Radiomics and Artificial Intelligence Applied to Carotid Ultrasound for Mortality Prediction in Sepsis: A Pilot Study
by Thais Ramos da Costa1-5*, Alice Abath Leite1,2,6, Camila Silva Bezerra1,2,6, Felipe Alves Mourato1,4,5, Esdras Marques Lins1,2,6, Emmanuelle Tenório Albuquerque Madruga Godoi1,7, Lúcia Helena de Oliveira Cordeiro3,7, Simone Cristina Soares Brandão1,4,7, Paula Regina Beserra Diniz5, Vinicius de Oliveira Menezes5
1Post-GraduationProgram in Surgery, Universidade Federal de Pernambuco (UFPE), Recife, PE, Brazil
2Instituto de Medicina Integral Professor Fernando Figueira (IMIP), Recife, PE, Brazil
3Hospital Barão de Lucena, Recife, PE, Brazil
4Department of Diagnostic Imaging, Hospital das Clínicas da Universidade Federal de Pernambuco (HC-UFPE), Recife, PE, Brazil
5Empresa brasileira de serviços hospitalares EBSERH - Hospital das Clínicas da Universidade Federal de Pernambuco (HC-UFPE), Recife, PE, Brazil
6Faculdade Pernambucana de Saúde, Recife, PE, Brazil
7Department of Internal Medicine, Universidade Federal de Pernambuco (UFPE), Recife, PE, Brazil
*Corresponding Author: Thais Ramos da Costa, Post-GraduationProgram in Surgery - Universidade Federal de Pernambuco (PPGC-UFPE), Hospital das Clínicas - Campus UFPE, Av. Prof. Moraes Rego,"s/nº - Bloco "A" - Cidade Universitária - Recife - PE, Brazil
Received Date: 31 January 2026
Accepted Date: 04 February 2026
Published Date: 06 February 2026
Citation: da Costa TR, Leite AA, Bezerra CS, Mourato FA, Lins EM, et al. (2026) Radiomics and Artificial Intelligence Applied to Carotid Ultrasound for Mortality Prediction in Sepsis: A Pilot Study. J Surg 11: 11558 https://doi.org/10.29011/2575-9760.011558
Abstract
Background: Endothelial dysfunction plays a central role in the pathophysiology of sepsis but is rarely assessed in routine clinical practice. Carotid ultrasound is a widely available bedside tool; however, conventional analysis has limited sensitivity for detecting early sepsis alterations. This pilot study evaluated the feasibility of applying radiomics to carotid ultrasound images combined with Artificial Intelligence (AI) to predict mortality in patients with viral and bacterial sepsis.
Methods: Clinical data and carotid ultrasound images from critically ill patients admitted to the intensive care unit were prospectively analyzed. Radiomic features were extracted from ultrasound images, and machine learning models were developed using clinical variables, radiomic features, and their combination. Model performance was assessed using area under the receiver operating characteristic curve (AUC), accuracy, sensitivity, precision, and F1-score.
Results: A total of 37 patients were included (mean age 61.3 ± 14.6 years; 62.1% male), of whom 27 had viral sepsis due to COVID-19 and 10 had bacterial sepsis. Among the evaluated models, the Naive Bayes classifier showed the best performance when combining clinical and radiomic data, achieving an AUC of 0.97, accuracy of 94%, sensitivity of 98%, and precision of 94%. The inclusion of radiomic features increased model robustness by approximately 9% compared with the clinical model alone.
Conclusion: Radiomics applied to carotid ultrasound imaging is feasible and demonstrates high predictive performance for mortality in patients with sepsis. The integration of radiomics and AI may enhance endothelial assessment and improve risk stratification in critically ill patients.
Keywords: Artificial Intelligence; Carotid Ultrasound; Mortality Prediction; Radiomics; Sepsis
Introduction
Sepsis remains one of the leading causes of morbidity and mortality worldwide and is characterized by a dysregulated systemic inflammatory response that frequently progresses to multiorgan failure. Despite advances in critical care, early identification of patients at increased risk of adverse outcomes remains a major clinical challenge [1-3]. Endothelial dysfunction plays a pivotal role in sepsis pathophysiology, contributing to microvascular injury, impaired tissue perfusion, and organ dysfunction. Nevertheless, endothelial assessment is not routinely incorporated into bedside evaluation [4-6]. During the COVID-19 pandemic, growing evidence highlighted the relevance of endothelial injury in critically ill patients, reinforcing the need for accessible tools capable of detecting vascular alterations [4,5,7]. Carotid ultrasound is a noninvasive, radiation-free, and widely available imaging modality. Conventional B-mode ultrasound enables assessment of the carotid Intima–Media Complex (IMC), a recognized marker of vascular health. However, traditional visual and morphometric analysis has limited ability to detect subtle or early endothelial changes [6,8,9]. Radiomics has emerged as an innovative approach that extracts high-dimensional quantitative features from medical images, capturing texture and spatial patterns beyond visual perception. When combined with artificial intelligence, radiomics enables the development of predictive models for clinical outcomes [10,11]. This pilot study aimed to evaluate the feasibility of applying radiomics to carotid ultrasound images, combined with AI-based modeling, to predict mortality in patients with viral and bacterial sepsis admitted to the intensive care unit.
