Response to ABCP Regimen in a ROS1Rearranged Lung Adenocarcinoma Patient with Brain Metastases and High PD-L1 Expression: A Case Report
Quan-Quan Tan 1,2# , Yu-Qing Chen 2,3# , Ming-Ying Zheng 2 , Yu-Fa Li 4 , Ke-Jun Liu 1,2 , Jun-Wei Su 2 , Hua-Jun Chen 2 , Jin-Ji Yang 1,2,3*
1 The Second School of Clinical Medicine, Southern Medical University, Guangzhou, China
2 Guangdong Lung Cancer Institute, Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
3 School of Medicine, South China University of Technology, Guangzhou, China
4 Department of pathology, Guangdong Provincial People’s Hospital and Guangdong Academy of Medical Sciences, Guangzhou, China #Contributed equally to this work.
*Corresponding author: Jin-Ji Yang, Guangdong Lung Cancer Institute, Guangdong Provincial People's Hospital and Guangdong Academy of Medical Sciences, Guangzhou, China.
Received Date: 19 November, 2022
Accepted Date: 26 November, 2022
Published Date: 29 November, 2022
Citation: Tan Q, Chen Y, Zheng M, Li Y, Liu K, et al. (2022) Response to ABCP Regimen in a ROS1-Rearranged Lung Adenocarcinoma Patient with Brain Metastases and High PD-L1 Expression: A Case Report. J Oncol Res Ther 7: 10151. DOI: https://doi.org/10.29011/2574-710X.10151
Abstract
Background: C-ROS oncogene 1 (ROS1) rearrangement is rare in non-small-cell lung cancer (NSCLC) while the ROS1 tyrosine kinase inhibitors (TKIs) have shown remarkable anti-tumor activity. However, drug resistance is inevitable. The next generation of targeted therapy or chemotherapy is a standard treatment recommended by the NCCN guidelines. Although the populations of high programmed cell death ligand 1 (PD-L1) expression (PD-L1>50%) were reported approximately 20.3%-60% in ROS1 rearranged NSCLC, there were few clinical trials focus on the immunotherapy among NSCLC patients with ROS1 rearrangement, and the efficacy is still controversial in the posterior line.
Case Description: We report a case of a 42-year-old female with ROS1-rearranged NSCLC and high PD-L1 expression treated with atezolizumab (1200 mg) / bevacizumab (7.5 mg/kg) / carboplatin (AUC 5) / pemetrexed (500 mg/m2) (ABCP) regimen after failure of targeted therapy. After 2 cycles, she achieved partial response (PR) and confirmed PR after 4 cycles of ABCP regimen not only in the primary lung lesion, but also in multiple metastatic lesions of the brain. Then the maintenance treatment of atezolizumab (1200 mg) / bevacizumab (7.5 mg/kg) / pemetrexed (500 mg/m 2) (ABP) was continued to have benefit. Moreover, the levels of serum tumor markers all showed a downward trend during treatment. During the treatment, the major adverse events (AEs) observed were nausea, dazzling and headache that were tolerable.
Conclusion: This case reminds us of new possibilities for further line therapy among NSCLC patients with ROS1-rearranged. The treatment of immune-chemotherapy plus antiangiogenic therapy may be a potential treatment option for advanced ROS1rearranged NSCLC especially with high PD-L1 expression after ROS1-TKI failure.
