Protein C Deficiency Associated with Postpartum Cerebral Thrombophlebitis: A Case Report with Literature Review
by Said Khallikane Sr1*, Monsef Elabdi2, Amine Bentaher3, Moncef Salek4, Hicham Kbiri5, Ilyass Masad6
1Anesthesiology and Critical Care, Hôpital militaire avicenne, Marrakech, Royaume du Maroc, Marrakech, MAR, Morocco
2Trauma and Orthopedic, Hassan II Military Hospital, Laayoune, MAR, Morocco
3Radiology, Moulay Ismail Military Hospital, Meknes, MAR, Morocco
4Diagnostic and interventional radiology department of the Moulay Ismail Military Hospital, Meknes, Morocco, Faculty of Medicine, Pharmacy and Dentistry, Sidi Mohamed Ben Abdellah University, Fes, Morocco, Meknes, MAR, Morocco
5Anesthesiology-ICU-Emergency, Avicenna Military Hospital, Marrakech, MAR, Morocco
6Anesthesiology-ICU-Emergemcy, HASSAN II Military Hospital of Laayun, Morocco
*Corresponding author: Said Khallikane Sr, Anesthesiology and Critical Care, Hôpital militaire avicenne, Marrakech, Royaume du Maroc, Marrakech, MAR, Morocco
Received Date: 12 November 2024
Accepted Date: 18 November 2024
Published Date: 19 November 2024
Citation: Sr Khallikane S, Elabdi M, Bentaher A, Salek M, Kabiri H, et al (2024) Protein C Deficiency Associated with Postpartum Cerebral Thrombophlebitis: A Case Report with Literature Review. Ann Case Report. 9: 2073. https://doi.org/10.29011/2574-7754.102073
Abstract
This article presents a comprehensive overview of a case report and literature review on postpartum cerebral thrombophlebitis revealing protein C deficiency. The case involves a 32-year-old woman who developed cerebral venous thrombosis (CVT) in the postpartum period, associated with severe headaches, visual disturbances, and lower limb weakness. Diagnostic investigations confirmed protein C deficiency, a rare genetic disorder that predisposes individuals to abnormal blood clot formation. The patient was treated with anticoagulation therapy with good outcome, highlighting the critical importance of early recognition and management of protein C deficiency to mitigate life-threatening thrombotic complications. The article emphasizes the significance of understanding the interplay between protein C deficiency and cerebrovascular disorders, particularly during pregnancy and the postpartum period. The findings underscore the need for heightened clinical awareness, timely intervention, and further research to enhance our understanding of protein C deficiency and its management, especially in the context of pregnancy- related complications.
Categories: Cardiology, Anesthesiology, Cardiac/Thoracic/Vascular Surgery.
Keywords: Human Protein C Concentrates; Anticoagulation Therapy; Thrombophilia; Protein C Deficiency; Postpartum Cerebral Venous Thrombosis.