Materials and Methods
Study Population
Patients admitted to the intensive care unit with a diagnosis of sepsis were included. Between July 2020 and February 2021, 59 patients with severe COVID-19 underwent carotid ultrasound during hospitalization. Of these, 27 had digitally stored images suitable for radiomic analysis. Additionally, 10 patients with nonviral (bacterial) sepsis admitted prospectively to the ICU were included. The final cohort comprised 37 patients. The study was approved by the institutional ethics committee (CAAE: 34736620.6.0000.8807), and informed consent was obtained from patients or their legal representatives.
Clinical Data Collection
Clinical data were obtained from physical and electronic medical records and included sex, age group, use of invasive mechanical ventilation, clinical outcome (hospital discharge or death), and comorbidities such as systemic arterial hypertension, diabetes mellitus, chronic kidney disease, liver disease, and cancer. Laboratory data included renal function parameters (urea and creatinine) and inflammatory markers, including leukocyte count.
Ultrasound Image Acquisition
All carotid ultrasound examinations were performed at the bedside using GE Logic ultrasound systems equipped with 12L-RS linear transducers (General Electric®). Examinations were conducted by three radiologists with 4–10 years of experience, with each patient evaluated by at least two operators. Images were stored in DICOM format. B-mode images were acquired in axial and longitudinal planes. The carotid intima–media complex was evaluated on the posterior wall of the common carotid artery in plaque-free segments. IMC thickness was defined as the distance between the lumen–intima and media–adventitia interfaces and classified as thickened according to ELSA-Brasil criteria [12,13] (Figure 1).
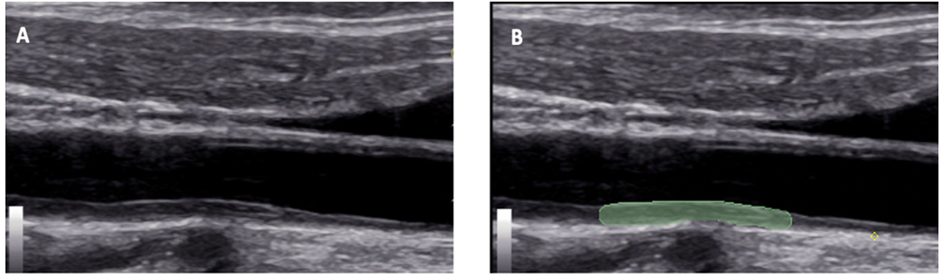
Figure 1: B-mode ultrasound image of the right carotid artery in the longitudinal direction of a study patient with severe Covid-19, showing in A the thickening of the intima-media complex (arrowhead) and in B the delimitation of the region of interest (green marking).
Radiomic Analysis
Radiomic analysis was performed using 3D Slicer software (version 4.11.20210226). Regions of interest were manually delineated along the carotid intima–media complex to ensure precise boundary definition. Radiomic features were extracted and exported in CSV format. Histogram-based analysis was performed, and features were categorized into first-order statistics (pixel intensity distribution) and second-order statistics (texture and spatial relationships) (Figure 2).
