Regenerative Reconstructive Treatment of A Peri- Implantitis Case: New Perspectives for Implant Surface Decontamination
by Giovanni Barbagallo1,3*, Federica di Gregorio1,3, Gianluca Monaca1,3, Gloria Alì1,3, Vanina Porto2,3, Giovanni Assenza1,3
1DDS, DMD, Italy
2BDH, MSAT, Italy
3Centro Odontoiatrico Mediterraneo in Agreement with NHS, Private Dental Practice, Catania, Sicily, Italy
*Corresponding author: Giovanni Barbagallo, Centro Odontoiatrico Mediterraneo in Agreement with NHS, Private Dental Practice, Catania, Sicily, Italy
Received Date: 18 January, 2024
Accepted Date: 22 January, 2024
Published Date: 24 January, 2024
Citation: Barbagallo G, di Gregorio F, Monaca G, Alì G, Porto V, et al. (2024) Regenerative Reconstructive Treatment of A PeriImplantitis Case: New Perspectives for Implant Surface Decontamination. J Surg 9: 1984. https://doi.org/10.29011/2575-9760.001984
Abstract
Introduction: Implantology is a therapeutic approach that has allowed the fixed prosthetic treatment of an increasing number of patients who would otherwise be destined for removable prosthetics. Peri-implant diseases are inflammatory conditions caused by biofilm that affect the tissues around dental implants; they are distinguished into peri-implant mucositis and peri-implantitis. Studies have shown a prevalence of 43-46.8% for mucositis and 19.83-22% for peri-implantitis. The primary goal of peri-implantitis treatment is to halt progressive bone loss by controlling bacterial infection.
Case Presentation: In 2013, a 68-year-old patient underwent rehabilitation with a fixed prosthesis on 5 implants in the lower arch. Despite regular check-ups, the implant in position 4.5 developed peri-implantitis. The patient is a non-smoker and maintains good plaque control. Reconstructive surgery was performed on the implant, with the treatment goal being not only conditioning and decontamination of the implant surface but also bone defect regeneration/reconstruction. Decontamination of implant threads, was carried out using ultrasonic instruments and 24% EDTA. At the 6-month postoperative follow-up, the implant showed no signs of inflammation (bleeding on probing and/or suppuration). Radiographic examination revealed clinically significant bone remineralization around the implant.
Conclusion: For the surgical treatment of peri-implantitis, reconstructive treatment involving mechanical debridement with ultrasonics and chemical decontamination, in addition to resolving peri-implant inflammation, allows for bone reconstruction around the implant. The use of multiple aids, both chemical and mechanical, for decontaminating the implant threads is key to achieving greater predictability in the procedure.
Introduction
The absence of proper oral health in the adult population is a condition that can have significant socio-economic implications. In fact, poor oral hygiene negatively impacts an individual’s quality of life, both in terms of interpersonal relationships and common daily activities such as eating or smiling. Furthermore, clinical studies have linked poor oral hygiene, particularly periodontal disease, to numerous acute and chronic systemic conditions, including pneumonia, cerebrovascular diseases, heart attack, and diabetes [1-2]. The lack of one or more teeth is still a relatively common situation in the global population [3]. We are still far from achieving the goals set by the World Health Organization (WHO), which aimed for less than 5% edentulism at ages 64-74 and 75% of individuals with at least 20 functional teeth by 2001 [4-5].
Osseointegrated implantology has offered new perspectives in the field of prosthetic rehabilitation, showing significant development in the last thirty years. Today, it is considered the preferred choice for replacing a single missing tooth due to decay, periodontal disease, trauma, or agenesis, as well as for restoring entire dental arches. The biological complications affecting osseointegrated implants are of great interest in contemporary dentistry.Two clinical varieties can be distinguished: periimplant mucositis and peri-implantitis. While the presence of an inflammatory lesion is a common characteristic of both conditions, only the latter involves a loss of supporting bone [6]. Implantology has modified the traditional therapeutic approach, making fixed prosthesis treatments possible for an increasing number of patients who would otherwise be destined for removable prosthetics. However, after the significant expansion of the last forty years, driven by the belief that osseointegrated implants could be considered a “definitive” treatment, clinical observations of technical and biological complications have multiplied [6]. Following implant placement, a trans-mucosal passage forms around the implant abutment. Peri-implant mucosa and the gum surrounding adjacent teeth share common characteristics but also have differences [7].It has been demonstrated that healed periimplant mucosa contains a core of connective tissue, primarily composed of collagen fibers and matrix elements (85%), few fibroblasts (3%), and vascular units (5%). The surface facing the implant consists of two distinct parts: a “coronal” part covered by sulcular epithelium and a thin epithelium similar in appearance to the junctional epithelium of the gum, and a more “apical” part where connective tissue seems to be in direct contact with the implant surface (connective tissue attachment zone). Around the implants, the main bundles of collagen fibers are anchored in the crestal bone and extend parallel to the implant surface [8].Renewal of exposed surfaces through the exfoliation process counteracts bacterial accumulation. Teeth are non-exfoliating surfaces and, as such, provide an excellent substrate for the deposition and proliferation of bacterial aggregates. Depending on the type of bacteria and the location of the accumulation, carious lesions, gingivitis, and periodontitis may develop. The ability to adhere to surfaces is characteristic of almost all bacteria and depends on the type of surface, the more or less fluid environment, and the type of bacteria. Plaque is defined as a deposit of numerous species, yet to be fully identified, of bacteria organized in a complex matrix of extracellular bacterial polymers and salivary exudate products [9].