Introduction
This article provides a comprehensive overview of the case report and literature review on postpartum cerebral thrombophlebitis revealing protein C deficiency. The significance of the topic lies in its association with life-threatening complications during pregnancy and the postpartum period, posing significant risks for both the mother and child. Cerebrovascular disorders during this period can be divided into thrombosis and ischemia, including arterial and venous strokes and hemorrhage, with cerebral venous thrombosis (CVT) accounting for a significant proportion of strokes related to pregnancy and post-partum period. The literature also highlights the rare nature of protein C deficiency, which is associated with an increased risk of developing abnormal blood clots due to disrupted coagulation systems. The purpose of the case report is to introduce a case of protein C deficiency in the postpartum period presenting with a set of symptoms of veinous cerebral thrombosis associated with veinous ischemia, localized cerebral edema and hemorrhagic infarction [1]. This sets the stage for the subsequent discussions, providing a foundation for understanding the complexities and challenges associated with postpartum cerebral thrombophlebitis revealing protein C deficiency. Protein C deficiency is a rare disorder associated with an increased risk of abnormal blood clot formation due to disruption in the coagulation system, primarily caused by a mutation in the PROC gene [1]. This deficiency can lead to various symptoms, ranging from severe cases in infants with disseminated intravascular coagulation (DIC) and fulminant purpura to moderate cases presenting with recurrent deep vein thrombosis (DVT) until adolescence. In mild and heterozygous cases, individuals are usually asymptomatic and may present with recurrent thrombosis, often associated with ischemic stroke or concomitant thrombosis during pregnancy and recurrent miscarriage. Pregnancy and the postpartum period are associated with physiological changes that can lead to altered homeostasis and a high risk of complications, including cerebral venous thrombosis (CVT) due to factors such as hypercoagulability, cesarean delivery, infections, blood loss, and dehydration, fluctuations of intracranial pressure, hypertensive complications, and loss of cerebrospinal fluid after dural puncture [2]. The physiological changes during pregnancy result in an increased level of procoagulant factors and decreased levels of anticoagulant factors, contributing to the prothrombotic state. Additionally, cesarean section and pregnancy-related anemia can further contribute to the risk of CVT, making it crucial to analyse the clinical characteristics, risk factors, and short-term prognosis of postpartum CVT.
Case Presentation
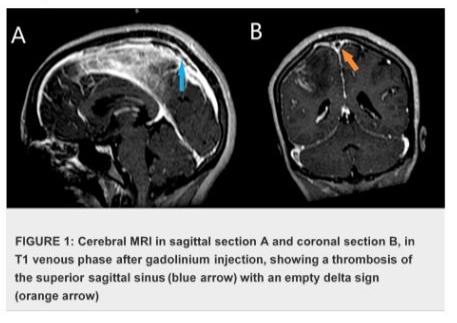
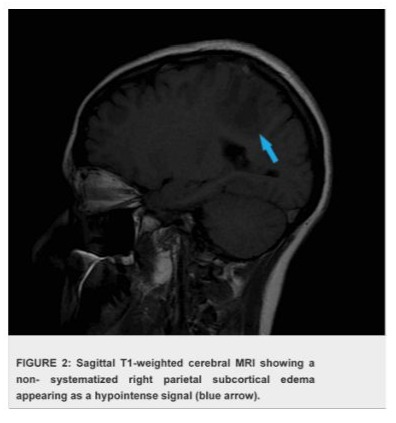
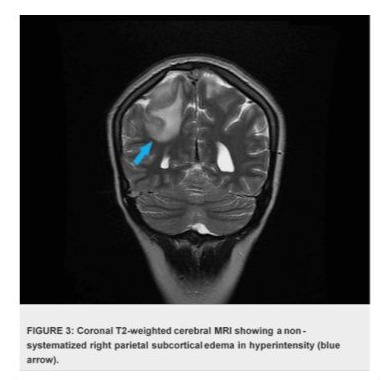
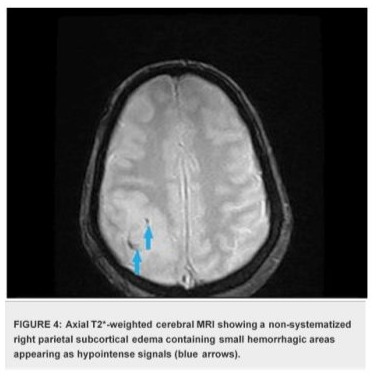
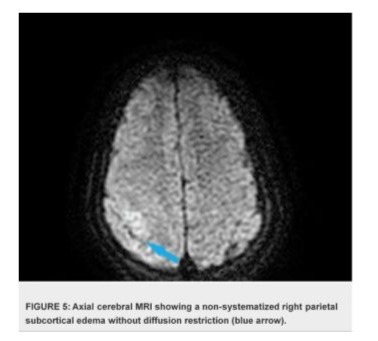
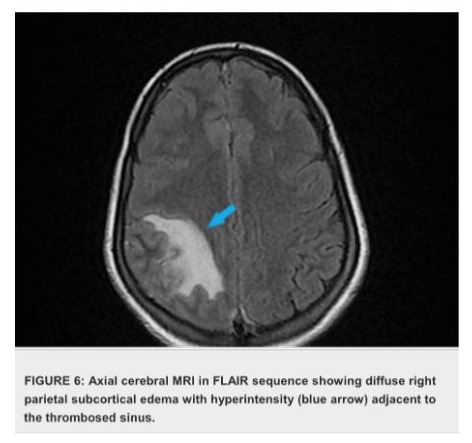