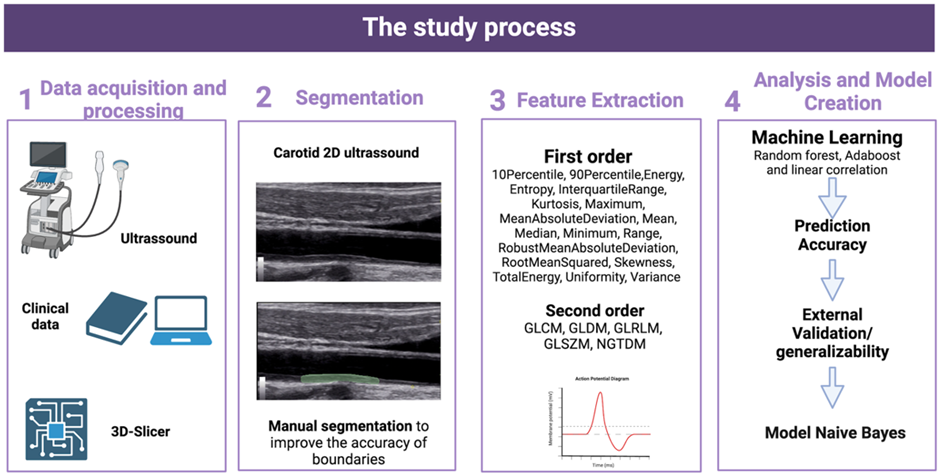
Figure 2: Stages of the study process.
Artificial Intelligence Model Development
Data preprocessing and model development were conducted using PyCaret version 3.0. Data cleaning, normalization, and merging were performed using the Pandas framework. Exploratory data analysis included assessment of feature distributions and correlation analysis with a 60% cutoff. The dataset was stratified and divided into 80% training and 20% testing sets. Class imbalance was addressed through resampling of the minority class. Sixteen machine learning algorithms were evaluated using clinical variables alone, radiomic features alone, and combined datasets. Dimensionality reduction, collinearity removal, and feature selection were performed using classical methods and permutation importance. Model performance was assessed using AUC, accuracy, sensitivity, precision, recall, and F1-score.
Statistical Analysis
Categorical variables were expressed as absolute numbers and percentages, while continuous variables were presented as mean ± standard deviation. Model performance with and without the inclusion of radiomic features was compared to assess the incremental value of radiomics in mortality prediction.
Results and Patient Characteristics
The cohort included 37 ICU patients with a mean age of 61.3 ± 14.6 years; 23 patients (62.1%) were male. Viral sepsis due to COVID-19 accounted for 27 cases (72.9%), while 10 patients (27.1%) had bacterial sepsis (Figure 3).
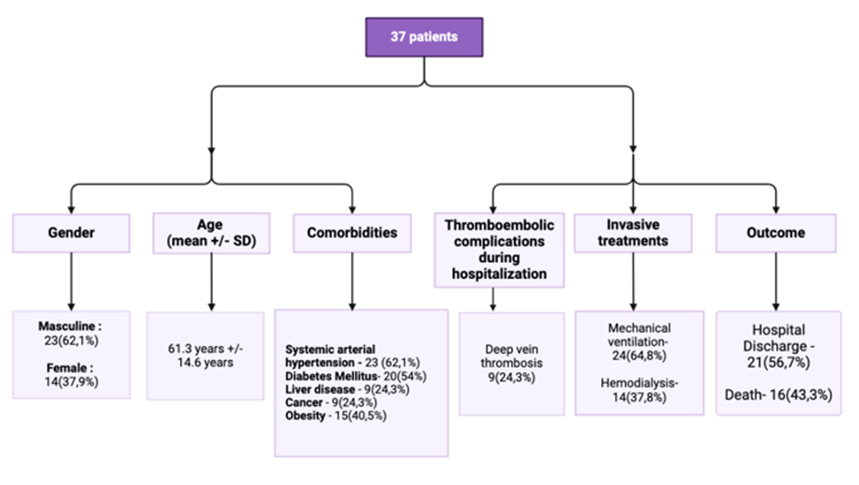
Figure 3: Clinical characteristics of the patients in the study.
Conventional Ultrasound Findings
On conventional analysis, 14 patients (37.8%) presented with IMC thickening, 13 (35.1%) showed irregularities of the inner arterial wall, and 10 patients (27.1%) demonstrated no detectable alterations (Figure 4).
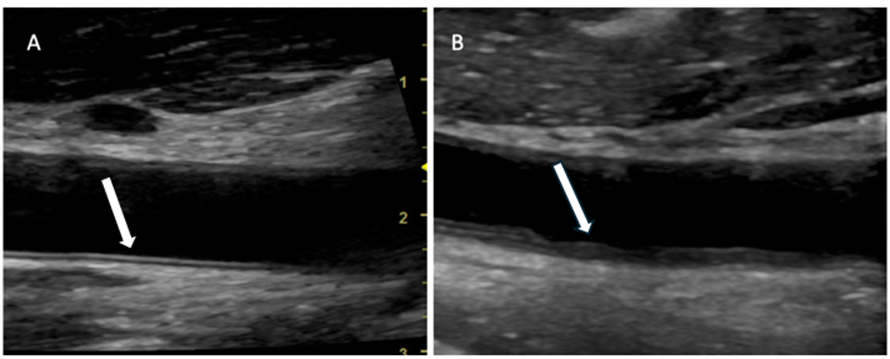
Figure 4: Carotid ultrasound of a patient from the study. In A, the intima-media complex without alterations and in B an example of a patient with thickening and irregularities (arrowhead).