Keywords: ROS1 rearrangement; Lung adenocarcinoma; Immunotherapy
Introduction
The overall prevalence of ROS1 rearrangement is approximately 1-2% in NSCLC [1]. Crizotinib, demonstrated by PROFILE 1001 trial, has shown an objective response rate (ORR) of 72% (95% CI, 58–83), a median progression-free survival (mPFS) of 19.3 months (95% CI, 15.2–39.1) and a median overall survival (OS) of 51.4 months (95% CI, 29.3–not reached) among patients with ROS1 rearrangement [2]. Due to the remarkable antitumor activity of crizotinib, it has been recommended as standard firstline therapy in NSCLC patients with ROS1 rearrangement. The next generation targeted agents or systemic therapies such as chemotherapy were recommended by the national comprehensive cancer network (NCCN) guidelines for NSCLC patients with ROS1 rearrangement who have failed first-line targeted therapy [3]. PD-L1 is a predictor of immunotherapy, and whether to add immunotherapy is based on its expression. The expression status of PD-L1 in ROS1-rearranged NSCLC patients have been reported in several previous studies as approximately 20.3%-60% patients were with high PD-L1 expression (PD-L1>50%). Though the proportion of PD-L1 high expression was more than that in patients with EGFR mutation or ALK fusion NSCLC [4-8], there were few studies on ROS1 rearranged NSCLC patients received immunotherapy, and thus the efficacy is still controversial. However, in our case, we challenged the combination regimen of chemo-immunotherapy and antiangiogenic therapy on a ROS1rearranged NSCLC patient after resistance to first-line targeted therapy.
Case report
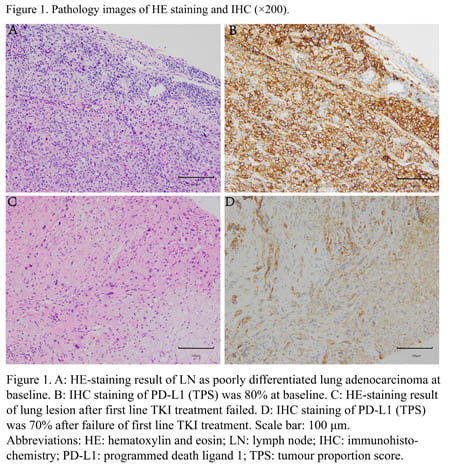
The patient, a 42-year-old female who was a never smoker, presented with neck pain. The chest computed tomography (CT) revealed a 68x52x77 mm lesion in her right upper lung with metastases to multiple mediastinal lymph nodes (LNs), the first and the second cervical vertebral body. Positive electron emission tomography (PET) - CT showed a strong fluorodeoxyglucose (FDG) accumulation in the primary lung lesion (SUVmax 22.3). Fortunately, the brain magnetic resonance imaging (MRI) was normal at baseline. The right supraclavicular LN biopsy showed poorly differentiated lung adenocarcinoma(Figure 1A) with immunohistochemistry (IHC) staining results as TTF-1 (+), CK (+++), CK7 (-), Napsin A (-), CK5/6 (-) and P40 (-) with the tumor proportion score (TPS) of PD-L1 (22C3, TPS) was 80% (Figure 1B). The next-generation sequencing (NGS) of the LN biopsy specimen and blood samples obtained at baseline revealed CD74-ROS1 rearrangement, TP53 mutation and TERT mutation. She was enrolled in a clinical trial of second-generation ALK and ROS1 tyrosine kinase inhibitor (TKI) SAF-189s. She achieved a partial response (PR) according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) after 1 month and confirmed PR at 3 months after the treatment of TKI and radiotherapy of the cervical vertebrae with 42Gy/14F.
However, after 10 months of TKI therapy, she underwent progressive disease (PD) due to the enlarged primary lung lesion and multiple brain metastases. Then, the lumbar puncture was performed, without any tumor cells in the cerebrospinal fluid (CSF). However,PD-L1 of lung tissue in second biopsy reduced from 80% to 70%(Figure 1D). And the lung biopsy showed poorly differentiated carcinoma (Figure 1C). NGS of blood and CSF still showed CD74-ROS1 rearrangement, TP53 mutation and TERT mutation. Besides, a FGFR3 amplification was found in CSF but not in blood (Table 1).