The case presentation involves a 32-year-old woman presented with a history of recurrent miscarriages and a previous episode of deep vein thrombosis (DVT) during her first pregnancy, who presented with postpartum cerebral thrombophlebitis, revealing an underlying protein C deficiency. The patient's history revealed a recent uncomplicated vaginal delivery and subsequent development of severe headaches, visual disturbances, and left lower limb weakness. Upon admission, she was found to have a low Glasgow Coma Scale score 10/15 and signs of increased intracranial pressure. Cerebral MRI shows venous thrombosis of the superior longitudinal sinus on T1 sequences with gadolinium injection in the venous phase (Figures 1), and diffuse, non-systematized right parietal subcortical cerebral edema, which appears hypo intense on T1 (Figure 2), hyper intense on T2 (Figure 3) and Flair (Figure 4), without restriction on diffusion sequence (Figure 5), indicating venous infarction in the area of the thrombosed sinus, in addition T2-star (Gradient Echo) (Figure 6) sequence reveals small petechial hemorrhagic areas appearing as hypo intense signals. Laboratory investigations revealed decreased levels of protein C, confirming the diagnosis of protein C deficiency, which is a rare disorder, associated with an increased risk of abnormal blood clot formation. The patient was promptly initiated on anticoagulation therapy despite the hemorrhagic infarction Levetiracetam 500 mg twice daily, and Mannitol She was not intubated and ventilated, In the diagnostic workup of postpartum cerebral thrombophlebitis revealing protein C deficiency, a comprehensive set of medical investigations and procedures were carried out in our patient to reach a precise diagnosis. The diagnostic process involved genetic profiling of thrombophilia confirmed a mutation in the PROC gene, indicating congenital protein C deficiency, which revealed severe inherited thrombophilia with combined thrombophilia, including heterozygous Factor V-H1299R, and heterozygous PAI-1 4G/5G. Furthermore, serologic measures of thrombophilia were employed, encompassing the assessment of various parameters such as anticardiolipin antibodies immunoglobulin G (IgG) and IgM, antigenic protein C, total and free protein S, antithrombin III, lupus anticoagulant, and homocysteine, which came back negative. Additionally, the lipid profile was evaluated, demonstrating specific lipid parameters such as total cholesterol, LDL, HDL, and triglycerides (Table 1). These meticulous diagnostic measures were crucial in uncovering the underlying protein C deficiency and its association with postpartum cerebral thrombophlebitis. An overlap with acenocoumarol (Sintrom) was initiated over 5 days after 8 days of treatment with enoxaparin. The enoxaparin was discontinued after two consecutive INR readings were within the therapeutic range, after 145 days of hospitalization, the patient condition improved favourably and she was discharged on Sintrom 4 mg, 1 tablet per day, with an INR check prescribed to be done one week later and was referred for a clinical hematology consultation for further management and family genetic investigation. The case highlights the critical importance of recognizing protein C deficiency as an underlying cause of postpartum cerebral thrombophlebitis and underscoring the importance of early recognition and the subsequent management of such conditions involving anticoagulation therapy and genetic testing for definitive diagnosis and contributes to the literature by presenting a rare but clinically significant manifestation of protein C deficiency, emphasizing the need for heightened awareness and timely intervention in similar clinical scenarios.
