Protection against Spread of Cancer in Breast Cancer and Melanoma using Aqueous Zinc Chloride to Kill Residual Malignant Tissue at the Time of Surgery
Norman A. Brooks*
Skin Cancer Medical Center in Encino, California, USA
*Corresponding author: Norman A. Brooks, Skin Cancer Medical Center in Encino, California, USA
Received Date: 11 April 2023
Accepted Date: 15 April 2023
Published Date: 17 April 2023
Citation: Brooks NA (2023) Protection against Spread of Cancer in Breast Cancer and Melanoma using Aqueous Zinc Chloride to Kill Residual Malignant Tissue at the Time of Surgery. Ann Case Report 8: 1264. DOI: https://doi.org/10.29011/2574-7754.101264
Abstract
Zinc chloride is a locally applied anti-cancer medicine that deeply penetrates and kills tissue, used since 1835 suspended in inactive paste vehicles to treat breast and skin cancers. The paste vehicle has become known as black salve. The U.S. Food & Drug Administration recognizes zinc chloride not suspended in paste to be a generally safe substance, and has approved aqueous zinc chloride in low concentration for intravenous use. The treatment of an advanced melanoma excision wound and a breast cancer lumpectomy wound with aqueous zinc chloride protects against the potential spread of cancer at the time of surgery. Surgery opens blood vessels and reduces cancer immunity. Case reports of 103 consecutive advanced melanomas treated with zinc chloride combined with surgery show a significant and statistical (p=0.003) improvement in cure rates over conventional surgery of melanomas. Cases are presented showing remarkable healing of locally advanced breast cancer tumors treated with zinc chloride. In breast cancer lumpectomy only a narrow rim of tissue is removed around the tumor with the inability to remove all the malignant tissue. Radiation has become the standard of treatment, and is used to kill residual malignant tissue that remains in the patient. Further research of a weak concentration of aqueous zinc chloride, the active ingredient of the black salve, is indicated as an alternative to radiation in breast cancer surgery. Radiation in breast cancer lumpectomy is time consuming, costly, and can cause severe radiotherapy related adverse events, including rare but serious second cancers, lung, and heart damage.
Keywords: Zinc chloride; Breast cancer; Melanoma; Lumpectomy; Radiation; Black salve
Introduction
History of Black Salve- Self Treatment, Skin Cancer, Melanoma and Breast Cancer
Black salve or zinc chloride, referring to the main ingredient of the salve, was first reported in 1835 for the treatment of breast cancers and skin cancers. The black salve is known as Mother Nature’s scalpel. It can be applied to the skin and can cure warts, tags, moles, skin cancers, and even early melanomas. The black salve containing zinc chloride can be purchased online for self- treatment, but is risky to use. The salve has been used by both practitioners of alternative medicine and doctors of conventional medicine. Two figures stand out in the use of the zinc chloride black salve, Harry Hoxsey and Frederic Mohs, MD.
Harry Hoxsey was born poor, worked in coal mines as a youth, then became an insurance salesman, and had no formal medical training, but with the black salve went on to build the world’s largest cancer clinic at the time in 1955, in Dallas Texas, employing 100 nurses, technicians, and 7 conventional medical doctors. There were also 17 other branches of the Hoxsey clinics distributed throughout the United States. Hoxsey advertised and had in person events to show his cancer cures. Figure 1 shows an event where 32,000 people were reportedly in attendance. The patient shown in Figure 1 had a cancer of the skull which had been resistant to treatments with radiation and surgery,but was cured by Hoxsey using the zinc chloride black salve.
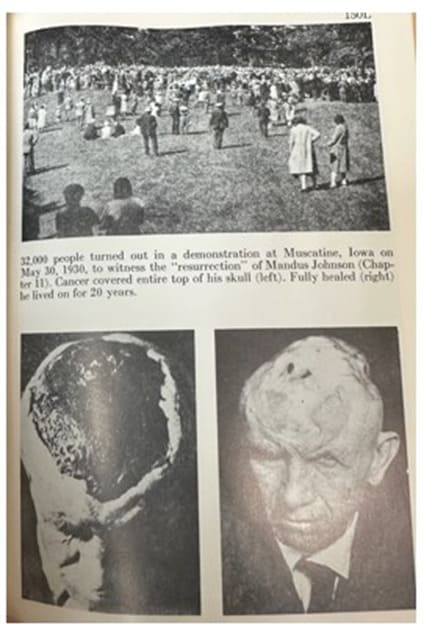
Figure 1: Zinc chloride cured this cancer, which was resistant to previous surgery and radiation. Copyright ©1956 Harry M. Hoxsey: Milestone Books, Inc.