Salivary glycoproteins are rich in amino acids that establish electrostatic bonds with the enamel surface. This film is bacteriafree and is considered a physiological coating of the dental surface. An intricate microbial aggregation develops on the acquired film, characterized by the secretion of an adhesive and protective matrix known as biofilm. Initially, the biofilm consists of bacterial microcolonies, followed by their multiplication and maturation into a complex structure where bacteria organize a system of canaliculi for the flow of water and substances, further maturing the biofilm. The mechanisms of bacterial biofilm formation on implantprosthetic surfaces are similar to those described for natural dentition. As soon as the implant surface is exposed to the oral cavity, it is covered by a salivary film containing various molecules (high molecular weight mucins, IgA, α-amylase, etc.) that provide an anchoring medium for microorganism attachment. Although the key role played by bacterial biofilm in the etiology of peri-implant diseases is clear, the role of individual bacterial species and/or their specific interaction in the onset and development of the disease is still not well understood. The complexity and high number of bacterial species present in peri-implant sites on one hand, and the difficulty in establishing a correct temporal relationship between the colonization of sites by certain pathogenic microorganisms and the formation of lesions on the other, have so far hindered a clearer understanding of the pathogenic role of individual species or their interaction within the biofilm. In any case, bacterial colonization of the peri-implant sulcus plays a key role and consequently represents the primary target of peri-implantitis therapy (Lindhe and Meyle 2008) [10]. Mucositis is characterized by inflammation of the peri-implant support tissues resulting from the accumulation of bacterial biofilm on implant and/or prosthetic surfaces [6].
In predisposed individuals, prolonged plaque accumulation and consequent inflammation of peri-implant mucosal tissues may eventually be followed by involvement in the lesion of supporting peri-implant bone tissue, leading to its coronal-apical resorption, defining the picture of peri-implantitis [6]. In this case as well, some similarities with periodontitis are evident, particularly the formation of a pocket at the peri-implant sulcus level, the presence of inflammation clinically manifested as bleeding on probing, and the loss of peri-implant bone tissue that develops coronally-apically [6]. The presence of non-physiological probing is related to the extent of the pathology: in advanced stages of peri-implantitis, probing may exceed 7-8 millimeters. The radiographic appearance is pathognomonic: peri-implant disease is evident in the form of a “bowl” lesion, an osseous crater surrounding the implant, possibly involving adjacent teeth. However, there are some important differences between the two pathologies, probably due to profound anatomical (absence of a ligamentous tissue between the implant surface and the bone) and histological differences (peri-implant mucosal tissues have less blood supply and exhibit characteristics more similar to scar tissue) [6].
Human histological studies have shown that the inflammatory infiltrate is more extensive in peri-implantitis, especially apically to the junctional epithelium, and that there is often a portion of connective tissue not covered by epithelial cells directly in contact with the bacterial biofilm. Furthermore, despite dominant cellular populations characterized by the presence of lymphocytes and plasma cells in both periodontal and peri-implant lesions, the latter also show a high number of macrophages and polymorphonuclear cells (Berglundh et al., 2011) [11]. Another very important difference is the rate of lesion progression. These differences have a relevant clinical impact and require a different therapeutic approach than periodontitis treatment [6]. Numerous epidemiological studies have presented data on the incidence of peri-implant biological complications. However, published data show high variability, a consequence of the fact that, despite a certain consensus on clinical definitions, diagnostic parameters vary significantly across studies [12,13]. This limitation can now be successfully addressed with the introduction of the new classification and agreement on diagnostic parameters. Various factors have been correlated with an increased incidence of peri-implant mucositis and periimplantitis, many of which are common to periodontal disease [14]. General risk factors that could influence host susceptibility to biofilm-induced peri-implant mucositis have been investigated. Cigarette smoking has been identified, in three studies, as a risk indicator for peri-implant mucositis. There is also evidence that radiation therapy is a possible risk indicator for mucositis. There is some evidence for diabetes mellitus; poorly controlled diabetes (HbA1c levels > 10.1) has been associated with increased bleeding on probing of implants. Although a history of cardiovascular disease has been associated with an increased risk of periimplantitis, there is no evidence of an association with mucositis [15]. Ferreira et al. have also reported an association between periimplant mucositis and systemic diseases. However, the described systemic diseases included “diabetes mellitus, hormonal changes, menopause, chemotherapy, thyroid alterations, heart problems, and alcohol consumption,” making the interpretation of the study results challenging [15]. Finally, among the main local risk factors are: oral hygiene; adherence/lack of adherence to implant-support therapy (IST); implant material and surface characteristics; design of implant-supported prostheses; size of peri-implant keratinized mucosa and excess cement [15].
Case Presentation
The case presented in this study focuses on a patient with peri-implantitis affecting an implant placed a decade earlier. The patient, B.E., 68 years old and a non-smoker, has a medical history of bilateral otosclerosis surgery and subsequent thyroidectomy, currently taking Eutirox. In 2013, the patient’s edentulous mandible was rehabilitated with a fixed prosthesis (Toronto Bridge) supported by 5 implants (Figure 1).
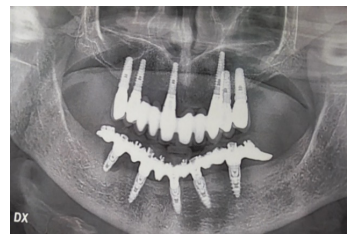
Figure 1
Despite regular check-ups over the years, peri-implantitis developed in the most distal implant in the right hemi-arch, despite the patient’s good oral hygiene. In January 2023, it was decided to intervene. The initial state of the affected implant pre-surgical treatment is visible in the intraoral image (Figure 2).