|
Parameters |
Patient Value |
Reference Range |
|
Lupus Anticoagulant |
Negative |
Negative (no detectable activity) |
|
Anti-cardiolipin Antibodies (IgG) |
15 GPL |
< 20 GPL (units) |
|
Anti-cardiolipin Antibodies (IgM) |
18 MPL |
< 20 MPL (units) |
|
Anti-β2 Glycoprotein I Antibodies (IgG) |
10 GPL |
< 20 GPL (units) |
|
Anti-β2 Glycoprotein I Antibodies (IgM) |
12 MPL |
< 20 MPL (units) |
|
Protein C Level |
60% |
70-150% of normal |
|
Protein S Level |
Free: 50% |
60-130% of normal |
|
Total: 55% |
65-140% of normal |
|
|
Antithrombin III Level |
90% |
80-120% of normal |
|
Homocysteine Levels |
12 µmol/L |
5-15 µmol/L |
|
Fibrinogen Levels |
300 mg/dL |
200-400 mg/dL |
|
D-dimer Test |
1.0 µg/mL |
< 0.5 µg/mL (may vary by lab) |
|
Total Cholesterol |
180 mg/dL |
< 200 mg/dL |
|
LDL Cholesterol |
95 mg/dL |
< 100 mg/dL (optimal) |
|
HDL Cholesterol |
45 mg/dL |
≥ 40 mg/dL (men), ≥ 50 mg/dL (women) |
|
Triglycerides |
130 mg/dL |
< 150 mg/dL |
Table 1: Serologic measures of thrombophilia and Lipid Profile Results.
Discussion
Cerebral thrombophlebitis in postpartum women is a critical concern due to the increased risk of cerebrovascular complications during this period. The postpartum period involves significant physiological changes, leading to a heightened risk of complications such as cerebral venous thrombosis (CVT). Studies have shown that CVT accounts for a notable percentage of strokes related to pregnancy, with most cases occurring in the postpartum period. The increased risk during this period can be attributed to factors such as hypercoagulability related to pregnancy, cesarean delivery, infections, and blood loss during delivery [2]. Additionally, the prothrombotic state during pregnancy, which is due to changes in the fibrinolytic and hemostatic system, persists for 6-8 weeks after delivery. The case report presented a rare occurrence of postpartum cerebral thrombophlebitis revealing protein C deficiency, shedding light on the association between these factors. The literature review supports the understanding that protein C deficiency is linked to an increased risk of abnormal blood clots due to disrupted coagulation, with manifestations ranging from recurrent thrombosis to ischemic stroke, especially during pregnancy and postpartum periods [1]. Moreover, the postpartum period is recognized as a time of heightened risk for cerebrovascular disorders, including thrombosis, attributed to physiological changes and hypercoagulability related to pregnancy and delivery [2]. investigating protein C levels in pregnant women has revealed insights into the association between protein C deficiency and adverse pregnancy outcomes. Research has shown that low protein C levels in pregnant women, particularly in combination with low protein S levels, may contribute to intrauterine growth restriction (IUGR) and a history of abortions, as was the case with our patient [3]. The interplay between protein C deficiency and postpartum cerebral thrombophlebitis highlights the urgent need for increased clinical awareness and proactive management to prevent potentially life-threatening complications and improve patient outcomes. Notably, timely intervention with low molecular weight heparin (LMWH) and progesterone support has been linked to positive results, such as healthy deliveries without thromboembolic events. These findings emphasize the importance of understanding the relationship between protein C deficiency and adverse pregnancy outcomes, especially in the context of thrombosis and postpartum cerebral thrombophlebitis [1-3]. The active form of protein C, known as activated protein C (APC), plays a pivotal role in regulating the coagulation system through its anticoagulant properties. [1] Consequently, a deficiency in protein C whether congenital or acquired can disrupt this delicate balance, leading to increased clot formation and subsequent thrombotic events. In cases of heterozygous protein C deficiency, there is a tenfold increased risk of thrombosis compared to the general population [1]. In contrast, homozygous or double heterozygous protein C deficiency represents a severe thromboembolic disorder, typically manifesting within hours of birth with life-threatening conditions such as purpura fulminans and extensive large-vessel thrombosis [4]. Furthermore, congenital protein C deficiency, resulting from mutations in the PROC gene, is also associated with an elevated risk of thromboembolic events. This deficiency can present in various forms, ranging from severe cases with disseminated intravascular coagulation (DIC) and fulminant purpura in infants requiring urgent therapeutic interventions including protein C substitution [4] to more common, milder forms of heterozygous deficiency that may remain asymptomatic until adulthood as was the case with our patient. These milder forms often lead to recurrent deep vein thrombosis (DVT) or ischemic stroke, particularly during pregnancy and in cases of recurrent miscarriage.