|
Date |
Sample type |
Gene |
Variant type |
cDNA |
Amino Acid |
Variable frequency |
|
Oct/2020 |
Tissue Tissue Tissue |
ROS1 |
Fusion |
CD74- ROS1 |
CD74: exon7~ ROS1: exon34 |
25.70% |
|
Oct/2020 |
TERT |
Mutation |
c.-146C>T |
|
11.89% |
|
|
Oct/2020 |
TP53 |
Mutation |
c.853G>A |
P. E285K |
20.07% |
|
|
Oct/2020 |
Plasma Plasma Plasma |
ROS1 |
Fusion |
CD74- ROS1 |
CD74: exon7~ ROS1: exon34 |
1.53% |
|
Oct/2020 |
TERT |
Mutation |
c.-146C>T |
|
0.40% |
|
|
Oct/2020 |
TP53 |
Mutation |
c.853G>A |
P. E285K |
1.79% |
|
|
Aug/2021 |
Plasma Plasma Plasma |
ROS1 |
Fusion |
CD74- ROS1 |
CD74: exon7~ ROS1exon34 |
0.99% |
|
Aug/2021 |
TERT |
Mutation |
c.-146C>T |
|
0.45% |
|
|
Aug/2021 |
TP53 |
Mutation |
c.853G>A |
P. E285K |
1.24% |
|
|
Aug/2021 |
CSF CSF CSF CSF |
ROS1 |
Fusion |
CD74- ROS1 |
CD74: exon7~ ROS1exon34 |
37.18% |
|
Aug/2021 |
TERT |
Mutation |
c.-146C>T |
|
16.28% |
|
|
Aug/2021 |
TP53 |
Mutation |
c.853G>A |
P. E285K |
29.81% |
|
|
Aug/2021 |
FGFR3 |
Amplification |
|
|
CN:5.5 |
|
|
The NGS results of
tissue and plasma when diagnosed lung adenocarcinoma showed ROS1 (+), TERT
(+), TP53 (+). After first-line TKI treatment failed, the NGS results of
plasma showed ROS1 (+), TERT (+), TP53 (+) and that of CSF showed ROS1 (+),
TERT (+), TP53 (+), FGFR3 (+). Abbreviations: NGS: next-generation
sequencing; ROS1: c-ros oncogene 1; TERT: telomerase reverse
transcriptase; TKI: tyrosine kinase inhibitor; CSF: cerebrospinal fluid;