Figure 2
The surgical intervention proceeded as follows: Local anesthesia with articaine was administered around the implant affected by peri-implantitis.
Removal of the prosthetic structure began by eliminating cement covering the prostethic abutment (Figures 3-5), followed by unscrewing the abutment (Figure 6) to facilitate the removal of the prosthesis.
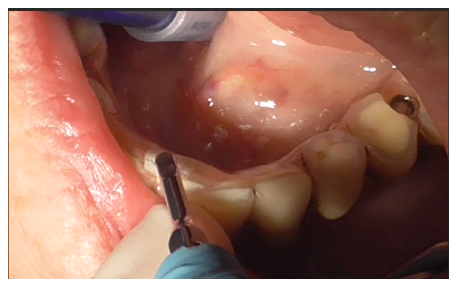
Figure 3
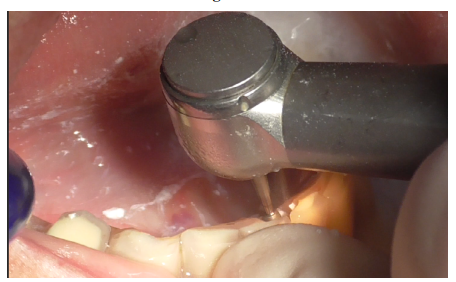
Figure 4
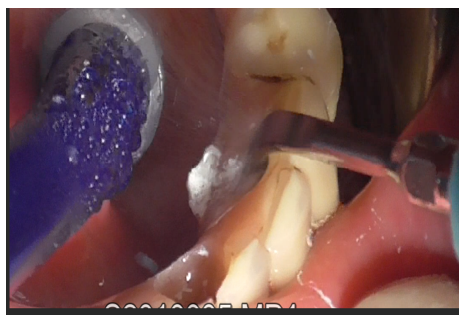
Figure 5
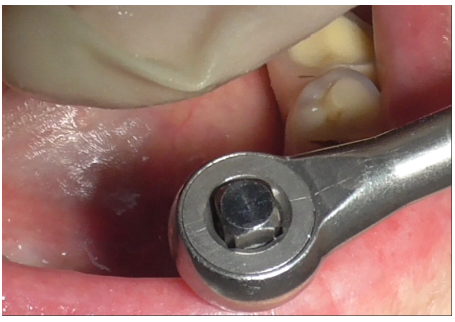
Figure 6
The lower arch without the prosthetic structure is revealed (Figure 7).
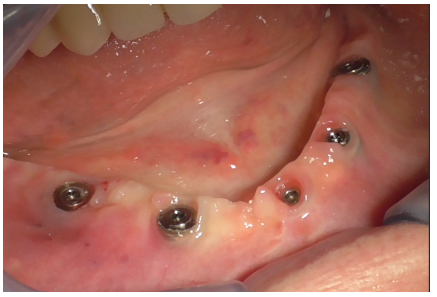
Figure 7
The peri-implantitis-affected implant was probed, revealing profuse bleeding (Figures 8-11).
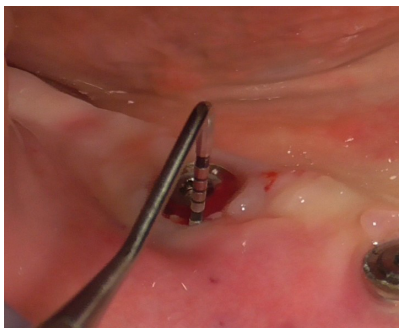
Figure 8
Figure 9
Figure 10
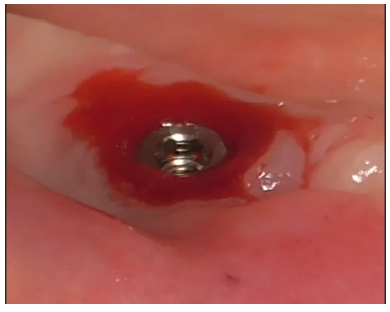
Figure 11
A full-thickness incision with a 15C blade was made both distally (Figure 12) and mesially to the implant (Figure 13), with more extension in the distal area to avoid a vertical incision.
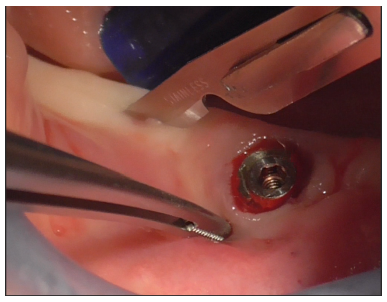
Figure 12
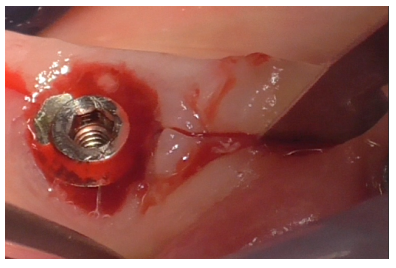
Figure 13
Full-thickness detachment was performed mesially and distally to the implant with a periosteal elevator (Figure 14), followed by careful detachment at the implant site (Figure 15), considering the tissue adhesions. The same detachment was performed lingually (Figure 16).