The occurrence of cerebral venous sinus thrombosis (CVST) in postpartum women is particularly noteworthy due to the rarity of this type of stroke and its potential impact on young individuals. CVST presents with a range of symptoms, from headaches to seizures and stroke, emphasizing the importance of early diagnosis and treatment for a favourable outcome. Given the heightened risk factors during the postpartum period, it is crucial to emphasize the need for increased awareness, timely diagnosis, and appropriate management to address the specific challenges and considerations related to CVST in postpartum women [5]. Treatment and management of postpartum cerebral thrombophlebitis revealing protein C deficiency involve addressing the underlying protein C deficiency and managing the complications associated with abnormal blood clot formation. In cases of severe congenital protein C deficiency, where patients are at an increased risk of thromboembolic events, the use of human protein C concentrates has been reported as an effective therapy leading to successful outcomes for several individuals. These concentrates, introduced in the late 1980s, have enabled successful treatment of patients with life-threatening manifestations of protein C deficiency, such as purpura fulminans and large-vessel thrombosis, by providing the necessary PC substitution [4]. Furthermore, the management of protein C deficiency involves addressing the increased risk of abnormal blood clots through anticoagulant therapy, particularly in cases of recurrent thrombosis associated with ischemic stroke and concomitant thrombosis during pregnancy. The objectives of anticoagulant therapy are to prevent the extension of thrombosis to other sinuses, allowing the development of collateral circulation and the prevention of venous infarcts. The theoretical risk is that of a massive hemorrhage within an infarct, which is often spontaneously hemorrhagic. A recent European multicenter trial [6], involving 59 patients (30 treated, 29 placebo), was completed. The treated patients received low-molecular-weight heparin (nadroparin) subcutaneously (180 anti-Xa/kg/24 hours) for three weeks followed by three months of oral anticoagulants. At three weeks, six out of 30 treated patients (20%) had an unfavourable outcome (defined by death or a Barthel index below 15) compared to seven out of 29 in the control group (24%). At three months, an unfavourable outcome was observed in 13% of the treated group and 21% of the control group. Complete recovery was noted in 12% and 28%, respectively. None of these differences were statistically significant. The same proportion of patients had hemorrhagic infarcts before treatment (50% and 48%, respectively). No patient experienced symptomatic hemorrhage. Therefore, this trial was overall negative, and it seems difficult to attribute this difference to the method of anticoagulant administration. It is worth noting, however, that 13% of treated patients had intracranial hypertension, compared to 28% in the control group, with these patients traditionally having a good prognosis. [7] A meta- analysis of these studies [6] showed a benefit of anticoagulant treatment on the patient's vital and functional prognosis, even if it is modest (and not statistically significant). The risk of severe hemorrhage within a hemorrhagic venous infarct is low. Another Two randomized therapeutic trials have evaluated the benefit/risk ratio of anticoagulant treatment versus placebo in patients with proven CVT. The first trial, a monocentric study [8], using intravenous, weight-adjusted unfractionated heparin, was stopped after the inclusion of 20 patients due to a statistically significant benefit in favor of heparin. At three months, eight out of ten patients in the treated group had fully recovered, and two had moderate deficits. In contrast, in the control group, three patients had died, six had moderate deficits, and only one had fully recovered.