Frederic Mohs, MD started practicing with the black salve containing zinc chloride in the 1930s, inventing a new surgery which combined standard surgery with zinc chloride. Discovering that zinc chloride not only deeply penetrated and killed tissue, but also microscopically preserved the tissue as if it had been processed in a pathology lab [1], he announced to the medical community that his new surgery combined with zinc chloride could cure cancers previously considered incurable. Mohs became an overnight success treating advanced skin cancers, advanced and recurrent melanomas, and recurrent breast cancers. Figure 2 shows a photograph taken in 1992 in Berlin, Germany presenting a poster on a statistical analysis of Mohs melanoma data. The analysis showed a 53% improved cure rate for the clinically incurable melanomas referred to Mohs for treatment with zinc chloride compared to conventional surgery.
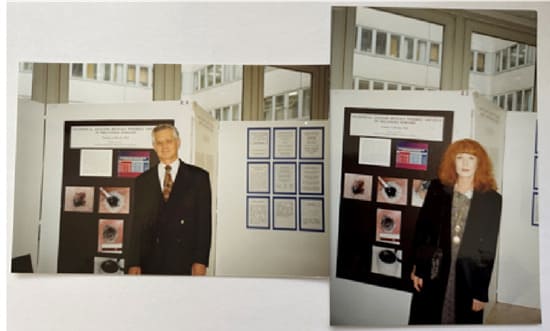
Figure 2: This figure is a photograph of a poster presented in Berlin, Germany in 1992 of a statistical analysis of Mohs melanoma data. The analysis showed a 53% improved cure rate for the clinically incurable melanomas treated with zinc chloride compared to conventional surgery
Figure 3 shows a melanoma under the great toenail and indicates the mechanism of action of zinc chloride in treating a melanoma. Such melanomas are aggressive and dangerous with a low cure rate even with toe amputation. The middle photograph shows the treatment site with zinc chloride black salve applied. Animal experiments have shown that a killed and preserved melanoma by zinc chloride makes the melanoma antigenic and stimulates immunity against further growth or spread in the body [2].
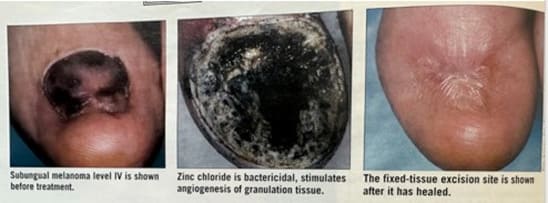
Figure 3: The middle photograph shows the site of the zinc chloride black salve treatment which stimulates immunity against further growth or spread of the melanoma. The caption in the third photograph refers to this as a fixed-tissue excision.
Lastly, self-treatment with black salve purchased online is risky. A survey of 340 adult patients in 2016 who purchased black salve for self-treatment showed that 60% were satisfied or very satisfied with the results [3]. There is serious risk in self-treatment with zinc chloride. Unscrupulous vendors online often falsely claim the black salve is cancer specific and destroys only cancerous tissue. The cartilage of the nose and the eye are particularly sensitive to black salve, and zinc chloride should never be allowed to get in the eye.
Materials and Methods
The Action of Zinc Chloride is to Protect against Perioperative Tumor Growth
“It’s very rare for surgery to cause cancer to spread” – American Cancer Society, October 2, 2019. An extensive literature review disputes this statement. Surgery has an inability to remove all microscopic cancer around the edges of a malignant tumor leaving tumor cells behind in the patient that can spread. The immune system also becomes compromised during surgery allowing cancer cells too much more easily spread further in the body.
The phenomenon of spread of cancer at the time of surgery has become known as perioperative tumor growth. Both breast cancer and melanoma are tumors that exist outside of the body cavity, and, in both breast cancer and advanced melanoma, molecular staging studies indicate that occult cancer may be prevalent at the surgical margins regardless of the size of a cancer excision [4,5]. In lumpectomy for breast cancer only a narrow rim is removed around the tumor, and radiation has become the standard of treatment to kill remaining malignant tissue after lumpectomy, but has radiation hazards.
