Rapid Reduction of Itch Intensity with An Omentum Purified Lipid-Based Topical Emollient Fluid Cream Containing Polidocanol, PEA, and Naturesensitive in Patients with Scabies Treated With Oral Ivermectin: A Pilot Prospective Non-Controlled Trial
by Stefano Veraldi1, Francesca Colombo2, Stefano Alfano2, Massimo Milani2
1Dermatological Center in Milan, Milan, Italy
2Medical Department Cantabria Labs Difa Cooper, Caronno P (VA) Italy.
*Corresponding author: Dr Massimo Milani, Medical Department Cantabria Labs Difa Cooper, Caronno P (VA) Italy.
Received Date: 13 January, 2026
Accepted Date: 21 January, 2026
Published Date: 24 January, 2026
Citation: Veraldi S, Colombo F, Alfano S, Milani M (2026) Rapid Reduction of Itch Intensity With An Omentum Purified LipidBased Topical Emollient Fluid Cream Containing Polidocanol, PEA, And Naturesensitive In Patients With Scabies Treated With Oral Ivermectin: A Pilot Prospective Non-Controlled Trial. Clin Exp Dermatol Ther 11: 245. https://doi.org/10.29011/2575-8268.100245
Abstract
Background: Oral ivermectin is the gold standard for scabies, yet itch-the primary cause of patient distress-often persists significantly during the first week of treatment. Standard systemic therapy typically achieves only a ~45% reduction in pruritus intensity seven days after the first dose. This pilot study evaluates the efficacy of a topical emollient fluid cream containing purified omental lipids (POL), polidocanol, palmitoylethanolamide (PEA), and Naturesensitive (POL Calm™) as an adjunctive therapy to accelerate itch relief. Methods: In an open-label, uncontrolled pilot study, 9 patients (mean age 48 ± 20 years) with confirmed non-crusted scabies were treated with oral ivermectin (200 µg/kg). Concurrently, patients applied the topical formulation twice daily. Itch was evaluated at baseline and Day 7 using a Numerical Rating Scale (NRS, 0-3) and a 10cm Visual Analogue Scale (VAS). Results: At baseline, all subjects reported a maximal NRS score of 3 and a mean VAS score of 8.0. By Day 7, the NRS score decreased to 1 in all subjects (a 75% reduction). The mean VAS score significantly decreased to 2.97 ± 0.3, representing a 63% reduction from baseline (p = 0.001). The formulation was well-tolerated with no reported adverse effects. Conclusion: The addition of a multi-component antipruritic cream to the standard ivermectin regimen resulted in a 63% reduction in itch intensity within the first week, compared to the approximately ~45% reduction typically reported with ivermectin alone. While limited by a small sample size, these results suggest that targeting the inflammatory pruritic response alongside parasitic eradication can bridge the symptomatic gap in scabies management. These findings provide a baseline for future randomized controlled trials.
Keywords: Scabies; Pilot Study; Purified Omental Lipids; Itch.
Introduction
Scabies remains a significant global public health challenge, profoundly impacting the quality of life of affected individuals [1]. Currently, oral ivermectin is considered a gold-standard treatment due to its efficacy and ease of administration [2]. However, the standard protocol typically requires two separate doses, spaced 7 to 10 days apart, to achieve complete parasite eradication and cure rates near 95% [3]. Despite this parasitological success, the management of clinical symptoms remains a critical issue [4]. Severe itch, often exacerbating at night, is the pathognomonic hallmark of the disease and the primary driver of patients’ psychological and physical distress [5]. A rapid resolution of itching is, therefore, a crucial clinical outcome. Existing literature indicates that systemic treatment alone does not provide immediate relief: seven days after the first dose of oral ivermectin, itch intensity evaluated by VAS score is reduced by only approximately 45% [6]. Consequently, there is a clear unmet medical need for therapeutic strategies capable of further reducing pruritus during the first week of treatment, prior to the second dose. In this context, integrating a targeted topical therapy could represent a significant clinical advancement. The aim of this pilot study is to evaluate the antipruritic efficacy of an innovative cream based on purified omentum lipids, enriched with three synergistic components: polidocanol, palmitoylethanolamide (PEA), and Naturesensitive™ (a mixture of Argania Spinosa Kernel Oil, Butyrospermum Parkii (Shea) Butter, Hordeum Vulgare Powder, and Tocopherol). This product has already demonstrated a relevant anti-itch activity in prurigo nodularis [7]. We hypothesize that this topical formulation can achieve a greater and rapid reduction in itch intensity during the first week after oral ivermectin. Proving this objective would provide clinicians with a valuable tool to enhance the quality of life of scabies patients, addressing a vital symptomatic gap in current standard-of-care protocols of subjects with scabies.