Before starting treatment, three patient in treated group and two in the control group had hemorrhagic infarcts on the CT scan. No symptomatic hemorrhage was observed in the heparin-treated group; whereas three patients experienced hemorrhage in the control group, (two of these patients did not have an initial hemorrhage). While these results are encouraging, the study has been subject to some methodological criticisms [9], as with a delayed initiation of treatment after the onset of symptoms (on average, one month). In practice, it is justified to propose anticoagulant treatment for all patients with confirmed CVT, including in the case of hemorrhagic infarct, provided there are no contraindications to this treatment, which aligns with our approach of administering therapeutic-dose subcutaneous enoxaparin to our patient despite the presence of small hemorrhagic infarcts on the MRI. Patients with protein C deficiency who suffer from deep vein thrombosis (DVT) or acute pulmonary embolism (PE) can be managed similarly to those without this deficiency, using direct oral anticoagulants (DOACs) or parenteral anticoagulation/warfarin therapy. However, since warfarin systematically lowers protein C levels, it will further reduce these levels in patients already deficient in this protein; potentially creating a thrombotic environment that can lead to warfarin- induced skin necrosis. Therefore, before discontinuing the initial treatment, low molecular weight heparin (LMWH), unfractionated heparin (UFH), or fondaparinux should overlap with warfarin therapy for at least 5 days and until the international normalized ratio (INR) is equal to or greater than 2.0 for at least two consecutive days. Warfarin should not be started in patients with protein C deficiency without first ensuring they are protected by a rapid-acting anticoagulant such as LMWH, UFH, or fondaparinux. Initial high doses of warfarin should be avoided [10,11], this aligns with our approach, which involved delaying the transition to acenocoumarol (Sintrom) until the 8th day of treatment with enoxaparin. The purpose of this delayed transition, as opposed to an early switch, is to minimize the prothrombotic risk initially induced by acenocoumarol which is a vitamin K antagonist similar to warfarin by reducing vitamin K-dependent coagulation inhibitors (proteins C and S), especially given that our patient has a protein C deficiency. The anticoagulation should last for at least 6 months and possibly long-term in many patients with protein C deficiency [11].
Additionally, for patients with postpartum cerebral thrombophlebitis, the treatment may also include addressing the specific complications related to cerebral thrombophlebitis, such as intracranial pressure management and neurological care Including medical treatment (such as corticosteroids, mannitol, acetazolamide) and/or the performance of subtractive lumbar punctures. Antiepileptic treatment, which is systematic in cases of epileptic seizures, may be considered prophylactically in cases of severe edema or hemorrhagic infarction, as was the case of our patient. [1] Overall, the management of postpartum cerebral thrombophlebitis revealing protein C deficiency requires a comprehensive approach that addresses both the underlying protein C deficiency and the specific complications associated with thrombosis. The clinical course and prognosis of cerebral venous thrombosis (CVT) are unpredictable on an individual basis. Indeed, some patients may initially be in a coma and survive without sequelae, while others may present with minor symptoms that worsen and result in significant lasting deficits. The mortality rate in developed countries is approximately 10 to 20% [12-14], compared to 30 to 50% in the 1960s. Among survivors, the functional prognosis is better than in arterial ischemia, with 10 to 20% of patients retaining sequelae [5] (epilepsy, focal deficit, optic atrophy). Several poor prognostic factors have been identified, including specific thrombosis locations (deep veins, cerebellar veins, and isolated cortical thrombosis), the nature of the underlying condition, the presence of focal signs, coma, age at onset (either in children or the elderly), the presence of hemorrhagic infarction or the delta sign on a CT scan, association with pulmonary embolism, or the rate of thrombosis progression. Homozygous or double heterozygous protein C deficiency represents a severe thromboembolic disorder. It has a poor prognosis, typically presenting within hours of birth with purpura fulminans and extensive thromboses in