In perioperative tumor growth there is increased circulating cancer cells in the blood stream and decreased immunity to the tumor occurring at the time of surgery [6-20]. Surgical excision opens blood vessels and allows malignant cells to enter the blood stream. The decrease in tumor immunity is related to the sudden surgical removal of the primary tumorous mass. The preserving of malignant tissue by zinc chloride has been shown in animal experiments to stimulate anti-tumor immunity [2].
The literature search also shows that perioperative chemotherapy has protected against perioperative tumor growth in breast cancer surgery. In perioperative chemotherapy one short course of chemotherapy is given immediately systemically after surgery. Perioperative chemotherapy has achieved a significant improvement in breast cancer survival, but this treatment has been all but abandoned for reasons of concern over possible systemic toxicity [6,21-25]. Thus, a lumpectomy excision wound is a treatable microscopic malignant wound with microscopic cancer cells surrounding the edges of the tumor excision. Treatments have been with systemic perioperative chemotherapy which has improved survival, but has been abandoned because of concerns of systemic toxicity, and radiation which has become the standard of treatment, but has radiation hazards.
Similarly, the excision wound of an advanced melanoma is also a microscopic malignant wound with microscopic malignant cells surrounding the excised tumorous site regardless of the size of the excision. In advanced melanoma, survival has been reported to be significantly improved over conventional surgery with zinc chloride therapy. A statistical analysis comparing survival in two historic melanoma cancer series was performed by the UCLA Department of Computational Medicine. In this analysis, a consecutive series of 103 melanoma cases treated with surgery and zinc chloride by Mohs, the inventor of Mohs surgery, and reported in his Chemosurgery textbook [1], was compared to a series of primary melanomas treated with conventional surgery. These primary melanomas treated with conventional surgery have become the foundation for the prognosis of melanoma known as Clark’s Level of Invasion that is still used today in pathology reports to indicate the prognosis of survival in melanoma.
Statistical Analysis of Melanoma Data Shows Zinc Chloride Improves Survival and Prevents Local Tumor Recurrence
The data for the 103 consecutive melanoma cases treated with zinc chloride and reported in Mohs Chemosurgery textbook was quantitatively compared for survival at the UCLA Department of Computational Medicine by the level of depth of invasion to a series of 162 primary melanoma cases treated with conventional surgery at the Massachusetts General Hospital also stratified by level of depth of invasion [1,26,27]. These cases at the Massachusetts General Hospital became the foundation for Clark’s melanoma survival by level of invasion with each deeper level having a worse prognosis [28]. In the zinc chloride series, 86 of the 103 cases had at least five years follow up. All cases in the Massachusetts General Series had at least five years follow up. The analysis showed a statistically significant 53% improvement in overall survival rate (p=0.003; HR = 0.53) for the zinc chloride treated melanomas. The 103 consecutive melanomas were advanced cases with 20% presurgical lymph node metastases and 40% recurrent tumors after previous surgery and radiation. The poor prognosis of the 20% metastatic lesions and 40% recurrent tumors was not factored into the analysis. The quantitative comparison of five-year survival by level of depth of melanoma invasion, ignoring the poor prognosis of metastases and recurrent tumors, was computed by comparing and weighting the differential rates of survival for each level of melanoma invasion in the two series (thin level II to deep level V). Surgery with zinc chloride had a five year 53% survival rate improvement vs. conventional surgery of primary non-metastatic melanomas despite 20% metastatic disease and 40% recurrent tumors.
In the Mohs zinc chloride series there were 4 five-year determinate cases of level II, with a survival rate 100%, 13 fiveyear determinate cases of level III, with a survival rate 92.3%, 14 five-year determinate cases of level IV, with survival rate 64.3%, and 55 five-year determinate cases of level V invading into the subcutaneous fat with the poorest prognosis, survival rate 32.7%.
There were 162 cases in the Clark series, all primary melanomas stratified by depth of invasion with 29 cases of level II, five-year survival rate 90.0%, 58 level III, five-year survival rate 57.0%, 59 level IV, five-year survival rate 40.7%, and 16 level V invading into the subcutaneous fat, five-year survival rate 18.8%.