Machine Learning Performance
Among the evaluated algorithms, the Naive Bayes classifier demonstrated the best performance when combining clinical and radiomic data, achieving an accuracy of 94%, AUC of 0.97, sensitivity of 98%, precision of 94%, and F1-score of 0.96. The incorporation of radiomic features improved model performance by approximately 9% compared with models based on clinical data alone (Table 1).
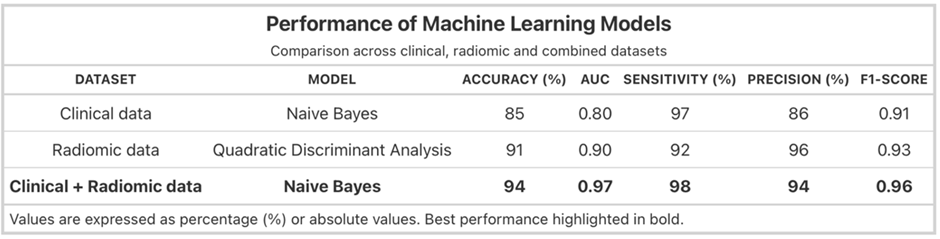
Table 1: Performance of machine learning models using clinical, radiomic, and combined datasets.
Discussion
This pilot study demonstrates the feasibility of applying radiomics to carotid ultrasound imaging combined with artificial intelligence to predict mortality in patients with sepsis. Although carotid ultrasound is widely used due to its accessibility and safety, previous studies have relied mainly on conventional IMC measurements [4,14,15]. To our knowledge, this is the first study to apply radiomic analysis of carotid ultrasound images for mortality prediction in septic patients using machine learning models. The most frequent conventional ultrasound findings were IMC thickening and irregularity, which have been described in inflammatory conditions such as vasculitis, HIV infection, and SARS-CoV-2 infection [16-19]. Our previous investigations demonstrated an association between these carotid alterations and mortality in patients with severe COVID-19 [5]. The present study extends these findings to bacterial sepsis, reinforcing the hypothesis that endothelial injury represents a shared pathophysiological mechanism across infectious etiologies. Among the evaluated algorithms, the Naive Bayes classifier achieved the best performance, particularly when radiomic features were combined with clinical data [20-22]. The improvement in predictive accuracy highlights the added value of radiomics in capturing subtle vascular alterations not detected by conventional ultrasound analysis. This study has limitations, including the small sample size and potential variability in the inflammatory phase at the time of imaging. However, as a pilot investigation, these findings provide a strong rationale for future multicenter studies with larger cohorts and external validation.
Conclusion
This study demonstrates the feasibility of developing an AI-based model for mortality prediction using radiomic features extracted from carotid ultrasound images in patients with viral and bacterial sepsis. The integration of radiomics and artificial intelligence shows promise for improving endothelial assessment and prognostic stratification in critically ill patients.
Ethics Statement: The study was approved by the institutional ethics committee (CAAE: 34736620.6.0000.8807). Informed consent was obtained from patients or their legal representatives.
Conflict of Interest: The authors declare no conflict of interest.
Funding: No external funding was received.
Ethics approval statement: The study was approved by the Research Ethics Committee of the Hospital das Clínicas of the Federal University of Pernambuco (CAAE: 34736620.6.0000.8807) and informed consent was obtained after being provided information regarding the study.
Disclosure: The authors declare no conflict of interest.
References
- Joffre J, Hellman J, Ince C, Ait-Oufella H (2020) Endothelial responses in sepsis. American Journal of Respiratory and Critical Care Medicine. American Thoracic Society 202: 361-370.
- Ince C, Mayeux PR, Nguyen T, Gomez H, Kellum JA, et al. (2016) The endothelium in sepsis. Vol. 45, Shock. Lippincott Williams and Wilkins 45: 259-270.
- Hattori Y, Hattori K, Machida T, Matsuda N (2022) Vascular endotheliitis associated with infections: Its pathogenetic role and therapeutic implication. Biochemical Pharmacology. Elsevier Inc 197: 114909.