large vessels [4]. In contrast, isolated heterozygous deficiency has a better prognosis, with thrombotic manifestations that may be sporadic, limited, and often occurring in adulthood [1]. Before ISCVT, only 6 prospective studies examined the long-term outcome after CVT. [15] These studies were based on a single Center or single country and had modest sample sizes. These features limit their statistical power and generalizability. Results from these series concerning death and disability were contradictory, perhaps related to referral bias. Total death rate at the end of follow-up in the aforementioned studies ranged from 0% [15]. to 39%. The death/dependence rate at the end of follow-up varied from 9% [16] to 44%, [15] while in ISCVT only 13% of the patients either had died or were dependent at the end of follow-up. Deaths during follow-up were as frequent as acute deaths but were predominantly related to underlying diseases, such as malignancies. Very few patients remained dependent. This finding contrasts with the outcome from arterial stroke types, in which the proportion of permanently dependent patients ranges between one third and two thirds of the survivors. Long-term prognostic factors were analysed by multivariate methods in 3 previous studies. ISCVT confirmed coma, cerebral hemorrhage, and malignancy as important prognostic factors for death or dependence [17]. In addition, ISCVT identified male sex, age >37 years, mental status disorder, thrombosis of the deep cerebral venous system, and CNS infection as variables that increase the risk of death or dependence. Seizures (10%) and new thrombotic events (4%) were the most frequent complications during follow-up. Recurrence of CVT and severe visual loss were exceptional but severe and potentially preventable occurrences. Except for spontaneous abortions, other complications rarely occurred during or after new pregnancies. These findings strongly support the evidence that past CVT (including puerperal CVT) is not a contraindication to pregnancy [17]. ISCVT results have implications for clinical practice concerning the investigation, treatment, and prognosis of patients with CVT. patients with CVT usually have multiple risk factors. Therefore, the identification of 1 risk factor (eg, contraceptives, infection) should not stop the search for additional risk factors, in particular inherited or acquired thrombophilia. More than 80% of the patients were treated with anticoagulants, indicating a consensus among most participants on the efficacy and safety of anticoagulation in the acute phase of CVT [17]. The ISCVT has identified easily available variables that predict an unfavourable outcome. Patients with these characteristics deserve additional close monitoring, and some may be candidates for more aggressive interventions, such as local thrombolysis and reduction of intracranial pressure.
Conclusions
Protein C deficiency is a rare genetic disorder that predisposes individuals to abnormal blood clot formation, leading to various clinical manifestations such as recurrent thrombosis, ischemic stroke, and thrombotic events during pregnancy. The deficiency of active protein C disrupts the coagulation system, resulting in an increased risk of clot production. This case report sheds light on the challenging nature of protein C deficiency, as evidenced by the diverse set of thrombotic symptoms observed in the patient, including cerebral thrombophlebitis. The findings underscore the critical importance of recognizing and addressing protein C deficiency in clinical practice, particularly in cases with atypical and multiorgan thrombotic presentations. Furthermore, this report emphasizes the need for further research to enhance our understanding of the clinical implications and management strategies for protein C deficiency, especially in the context of pregnancy and post-partum period. This case report and literature review highlight the complex nature of protein C deficiency and its clinical implications, underscoring the importance of early recognition and management of this rare disorder to mitigate the risk of thrombotic complications. Future studies should focus on elucidating the underlying mechanisms of thrombosis in protein C deficiency and developing tailored therapeutic approaches to improve patient outcomes.
Disclosures
Human Subjects: Consent was obtained or waived by all participants in this study. The ethics committee of the Hassan II Military Hospital in Marrakech, Morocco issued approval 124578. The ethical committee has agreed this work for publication.