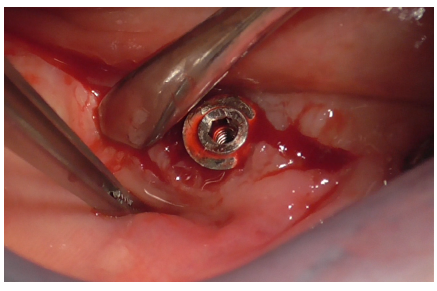
Figure 14
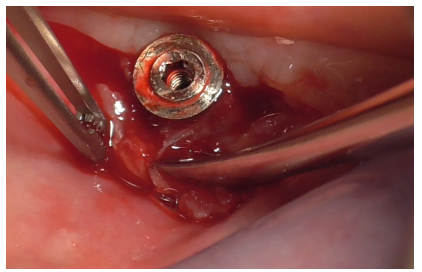
Figure 15
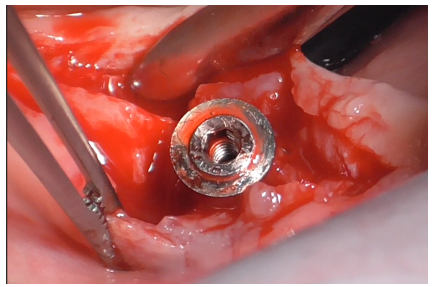
Figure 16
Implant decontamination commenced with an ultrasonic tip (Figure 17) and continued with the diamond ball insert (ES015T) of the Ultrasonic Device (Figure 18).
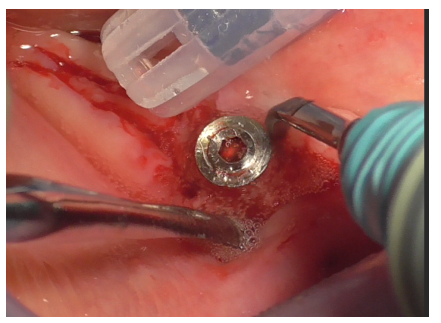
Figure 17
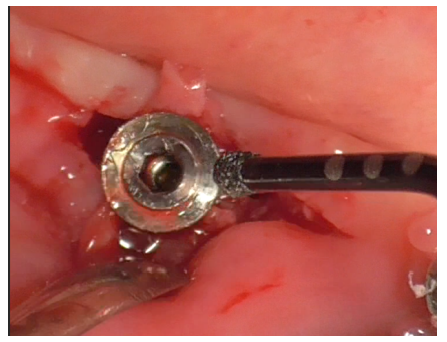
Figure 18
Granulation tissue surrounding the peri-implant soft tissue was excised with a scalpel (Figure 19), exposing the characteristic bowl-shaped defect of peri-implantitis (Figure 20).
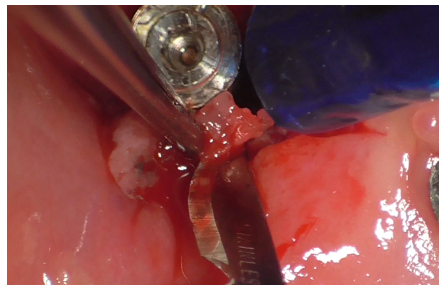
Figure 19
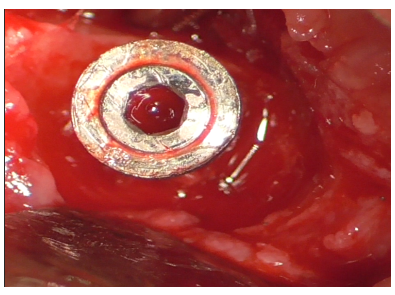
Figure 20
A healing abutment was then screwed into the implant to preserve the connection during subsequent stages (Figure 21).
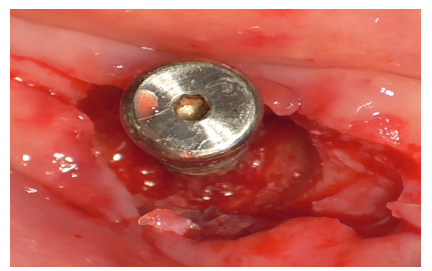
Figure 21
Additional decontamination was performed with the previously used diamond ball insert for the Ultrasonic Device (Figure 22).
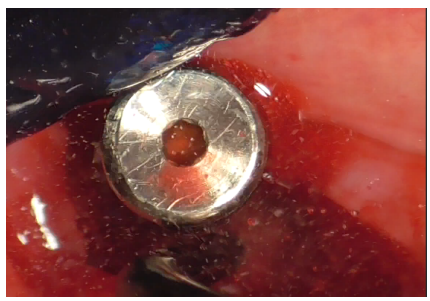
Figure 22
The Ultrasonic Device was equipped with the ES004E insert (patented by Esacrom, Dr. Tarquini), creating an ultrasonic bath (Figure 23).
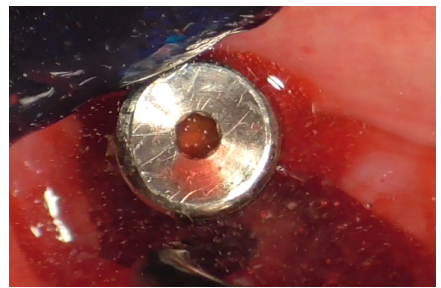
Figure 23
Initially, this insert was used with physiological saline flow (Figure 24) for approximately 1 minute. Subsequently, Ambramicin was applied to the site (Figure 25), capsules were specially opened to use the powdered antibiotic inside, activated for 1 minute using the same bell-shaped insert with physiological saline flow (Figure 26).