- Brandão SCS, Godoi ETAM, Ramos J de OX, de Melo LMMP, Dompieri LT, et al. (2020) The role of the endothelium in severe COVID-19. Arquivos Brasileiros de Cardiologia. Arquivos Brasileiros de Cardiologia 115: 1184-1189.
- Bezerra CS, Leite AA, da Costa TR, Lins EM, Godoi ETAM, et al. (2022) Ultrasound findings in severe COVID-19: a deeper look through the carotid arteries. Radiol Bras 55: 329-336.
- Wang X, Luo P, Du H, Li S, Wang Y, et al. (2022) Ultrasound Radiomics Nomogram Integrating Three-Dimensional Features Based on Carotid Plaques to Evaluate Coronary Artery Disease. Diagnostics 12: 256.
- Ke Z, Li L, Wang L, Liu H, Lu X, et al. (2022) Radiomics analysis enables fatal outcome prediction for hospitalized patients with coronavirus disease 2019 (COVID-19). Acta radiol 63: 319-327.
- Mitchell C, Korcarz CE, Zagzebski JA, Stein JH (2021) Effects of ultrasound technology advances on measurement of carotid intima–media thickness: A review. Vascular Medicine (United Kingdom). SAGE Publications Ltd 26: 81-85.
- Liu Y, Kong Y, Yan Y, Hui P (2024) Explore the value of carotid ultrasound radiomics nomogram in predicting ischemic stroke risk in patients with type 2 diabetes mellitus. Front Endocrinol (Lausanne) 15: 1357580.
- Li M De, Cheng MQ, Chen L Da, Hu HT, Zhang JC, et al. (2022) Reproducibility of radiomics features from ultrasound images: influence of image acquisition and processing. Eur Radiol 32: 5843-5851.
- Lambin P, Leijenaar RTH, Deist TM, Peerlings J, De Jong EEC, et al. (2017) Radiomics: The bridge between medical imaging and personalized medicine. Nature Reviews Clinical Oncology. Nature Publishing Group 14: 749-762.
- Albricker ACL, Freire CMV, Santos SN Dos, Alcantara ML de, Cantisano AL, et al. (2023) Atualização da Recomendação para Avaliação da Doença das Artérias Carótidas e Vertebrais pela Ultrassonografia Vascular: DIC, CBR, SBACV – 2023. Arq Bras Cardiol 120: e20230695.
- De Korte CL, Fekkes S, Nederveen AJ, Manniesing R, Hansen HRHG (2016) Review: Mechanical Characterization of Carotid Arteries and Atherosclerotic Plaques. IEEE Trans Ultrason Ferroelectr Freq Control 63: 1613-1623.
- Zanini G, Selleri V, Roncati L, Coppi F, Nasi M, et al. (2024) Vascular “Long COVID”: A New Vessel Disease? Angiology. SAGE Publications Inc 75: 8-14.
- Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, et al. (2021) Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol 21: 319-329.
- Godoi ETAM, Brandt CT, Lacerda HR, Godoi JTAM, De Oliveira DC, et al. (2017) Intima-media thickness in the carotid and femoral arteries for detection of arteriosclerosis in human immunodeficiency virus-positive individuals. Arq Bras Cardiol 108: 3-11.
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, et al. (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. Lancet Publishing Group 395: 1033-1034.
- Pelle MC, Zaffina I, Lucà S, Forte V, Trapanese V, et al. (2022) Endothelial Dysfunction in COVID-19: Potential Mechanisms and Possible Therapeutic Options Life. MDPI 12: 1605.
- Lecler A, Obadia M, Savatovsky J, Picard H, Charbonneau F, et al. (2017) TIPIC syndrome: Beyond the myth of carotidynia, a new distinct unclassified entity. American Journal of Neuroradiology 38: 1391-1398.
- Hou C, Li S, Zheng S, Liu LP, Nie F, et al. (2024) Quality assessment of radiomics models in carotid plaque: a systematic review. Quantitative Imaging in Medicine and Surgery. AME Publishing Company 14: 1141-1154.
- Attenberger U, Reiser MF (2022) Future perspectives: how does artificial intelligence influence the development of our profession? Radiologe 62: 267-270.
- Attenberger UI, Langs G (2021) How does Radiomics actually work? - Review. RoFo Fortschritte auf dem Gebiet der Rontgenstrahlen und der Bildgebenden Verfahren 193: 652-657.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.