Conflicts of Interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: that they have no conflicts of interest
Payment/Services Info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial Relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other Relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Participants Consent: All participants have given their explicit consent for the publication of personal data concerning themselves and their colleagues as part of this study. They understand that this data may include information that could identify them in the context of the research findings. They have been informed about the purpose of this publication, the type of data that will be disclosed, and the potential implications. They acknowledge that this information will be publicly accessible after publication. They confirm that their consent is voluntary and that they have the right to withdraw it at any time prior to publication.
Authors Contributions: All authors contributed to the production of this article. They also declare that they have read and approved the final version of this manuscript.
References
- Afghani R, Gharib H, Kor F, Kharazam P (2021) Protein C deficiency: Report of a challenging case with recurrent multiorgan thrombosis. Int J Surg Case Rep. 86:106361-10.
- Bajko Z, Motataianu A, Stoian A, Barcutean L, Andone S, et al (2021) Gender Differences in Risk Factor Profile and Clinical Characteristics in 89 Consecutive Cases of Cerebral Venous Thrombosis. J Clin Med. 10: 1382.
- Mukhtar B, Garg R, Ibrahim G, Batra J (2023) Investigating protein C and S levels in pregnant women with recurrent early pregnancy loss versus normal pregnancy. J Med Life. 16:160-166.
- Kroiss S, Albisetti M (2010) Use of human protein C concentrates in the treatment of patients with severe congenital protein C deficiency. Biologics. 24:51-60.
- Jain S, Bhushan M, Talwar V (2024) Post-partum cerebral venous sinus thrombosis: A case report. Qatar Med J. 11:2024.
- De Bruijn SF, Stam J (1999) Randomized, placebo-controlled trial of anticoagulant treatment with low-molecular- weight heparin for cerebral sinus thrombosis. Stroke. 30:484-8.
- Bousser MG (1999) Cerebral venous thrombosis: nothing, heparin, or local thrombolysis? . Stroke. 30:481-3.
- Einhäupl KM, Villringer A, Meister W, Mehraein S, Garner C, et al (1991) Heparin treatment in sinus venous thrombosis . Lancet. 8767:958-1679154.
- Easton, E. J. (1993) Treatment of cerebral venous thrombosis. 3:329-332.
- James AH, Bates SM, Bauer KA, Branch W, Mann K, et al (2017) Management of hereditary antithrombin deficiency in pregnancy. Thromb Res. 157:41-45.
- Lipe B, Ornstein DL: (2011) Deficiencies of natural anticoagulants, protein C, protein S, and antithrombin. Circulation. 14:365-8.
- Rondepierre P, Hamon M, Leys D, Leclerc X, Vehier FM, et al (1995) Thromboses veineuses cérébrales: étude de l'évolution [Cerebral venous thromboses: study of the course]. Rev Neurol (Paris. 151:100-4.
- Dey S, Biswas SC, Gangopadhyay G (2021) "Study of Clinical and Etiological Profile and Outcome of Cerebral Venous Thrombosis in Pregnancy and Puerperium in a Tertiary Care Hospital in Eastern India,". IJOPARB; Indian Journal of Perinatology and Reproductive Biology 11:2021-2249.
- Zöller B, Svensson PJ, Dahlbäck B, Lind-Hallden C, Hallden C, et al (2020) Genetic risk factors for venous thromboembolism. Expert Rev Hematol. 13:971-981.
- Cakmak S, Derex L, Berruyer M, Nighoghossian N, Philippeau F, et al (2003) Cerebral venous thrombosis: clinical outcome and systematic screening of prothrombotic factors. Neurology. 8:1175-8.
- Ferro JM, Lopes MG, Rosas MJ, Ferro MA, Fontes J, et al (2002) Long-term prognosis of cerebral vein and dural sinus thrombosis. results of the VENOPORT study. Cerebrovasc Dis. 2002:272-8.
- Ferro JM, Canhão P, Stam J, Bousser M, Berinagarrementeria F, et al (2004) Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 35:664-70.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.