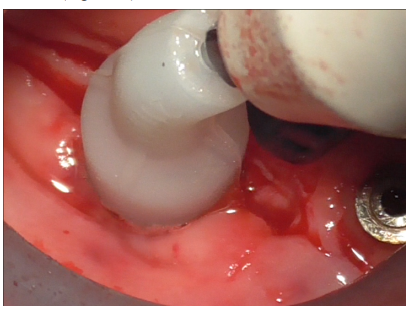
Figure 24
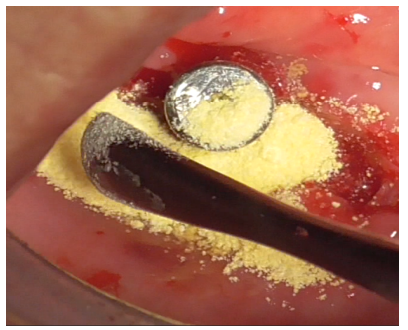
Figure 25
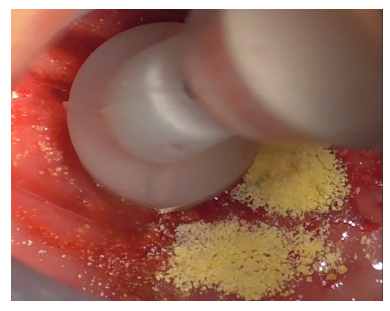
Figure 26
After thorough rinsing, EDTA gel was applied to the site (Figure 27) and activated for 1 minute using the bell-shaped insert (Figure 28).
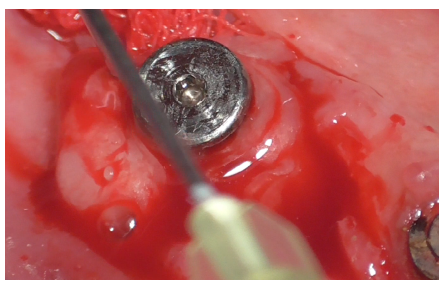
Figure 27
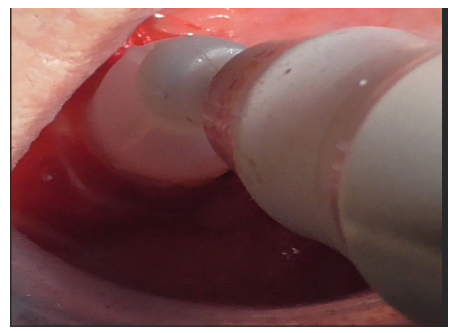
Figure 28
The decontaminated implant with exposed threads is revealed (Figure 29).
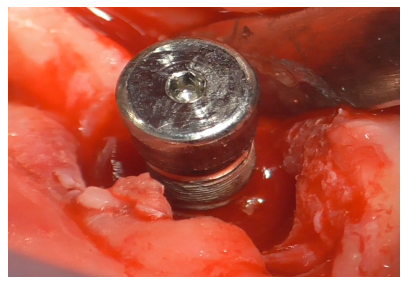
Figure 29
The flap was detached distally to the implant both vestibularly and lingually (Figure 30) to expose the bone crest needed for autologous bone grafting, performed with a specific bone file (Figure 31).
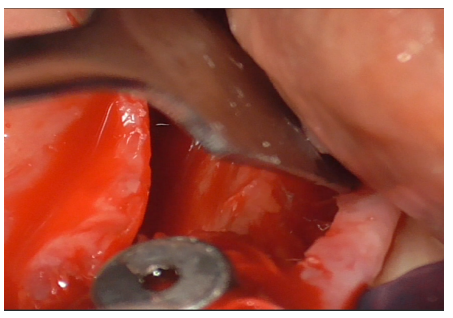
Figure 30
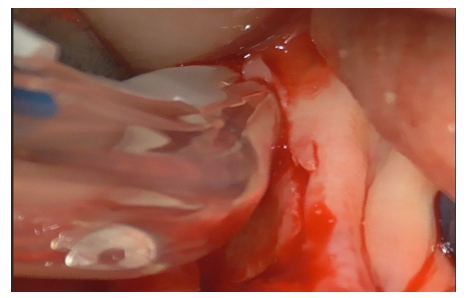
Figure 31
Both allograft bone (DBBM, Deproteinized Bovine Bone Matrix) (Figure 32) and freshly harvested autologous bone (Figure 33) were prepared.

Figure 32
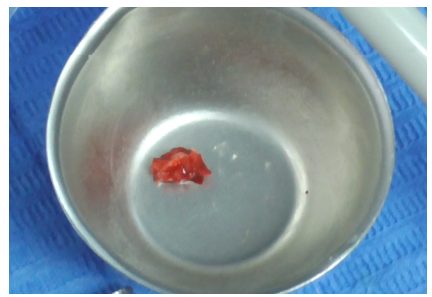
Figure 33
Fibrin sponges were applied to the defect bottom for observational purposes, intending to monitor bone regeneration over time. Autologous bone (Figure 34) and xenogenic bone mixed with patient blood (Figure 35), hyaluronic acid enriched with polynucleotides (Figure 36), were applied as first layer; the remaining DBBM, mixed with hyaluronic acid were successively applied as second layer.