FGFR3: fibroblast growth factor receptor 3. |
||||||
Table 1: The NGS results when diagnosed and first-line treatment failed.
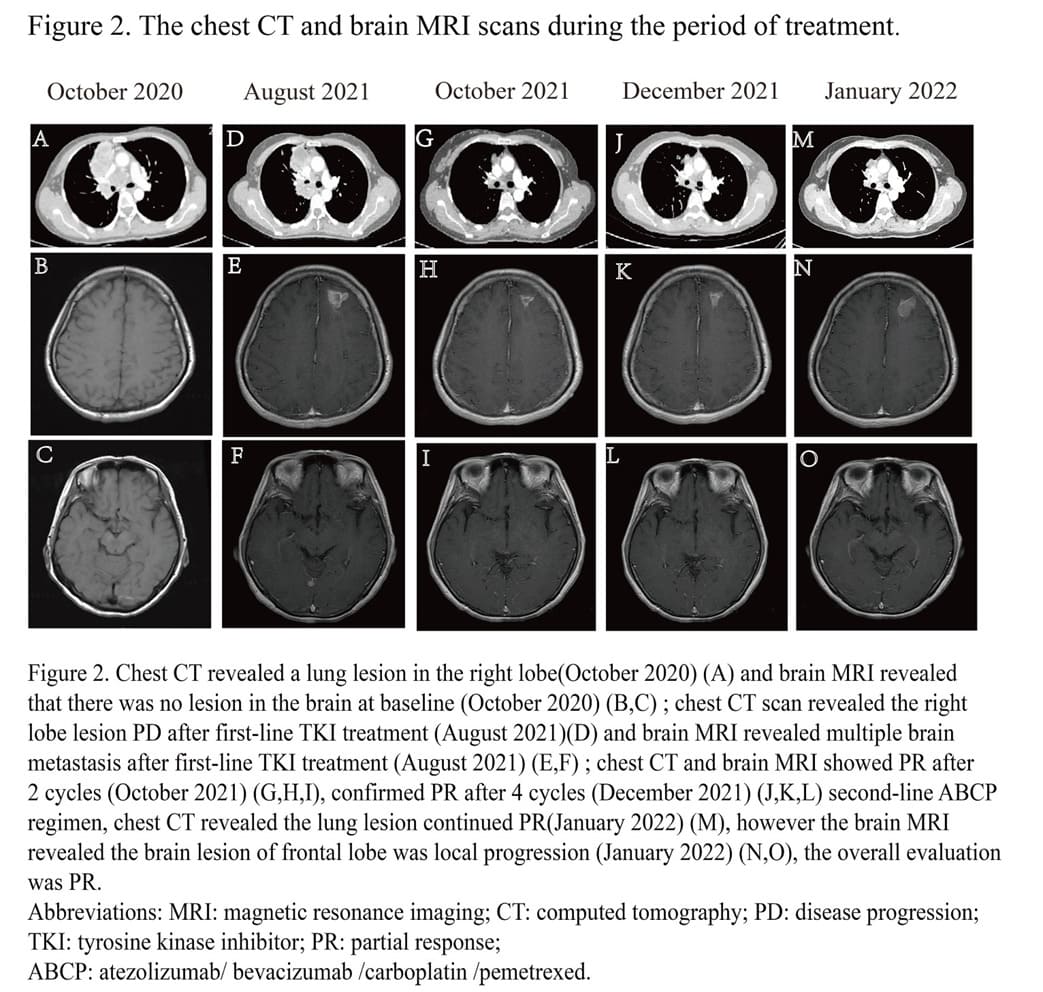
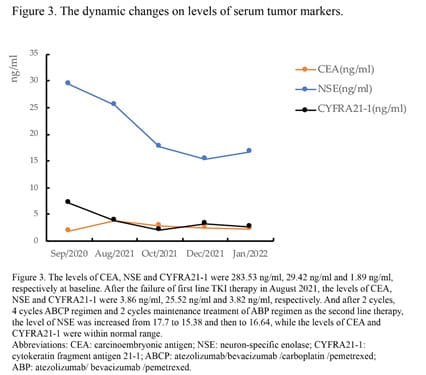
Due to the high PD-L1 expression, we challenged a combined treatment of immunochemotherapy and antiangiogenic therapy consisting of atezolizumab (1200 mg) / bevacizumab (7.5 mg/kg) / carboplatin (AUC 5) / pemetrexed (500 mg/m2) (ABCP). And after two cycles’ treatment, not only the primary lung lesion, but also the multiple brain metastases lesions were evaluated as PR (Figure 2). However, the patient had some central nervous system symptoms, including nausea, dazzling and headache after 1 cycle treatment. And after 4 cycles, the lung primary lesion and brain lesions were assessed as confirmed PR with chest CT and brain MRI. The lung lesion was evaluated PR while the brain metastases lesion in frontal lobe a little bit enlarged by brain MRI after 2 more cycles maintenance treatment of atezolizumab (1200 mg) / bevacizumab (7.5 mg/kg) / pemetrexed (500 mg/m2) (ABP), the overall evaluation was PR. The level of carcinoembryonic antigen (CEA), neuron-specific enolase (NSE) and cytokeratin fragment antigen 21-1 (CYFRA21-1) all showed a downward trend in the course of treatment (Figure 3). Until now, the patient had received 4 cycles ABCP regimen and 2 cycles ABP regimen treatments and will receive whole brain radiotherapy before next cycle of treatment.