Study Aim
We wanted to evaluate in a pilot open, non-controlled study the effect on Itch during the first week in subjects with no-crusted scabies treated with oral ivermectin (200 µg/Kg) with the concomitant use of a purified omental lipids base cream containing also 3 anti-itching components (Polidocanol, Palmitoyl ethanol amide and Natursensitive).
Subjects and Methods
This was an open-label, uncontrolled pilot study. We enrolled 9 subjects (7 men, 2 women; mean age 48 ± 20 years; mean BMI 25 ± 4) with clinically and instrumentally confirmed diagnoses of noncrusted scabies at an outpatient dermatological service in Milan, Italy, after their written informed consent. The Inclusion Criteria were Subjects aged 18 or older with scabies eligible for ivermectin treatment. The Exclusion Criteria were Crusted (Norwegian) scabies; known allergy to study components; presence of clinically relevant systemic diseases (e.g., renal failure, hepatic disease, or hematologic malignancies) associated with generalized pruritus. Patients received oral ivermectin (200 µg/kg; mean dosage 5 ± 1 tablets of 3 mg) and were instructed to apply the POL-based cream twice daily. On average, symptoms were present for 37 ± 21 days prior to diagnosis and treatment (range: 20-90 days). Outcome Assessment: Itch intensity was evaluated at baseline and at Day 7 (immediately prior to the second ivermectin dose). We utilized a 4-point Numerical Rating Scale (NRS: 0 = no itch to 3 = severe itch) and a 10cm VAS (0 = no itch, 10 = extreme itch).
Statistical Analysis
The data analysis was performed using GraphPad software. Data were expressed as mean ± SD. No sample size calculation was performed due to the pilot nature of the study
Results
At baseline the NRS score was 3 in all the 9 subjects, and the VAS score was 8. The NRS score at day 7 was reduced to 1 in all treated subjects. This represents a 75% reduction in the severity of itch score. The mean VAS score was 2.97 ± 0.3 (-63% in comparison with baseline values; p = 0.001) (Figure 1). The cream was very well tolerated.
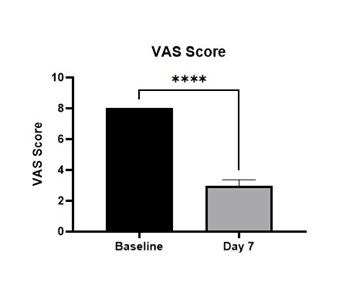
Figure 1: Evolution of VAS score at baseline and after 7 days (****: p=0.001).
Discussion
The results of this pilot study suggest that the concomitant use of POL Calm-a topical formulation containing three anti-pruritic components-alongside oral ivermectin may accelerate the reduction of itch intensity in patients with scabies as soon as the first 4-7 days after the first oral ivermectin intake. While oral ivermectin is the gold standard for systemic treatment, clinical experience and available literature indicate that pruritus often persists significantly after the initial dose [8]. Strategies to reduced scabies-associated itch or post scabietic itch are considered a relevant clinical outcome [9-10]. Specifically, published data suggest that oral ivermectin alone typically yields a reduction in the Visual Analogue Scale (VAS) for pruritus of approximately 45% seven days after the first administration. In our cohort, however, we observed a more pronounced clinical improvement, with a 63% reduction in VAS scores at the same time point. This difference could be clinically relevant. Standard ivermectin protocols often achieve definitive symptomatic relief only after the second dose, usually administered 7 to 10 days after the first. By bridging this gap with a targeted anti-pruritic agent like POL Calm, clinicians may improve patient quality of life and treatment adherence during the first week of therapy. Furthermore, the observed acceleration in pruritus relief is of paramount clinical importance, as it may significantly reduce scratching-induced skin trauma. By preserving skin barrier integrity during the critical first week of treatment, the adjunct use of POL Calm could potentially lower the risk of secondary bacterial superinfections, such as impetigo caused by Staphylococcus aureus or Streptococcus pyogenes, which represent a common and severe complication of scabies infestations [11]. Furthermore, the topical cream has provided immediate and sustained relief from pruritus regardless of symptom duration prior to diagnosis and treatment. This product has demonstrated a relevant anti-itching effect also in another dermatological condition characterized by sever itch as prurigo nodularis. In this clinical setting the use of this cream was associated with a reduction in the VAS score of 76% in comparison with baseline7. Some study limitations should be taken in account in evaluating our results. We acknowledge that this study has several limitations that must be considered when interpreting the data:
- Small Sample Size: The study was conducted on a limited cohort of 9 subjects.