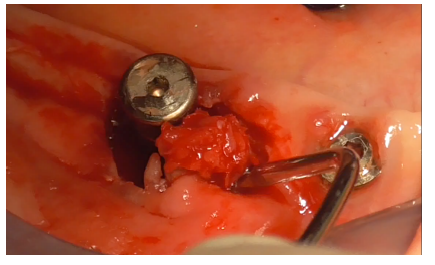
Figure 34
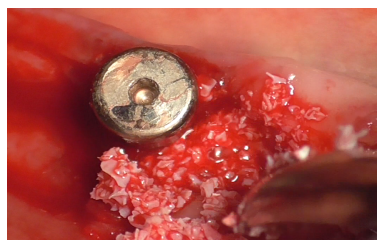
Figure 35
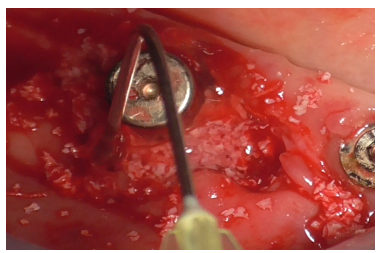
Figure 36
Suturing followed, using interrupted PTFE 4/0 sutures mesially to the implant (Figures 37-38).
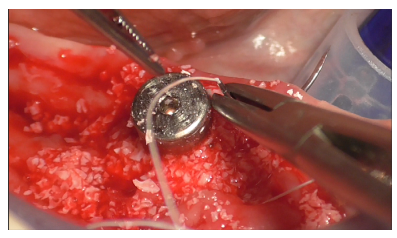
Figure 37
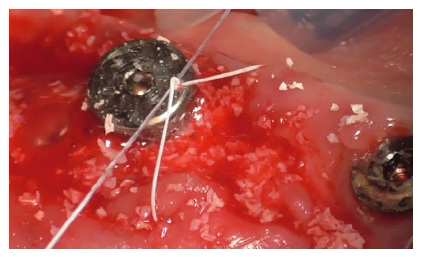
Figure 38
The same suture was given distally (Figure 39), followed by reinforcement sutures mesially and distally (Figure 40). Finally, simple interrupted silk 3/0 sutures were given distally in the autologous bone harvesting site. All sutures removed after 15 days.
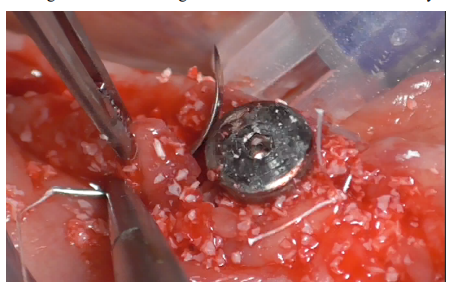
Figure 39
Figure 40
The immediate post-intervention site condition is illustrated (Figures 41-42).
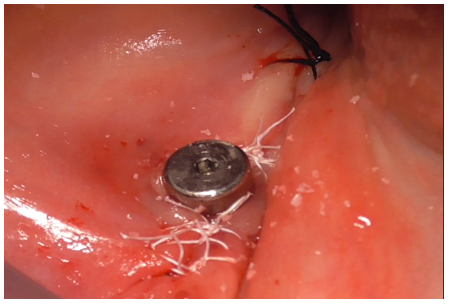
Figure 41

Figure 42
At 15 days post-surgery after suture removal (Figures 43) and at six months during the follow-up endoral X-ray (Figure 44).
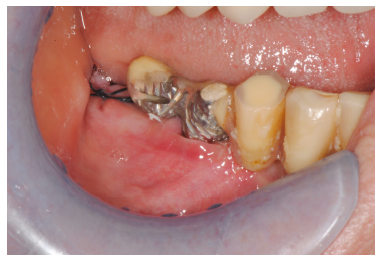
Figure 43
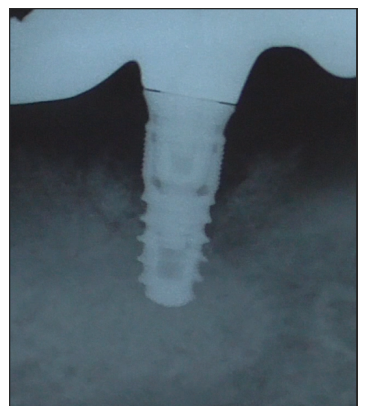
Figure 44
The condition of the soft tissues one year after surgery (Figure 45).
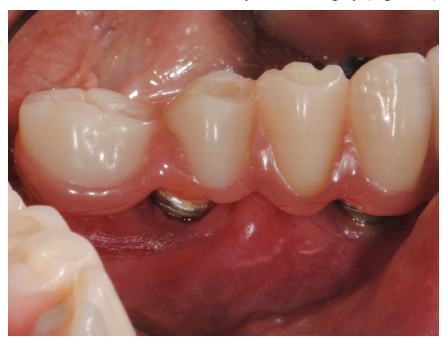
Figure 45
Radiographically, we can observe an increase in bone level around the implant (Figure 46).

Figure 46
Furthermore, a probing around the implant is performed, showing a reduction in pocket depth and bleeding (Figures 47,48).
Figure 47
Figure 48
Discussion
Peri-implant diseases are inflammatory conditions caused by biofilm affecting the tissues around dental implants; they are distinguished as peri-implant mucositis and peri-implantitis [16]. Peri-implant mucositis is an inflammatory lesion of the periimplant mucosa in the absence of continuous marginal bone loss [15]. Clinically, it is characterized by bleeding upon probing. In addition to bleeding, other signs of inflammation such as redness, swelling, and/or suppuration may be present [17].Peri-implantitis is a “pathological condition associated with peri-implant biofilm occurring in the tissues around dental implants. It is characterized by inflammation of the peri-implant mucosa and progressive loss of supporting bone” [17]. Clinically, it presents with bleeding upon probing and/or suppuration, increased probing depth, and/ or recession of the mucosal margin. Radiographically, there is radiographic bone loss compared to previous radiographs [17].