Discussion
This case of ROS1-rearranged NSCLC with high PD-L1 expression was treated with ABCP regimen after the failure of TKI therapy and achieved efficacy not only in the primary lung lesion, but also in brain metastases. However, no systematic study has shown response to the chemo-immunotherapy plus antiangiogenic therapy in ROS1 -rearranged NSCLC patients with brain metastases.
The incidence of brain metastases for untreated ROS1rearranged NSCLC patients was reported as 36% according to Patil T et al., which was higher than wild-type NSCLC [9]. Many current studies and clinical trials have demonstrated that some targeted therapies not only have a certain effect on lung lesions but also have better control on brain metastases, and the intracranial efficacy of TKIs therapy has been evaluated. For instance, the intracranial ORR of lorlatinib was 64% (95%CI, 31-89) in TKInaive patients and 50% (95%CI, 29-71) in crizotinib-pretreated patients [10]. The intracranial ORR of brigatinib achieved 50.0% (95%CI, 11.8-88.2) in crizotinib-pretreated patients as well [11]. Thus, the further-line treatment of ROS1-rearranged NSCLC patients with intracranial progression still mainly focuses on targeted therapy, especially the next-generation TKIs. Whereas the patient in our study refused to receive the next-generation ROS1-TKI due to financial problem. Considering the high PDL1 expression, she was likely to be sensitive to immunotherapy. Although the effects of chemotherapy have been demonstrated by several studies [12-13], there were fewer studies to illustrate the efficacy of chemo-immunotherapy plus antiangiogenic therapy on ROS1-rearranged patients.
As for Immune-Checkpoint Inhibitors (ICIs) monotherapy in ROS1 rearrangement patients, the previous studies all showed a poor efficacy, the IMMUNOTARGET showed an ORR of only 16.7% (1 out of 6, median PFS was not estimated) in ROS1rearranged NSCLC patients with PD-L1>20% after the treatment of single-agent ICI [14]. Another multi-institutional retrospective study also had suboptimal results that the ORR was 13% in ROS1 fusion-positive NSCLCs after ICI monotherapy [15]. Thus, the ICI monotherapy maybe not indicated for ROS1-rearranged NSCLC. And the regimen of chemo-immunotherapy plus antiangiogenic therapy, it was not reported in NSCLC with other driver genes except for EGFR mutation. As in the subgroup analyses of IMpower150 study, the atezolizumab/bevacizumab /carboplatin/ paclitaxel regimen had an improvement in PFS compared with the bevacizumab/carboplatin/paclitaxel regimen in NSCLC patients with EGFR mutation [16]. Furthermore, a phase 2 single-arm study also demonstrated good efficacy of modified ABCP regimen in metastatic EGFR-mutated NSCLC after TKI failure [17]. Surprisingly, we found that such a case with ROS1 rearrangement and high PD-L1 expression also showed anti-tumor activity in both lung tumors and brain metastases with ABCP regimen.
In conclusion, the successful treatment of immunechemotherapy plus antiangiogenic therapy in this case provided a potential treatment option for advanced ROS1-rearranged NSCLC with high PD-L1 expression after ROS1-TKI failure. Nevertheless, further studies of immune-chemotherapy plus antiangiogenic therapy are warranted in a larger sample size of ROS1-rearranged NSCLC patients. Additionally, further studies on how the immune microenvironment influenced by immunotherapy in ROS1 positive NSCLC patients are also needed.
Declarations
Consent for publication: Written informed consent was obtained from the patient for using tissue samples.
Ethical approval: Ethical approval was obtained from Guangdong Provincial People's Hospital and Guangdong Academy of Medical Sciences.
Declaration of Competing Interest: All the authors declare that they have no competing interest. This article has not been published previously.
Funding
Funding from National Natural Science Foundation of China [Grant No. 81972164, JJ Yang], Provincial Natural Science Foundation of Guangdong Province [Grant No. 2019A1515010931, JJ Yang] and High-level Hospital Construction Project [Grant No. DFJH201809, JJ Yang].
