cancers: What radiologists and radiation oncologists need to know. Frontiers in oncology 12: 1013801.
- Mattavelli D, Schreiber A, Villaret AB, Accorona R, Turri-Zanoni M, et al. (2018) Complications and donor site morbidity of 3-layer reconstruction with iliotibial tract of the anterior skull base: Retrospective analysis of 186 patients. Head & neck 40: 63-69.
- Sun Y, Huang Q, Cui S, Wang M, Zhang N, et al. (2021) Outcomes and Quality-of-Life Measures after Endoscopic Endonasal Resection of Kadish Stage C Olfactory Neuroblastomas. World neurosurgery 151: e58-e67.
- Dolci RLL, Todeschini AB, Santos A, Lazarini PR (2019) Endoscopic endonasal double flap technique for reconstruction of large anterior skull base defects: technical note. Brazilian journal of otorhinolaryngology 85: 427-434.
- Gallia GL, Reh DD, Salmasi V, Blitz AM, Koch W,et al. (2011) Endonasal endoscopic resection of esthesioneuroblastoma: the Johns Hopkins Hospital experience and review of the literature. Neurosurgical review 34: 465-475.
- Conger A, Zhao F, Wang X, Eisenberg A, Griffiths C, et al. (2018) Evolution of the graded repair of CSF leaks and skull base defects in endonasal endoscopic tumor surgery: trends in repair failure and meningitis rates in 509 patients. Journal of neurosurgery 130: 861875.
- Kim CS, Patel U, Pastena G, Higgins M, Peris-Celda M, et al. (2019) The Magnetic Resonance Imaging Appearance of Endoscopic Endonasal Skull Base Defect Reconstruction Using Free Mucosal Graft. World neurosurgery 126: e165-e172.
- Eloy JA, Patel SK, Shukla PA, Smith ML, Choudhry OJ, et al. (2013) Triple-layer reconstruction technique for large cribriform defects after endoscopic endonasal resection of anterior skull base tumors. International forum of allergy & rhinology 3: 204-211.
- Qi Q, Zhang Y, Wang J, Zhong H, Chen H, et al. (2021) Deployment of a bioabsorbable plate as the rigid buttress for skull base repair after endoscopic pituitary surgery. Gland surgery 10: 1010-1017.
- Youngerman BE, Kosty JA, Gerges MM, Tabaee A, Kacker A, et al. (2020) Acellular dermal matrix as an alternative to autologous fascia lata for skull base repair following extended endoscopic endonasal approaches. Acta neurochirurgica 162: 863-873.
- Ramakrishnan VR, Terella AM, Poonia S, Chiu AG, Palmer JN (2017) Osseous Repair in Minimally Invasive Reconstruction of Anterior Skull Base Defects. The Journal of craniofacial surgery 28: 36-39.
- Fiacchini G, De Santi S, Trico D, Cambi C, Seccia V, et al. (2020) Comparison of a purely endoscopic three-layer technique versus pericranial flap for reconstruction of anterior skull base defects after sino-nasal tumor resection: assessment of postoperative frontal lobe sagging and frontal lobe falling. Rhinology 58: 482-488.
- Gabriel PJ, Kohli G, Hsueh WD, Eloy JA, Liu JK (2020) Efficacy of simultaneous pericranial and nasoseptal “double flap” reconstruction of anterior skull base defects after combined transbasal and endoscopic endonasal approaches. Acta neurochirurgica 162: 641-647.
- Luginbuhl AJ, Campbell PG, Evans J, Rosen M (2010) Endoscopic repair of high-flow cranial base defects using a bilayer button. The Laryngoscope 120: 876-880.
- Garcia-Navarro V, Anand VK, Schwartz TH (2013) Gasket seal closure for extended endonasal endoscopic skull base surgery: efficacy in a large case series. World neurosurgery 80: 563-568.
- Kim N, Lee CG, Kim EH, Kim CH, Keum KC, et al. (2019) Patterns of failures after surgical resection in olfactory neuroblastoma. Journal of neuro-oncology 141: 459-466.
- Eloy JA, Shukla PA, Choudhry OJ, Singh R, Liu JK (2012) Assessment of frontal lobe sagging after endoscopic endonasal transcribriform resection of anterior skull base tumors: is rigid structural reconstruction of the cranial base defect necessary? The Laryngoscope 122: 26522657.
- Saroul N, Rumeau C, Verillaud B, Patron V, Righini C, et al. (2021) Failure of anterior skull base reconstruction for sinonasal carcinoma: consequence on the postoperative follow up. A multicentre evaluation of management. Acta oto-laryngologica 141: 630-634.
- Al-Asousi F, Okpaleke C, Dadgostar A, Javer A (2017) The use of polydioxanone plates for endoscopic skull base repair. American journal of rhinology & allergy 31: 122-126.
- Guo S, Dipietro LA (2010) Factors affecting wound healing. Journal of dental research 89: 219-229.
- Seaman SC, Moline MJ, Graham SM, Greenlee JDW (2021) Endoscopic extrasellar skull base reconstruction using bioabsorbable plates. American journal of otolaryngology 42: 102750.
- Hu F, Gu Y, Zhang X, Xie T, Yu Y, et al. (2015) Combined use of a gasket seal closure and a vascularized pedicle nasoseptal flap multilayered reconstruction technique for high-flow cerebrospinal fluid leaks after endonasal endoscopic skull base surgery. World neurosurgery 83: 181-187.
- Greenfield JP, Anand VK, Kacker A, Seibert MJ, Singh A, et al. (2010) Endoscopic endonasal transethmoidal transcribriform transfovea ethmoidalis approach to the anterior cranial fossa and skull base. Neurosurgery 66: 883-892.
- Prickett KK, Wise SK, Delgaudio JM (2011) Choice of graft material and postoperative healing in endoscopic repair of cerebrospinal fluid leak. Archives of otolaryngology--head & neck surgery 137: 457-461.
- El-Banhawy OA, Halaka AN, Altuwaijri MA, Ayad H, El-Sharnoby MM (2008) Long-term outcome of endonasal endoscopic skull base reconstruction with nasal turbinate graft. Skull base : official journal of North American Skull Base Society [et al] 18: 297-308.
- Zeng Q, Tian Y, He Y, Xie Q, Ou L, et al. (2021) Long-Term Survival Outcomes and Treatment Experience of 64 Patients With Esthesioneuroblastoma. Frontiers in oncology 11: 624960.
- Herle P, Shukla L, Morrison WA, Shayan R (2015) Preoperative radiation and free flap outcomes for head and neck reconstruction: a systematic review and meta-analysis. ANZ journal of surgery 85: 121-127.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.