Various epidemiological studies have been conducted to establish the prevalence of peri-implant diseases. In a systematic review and meta-analysis of 11 studies, a prevalence estimate of 43% for peri-implant mucositis and 22% for peri-implantitis was demonstrated. Another systematic review, comprising 47 studies, reported a prevalence of 46.83% for peri-implant mucositis and 19.83% for peri-implantitis [16]. Mucositis is a condition that, if treated, leads to the complete restoration of peri-implant health; its neglect, however, can lead to the onset of peri-implantitis. The progression of the latter can result in the loss of the affected implant and consequently, the prosthesis supported by the implant itself [16].The goal of peri-implantitis treatment is to halt progressive bone loss by controlling the bacterial infection responsible for tissue destruction. To achieve this, it is necessary to reduce pathogenic bacterial flora in the peri-implant pocket, condition, and decontaminate the implant surface. In cases where sites cannot be adequately maintained through home and professional oral hygiene procedures, corrective and/or site reduction procedures are necessary. Additionally, bone regeneration of osseous defects and inclusion of the patient in a support program are advisable [18].
Current peri-implantitis treatment is based on approaches developed for periodontitis treatment. Indeed, the management of peri-implantitis is still a new field of research and clinical practice. The treatment phases consist of non-surgical and surgical therapies. The primary goal of non-surgical therapy is to control peri-implant biofilm and inflammation through instrumentation both supra- and subgingivally. This treatment currently does not show significant results [16]. The decision to perform a non-surgical initial phase is based on attempting to control biofilm and inflammation with initially less invasive approaches before moving, if necessary, to surgical treatment when the patient has better plaque control and risk factor management [16].The aim of surgical therapy is to gain access to the implant to allow decontamination of surfaces and the creation of a positive anatomy of hard and soft peri-implant tissues. Where possible, osseous defect regeneration is advisable [14]. Surgical approaches described for peri-implantitis treatment include open-flap debridement (OFD), resective approach (apically positioned flap, APF), and regenerative surgery.OFD is a surgical technique that, through flap elevation, allows decontamination of implant surfaces with mechanical decontamination instruments [19]. It has been shown that OFD is more effective than nonsurgical treatment and can promote new osseointegration, especially on rough implant surfaces [20].
In the case of peri-implant defects in non-aesthetic sites, a resective approach including osteoplasty and/or ostectomy, decontamination, and possible polishing of the exposed portion of the implant surface and apical repositioning of the flap can be used. The FDI World Dental Federation consensus meeting on periimplantitis stated that resective surgical therapy has limited longterm value and should only be performed in shallow infraosseous defects where regenerative procedures are not recommended [21]. Reconstructive procedures aim to regenerate osseous defects, achieve re-osseointegration, and limit soft peri-implant tissue resorption [16]. The technique involves flap elevation to access the defect, decontamination of the implant surface, and the use of materials to fill the defect [22]. Reconstructive therapy for periimplant osseous defects includes the use of autologous bone grafts, allogeneic bone substitutes, protective membranes, bioactive agents (growth factors, autologous platelet concentrates, and amelogenin), or combinations of these [16].
In our case report, a regenerative/reconsructive surgical procedure was chosen with the goal of not only conditioning and decontaminating the implant surface but also resolve the osseous defect [16]. In accordance with the literature, a flap was raised to access the defect and perform debridement [22]. Decontamination of implant threads was performed using an ultrasonic instrument to eliminate biofilm adhering to surfaces and reduce bacterial colonization [23]. To enhance site debridement and minimize colonization by pathogenic microbial agents, the ES004E insert patented by Dr. Tarquini for Esacrom was chosen. Thanks to its shape, a strong cavitation effect is achieved, improving site disinfection. The goal of peri-implantitis therapy is to control the bacterial infection causing tissue destruction [18]. For this reason, additional disinfection procedures were performed. In accordance with the literature, 24% EDTA was chosen as an adjunct to mechanical biofilm decontamination [24]. Given the importance of maintaining a pH ≥ 3 to prevent morphological changes and corrosion of the implant surface that can hinder re-osseointegration, the exposure duration to this chemical agent was one minute [25]. To further reduce microbial load, ambramicin powder was also used. Clinical studies have indeed demonstrated the effectiveness of tetracyclines as adjuncts in regenerative surgery procedures [26]. Autologous bone, harvested from an adjacent area to the implant site, allogenic bone, hyaluronic acid, and polynucleotides were used as bone fillers [16]. Before proceeding with osseous defect filling, fibrin sponges were placed at the site’s bottom, allowing radiographic assessment of effective bone regeneration, leveraging the radiotransparency of this material. Results in the 6-month post-operative follow-up showed the absence of clinical signs of inflammation (bleeding and suppuration) detectable through objective examination and use of a periodontal probe. Radiographically, significant bone remineralization around the implant was visible clinically. Results at 1-year post-operative follow-up showed a clinically significant reduction in probing depth with an absence of bleeding. Additionally, radiographically, there is a clinically significant increase in bone around the implant threads.