CRediT authorship contribution statement
Quan-Quan Tan: Data collection, writing the paper. Yu-Qing Chen: Methodology, writing the paper. Ming-Ying Zheng:
Investigation, reviewing the paper. Yu-Fa Li: Data collection. KeJun Liu: Validation. Jun-Wei Su: Validation. Hua-Jun Chen: Study concept, validation. Jin-Ji Yang: Study concept, Supervision.
Acknowledgments: None.
References
- Gainor JF, Shaw AT (2013) Novel targets in non-small cell lung cancer: ROS1 and RET fusions. Oncologist 18: 865-875.
- Shaw AT, Riely GJ, Bang YJ, et al. (2019) Crizotinib in ROS1rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol 30: 1121-1126.
- Ettinger DS, Wood DE, Aisner DL, et al. (2021) NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J Natl Compr Canc Netw 19: 254-266.
- Dudnik E, Bshara E, Grubstein A, et al. (2018) Rare targetable drivers (RTDs) in non-small cell lung cancer (NSCLC): Outcomes with immune check-point inhibitors (ICPi). Lung Cancer 124: 117-124.
- Mazieres J, Drilon A, Lusque A, et al. (2019) Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 30: 1321-1328.
- Negrao MV, Skoulidis F, Montesion M, et al. (2021) Oncogenespecific differences in tumor mutational burden, PD-L1 expression, and outcomes from immunotherapy in non-small cell lung cancer. J Immunother Cancer 9: e002891.
- Karatrasoglou EA, Chatziandreou I, Sakellariou S, et al. (2020) Association between PD-L1 expression and driver gene mutations in non-small cell lung cancer patients: correlation with clinical data. Virchows Arch 477: 207-217.
- Jin Y, Xue Q, Shen X, et al. (2022) PD-L1 Expression and Comprehensive Molecular Profiling Predict Survival in Nonsmall Cell Lung Cancer: A Real-World Study of a Large Chinese Cohort. Clin Lung Cancer 23: 43-51.
- Patil T, Smith DE, Bunn PA, et al. (2018) The Incidence of Brain Metastases in Stage IV ROS1-Rearranged Non-Small Cell Lung Cancer and Rate of Central Nervous System Progression on Crizotinib. J Thorac Oncol 13: 1717-1726.
- Shaw AT, Solomon BJ, Chiari R, et al. (2019) Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: a multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol 20: 1691-1701.
- Daga H, Niho S, Sakakibara-Konishi J, et al. (2021) Phase II study of brigatinib in ROS1 positive non-small cell lung cancer (NSCLC) patients previously treated with crizotinib: Barossa cohort 2. In: Wolters Kluwer Health. J Thorac Oncol 16: S1086-S1086.
- Shen L, Qiang T, Li Z, et al. (2020) First-line crizotinib versus platinum-pemetrexed chemotherapy in patients with advanced ROS1rearranged non-small-cell lung cancer. Cancer Med 9: 3310-3318.
- Xu H, Zhang Q, Liang L, et al. (2020) Crizotinib vs platinum-based chemotherapy as first-line treatment for advanced non-small cell lung cancer with different ROS1 fusion variants. Cancer Med 9: 3328-3336.
- Mazieres J, Drilon A, Lusque A, et al. (2019) Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 30: 1321-1328.
- Choudhury NJ, Schneider JL, Patil T, et al. (2021) Response to Immune Checkpoint Inhibition as Monotherapy or in Combination with Chemotherapy in Metastatic ROS1-Rearranged Lung Cancers. JTO Clin Res Rep 2: 100187.
- Reck M, Mok TSK, Nishio M, et al. (2019) IMpower150 Study Group. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, openlabel phase 3 trial. Lancet Respir Med 7: 387-401.
- Lam TC, Tsang KC, Choi HC, et al. (2021) Combination atezolizumab, bevacizumab, pemetrexed and carboplatin for metastatic EGFR mutated NSCLC after TKI failure. Lung Cancer 159: 18-26.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.