- Study Design: This was an open-label, uncontrolled pilot study, which lacks a placebo or head-to-head comparator group.
- Bias: The absence of blinding may introduce observer or participant bias regarding symptom reporting.
Despite these weaknesses, the primary value of this pilot investigation lies in its role as a foundational step for future research. The preliminary data collected regarding the mean reduction and variance of VAS scores provide the necessary parameters for an accurate sample size calculation in subsequent, robustly powered randomized controlled trials (RCTs).
Conclusion
In conclusion, our preliminary findings indicate that adding POL Calm to the standard oral ivermectin regimen may facilitate a faster resolution of pruritus in patients affected by scabies. Given that systemic treatment alone often requires a second administration to achieve significant symptomatic control, the immediate introduction of a triple-component anti-pruritic agent represents a valuable supportive strategy. This “take-home message” suggests that integrated management-addressing both the parasitic infestation and the inflammatory pruritic response-is essential for rapid patient relief. Larger, controlled studies are now warranted to confirm these observations.
Ethical Issues
The study was conducted in accordance with the Declaration of Helsinki (Ethical Principles for Medical Research Involving Human Subjects).
Conflict of Interest
The study was a sponsor-free trial. FC, SA and MM are employees of CantabriaLabs Difa Cooper.
References
- Heukelbach J, Feldmeier H Scabies (2006) The Lancet 367: 17671774.
- Meinking TL, Taplin D, Hermida JL, Pardo R, Kerdel FA (1995) The treatment of scabies with ivermectin. N Engl J Med 333: 26-30.
- Fawcett RS (2003) Ivermectin use in scabies. Am Fam Physician 68: 1089-1092.
- Sunderkötter C, Wohlrab J, Hamm H (2021) Scabies: epidemiology, diagnosis, and treatment. Dtsch Ärztebl Int 118: 695-704.
- Johnston G, Sladden M (2005) Scabies: diagnosis and treatment. BMJ 331: 619-622.
- Mohsena A (2020) Efficacy and Safety of Oral Ivermectin and Topical Permethrin in the Treatment of Scabies. TAJ: Journal of Teachers Association 33: 41-47.
- Ardigò M, Franceschini C, Campione E, Cosio T, Lanna C, et al. (2020) Efficacy of a topical product containing purified omental lipids and three anti-itching compounds in the treatment of chronic pruritus/prurigo nodularis in elderly subjects: A prospective, assessor-blinded, 4-week trial with transepidermal water loss and optical coherence tomography assessments. Clin Cosmet Investig Dermatol 13: 1051-1058.
- Jannic A, Bernigaud C, Brenaut E, Chosidow O (2018) Scabies itch. Dermatol Clin 36: 301-308.
- Veraldi S, Schianchi R, Esposito L, Pontini P, Nazzaro G (2019) Treatment of postscabies prurigo with diflucortolone and chlorquinaldol in a group of African refugees. Trop Doct 49: 268-270.
- Veraldi S, Nazzaro G, Schianchi R, Anton Ionescu M (2025) Treatment of postscabies itching with calamine: results of a retrospective, multicenter, sponsor-free study. Italian Journal of Dermatology and Venereology 160: 459-461.
- Leung AKC, Lam JM, Leong, KF (2020) Scabies: a neglected global disease. Curr Pediatr Rev 16: 33-42.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.