Conclusion
In conclusion, the research conducted on the therapy of periimplantitis has highlighted significant progress in understanding and addressing this clinical complication. Current therapeutic approaches, including both surgical and non-surgical interventions, show considerable potential in improving peri-implant health. Looking ahead, it is crucial to promote awareness among dental professionals about the importance of peri-implantitis prevention and emphasize the significance of long-term follow-up protocols to monitor implant stability. Therefore, peri-implantitis therapy represents a dynamic and evolving field with multiple opportunities for enhancing clinical management. Continuous commitment to research and education will contribute to optimizing clinical practices, thus improving the quality of care provided to patients with dental implants.
References
- Cinar AB and Schou L (2014) Impact of Empowerment on Toothbrushing and Diabetes Management. Oral health & preventive dentistry 12: 337-344.
- Tavares M, Lindefjeld Calabi KA, San Martin L (2014) Systemic Diseases and Oral Health. Dental clinics of North America 58: 797
- Musacchio E, Perissinotto E, Binotto P, Sartori L, Silva-Netto F, et (2007) Tooth loss in the elderly and its association with nutritional status, socio-economic and lifestyle factors. Acta odontologica Scandinavica 65: 78-86.
- Muller F, Naharro M, Carlsson GE (2007) What are the prevalence and incidence of tooth loss in the adult and elderly population in Europe?. Clin Oral Implants Res 18: 2-14.
- (2008) The World Oral Health Report 2003: continuous improvement of oral health in the 21st century – the approach of the WHO Global Oral Health Programme Poul Erik Petersen First published: 09 October 2008.
- Carassi A, Cortellini P, Trombelli L (2016) Parodontologia e Terapia Implantare, Milano, Quintessenza Edizioni 6: 2.
- Lang NP, Lindhe J (2016) Parodontologia clinica e implantologia orale, Milano, Edi.Ermes 2.
- Araujo MG, Lindhe J (2018) Peri-implant health. J Clin Periodontol 45: S230-S236.
- Theilade E, Wright WH, Jensen SB, Loe H (1966) Experimental gingivitis in man. II. A longitudinal clinical and bacteriological Journal of periodontal research 1: 1-13.
- Lindhe J, Meyle J, Group D of European Workshop on Periodontology (2008) Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology, J Clin Periodontol 35: 282-285.
- Berglundh T, Gotfredsen K, Zitzmann NU, Lang NP, Lindhe J (2007) Spontaneous progression of ligature induced peri-implantitis at implants with different surface roughness; an experimental study in Cli Oral Implants Res 18: 655-661.
- Karoussis IK, Salvi GE, Heitz-Mayfield LJ, Bragger U, Hammerle CH, et al. (2003) Long-term implant prognosis in patients with and without a history of chronic periodontitis: a 10-year prospective cohort study of the Dental Implant System. Clin Oral Implants Res 14: 329-339.
- Papathanasiou E, Finkelman M, Hanley J, Parashis AO (2016) Prevalence, Etiology and Treatment of Peri-Implant Mucositis and Peri-Implantitis: A Survey of Periodontists in the United States, J Periodontol 87: 493-502.
- Renvert S, Polyzois I, Maguire R (2009) Re-osseointegration on previously contaminated surfaces: a systematic review. Clin Oral Implants Res 20: 216-227.
- Heitz-Mayfield LJA, Salvi GE (2018) Peri-implant mucositis. J Clin Periodontol 45: S237-S245.
- Herrera D, Berglundh T, Schwarz F, Chapple I, Jepsen S, et al. (2023) Prevention and treatment of peri-implant diseases-The EFP S3 level clinical practice guideline. J Clin Periodontol 50: 4-76.
- Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J, et al. (2018) Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. Journal of Clinical Periodontology 45: S286-S291.
- Mombelli A (2002) Microbiology and antimicrobial therapy of peri Periodontol 2000. 28: 177-189.
- Heitz-Mayfield LJA, Salvi GE, Mombelli A, Faddy M, Lang NP (2012) Anti-infective surgical therapy of peri-implantitis. A 12-month prospective clinical study. Clin Oral Implants Res 23: 205-210.
- Máximo MB, de Mendonça AC, Renata Santos V, Figueiredo LC, Feres M, Duarte PM (2009) Short-term clinical and microbiological evaluations of peri-implant diseases before and after mechanical antiinfective therapies. Clin Oral Implants Res 20: 99-108.
- Khoury F, Keeve PL, Ramanauskaite A, Schwarz F, Koo KT, et al. (2019) Surgical treatment of peri-implantitis - Consensus report of working group 4. Int Dent J 69: 18-22.
- Mercado F, Hamlet S, Ivanovski S (2018) Regenerative surgical therapy for peri-implantitis using deproteinized bovine bone mineral with 10% collagen, enamel matrix derivative and Doxycycline-A prospective 3-year cohort study. Clin Oral Implants Res 29: 583-591.
- Figuero E, Graziani F, Sanz I, Herrera D, Sanz M (2014) Management of peri-implant mucositis and peri-implantitis. Periodontology 66: 255
- Garaicoa-Pazmino C, Sinjab K, Wang H-L (2019) Current Protocols for the Treatment of Peri-implantitis. Current Oral Health Reports 6: 209-217.
- Wheelis SE, Gindri IM, Valderrama P, Wilson Jr TG, Huang J, et al. (2016) Effects of decontamination solutions on the surface of titanium: investigation of surface morphology, composition, and roughness. Clinical Oral Implants Research 27: 329-340.
- Emanuel N, Machtei EE, Reichart M, Shapira L (2020) D-PLEX500: a local biodegradable prolonged release doxycycline-formulated bone graft for the treatment for peri-implantitis. A randomized controlled clinical study. Quintessence Int 51: 546-553.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.