In addition to the Mohs data nine physicians contributed to a melanoma registry of 179 cases between 1981 and 1991. In 64 five-year determinate cases of thin melanomas (<0.85mm) survival was improved 60%. There was a 95.7% five-year survival with zinc chloride applied to the excision wound vs. 88.9% fiveyear survival with conventional fresh-tissue excision only, hazard ratio 0.37 [29,30]. There were not enough patients in the registry to make statistical conclusions. For the thin melanomas, a sample size of 240 per group is needed. In the Mohs technique the excision wound is treated after a layer by layer removal.
Results
Mohs, the inventor of Mohs surgery, reported data in his 1978 Chemosurgery textbook for 103 consecutive cases of melanoma and 22 cases of locally advanced breast cancers treated with surgery and zinc chloride [1]. 18 of the 22 cases of locally advanced breast cancer were recurrent tumors after previous surgical mastectomy (complete removal of the breast), and significant palliative benefit and healing of the cancer wounds was achieved. Locally advanced breast cancers are often characterized by bleeding wounds, oozing, and odor. Such wounds are referred to as malignant wounds. Mohs paste containing zinc chloride has been successfully used by many others besides Mohs to treat and heal malignant wounds in locally advanced breast cancers [31-39]. Figures 4 and 5 illustrate a malignant wound with a large tumorous mass in a locally advanced breast cancer that was successfully treated with strong 50% zinc chloride with complete healing of the malignant wound. As previously discussed in Methods a lumpectomy excision wound is a malignant wound but with far less tumor burden than the tumorous mass shown in Figure 4. A lumpectomy can have remaining microscopic cancerous cells surrounding the edges of the tumor excision. A picture can be worth 1,000 words. Figure 5 shows the remarkable effectiveness of zinc chloride in healing and curing a massive malignant wound as compared to the microscopic malignant wound of a lumpectomy which would be much simpler to heal. In this case the patient was a 90-yearold woman who presented with an 8cm bleeding malignant wound extending from the nipple. The zinc chloride was used to make the malignant tumorous wound amenable to radical mastectomy which was subsequently performed. [31]
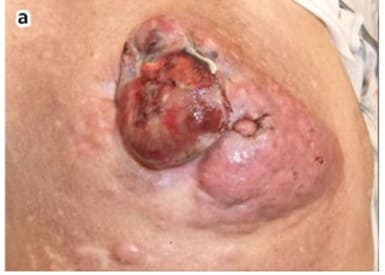
Figure 4: Malignant wound in locally advanced breast cancer. Copyright ©2012 Tsukada et al.; licensee BioMed Central Ltd
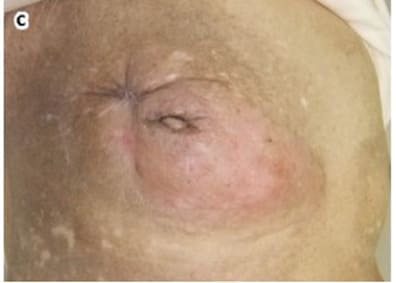
Figure 5: Healed malignant breast cancer wound. The malignant wound was healed with zinc chloride. Copyright ©2012 Tsukada et al.; licensee BioMed Central Ltd
Discussion
Zinc Chloride vs Radiation in Breast Cancer Lumpectomy
In breast cancer lumpectomy only the tumor and a narrow rim of tissue is removed around the tumor. A standard of treatment in lumpectomy is excision followed by radiation to treat malignant tissue and cancerous roots that can extend beyond the narrow margins of the excision. A trend in breast cancer surgery has been intraoperative radiation therapy. An advantage of intraoperative radiation over delayed radiation in lumpectomy is the delivery of radiation to treat perioperative tumor growth by immediately killing remaining tumorous cells around the narrow margins of a lumpectomy wound before these malignant cells have a chance to proliferate [40,41]. Intraoperative radiation is cumbersome to administer and requires lead shielding. Lead shielding is a procedure with deep dissection down to the pectoralis fascia (the deep band of tissue that covers the muscles of the chest) to accommodate the shield to protect the ribs, lung, and heart from scatter radiation. Generally, the anesthesiologist, surgeon, and radiation oncologist remain in the room during the radiation delivery either wearing lead aprons or standing behind a radiation shield. Radiation can have severe adverse effects and can damage the heart, lungs, bones, and nerves, and can induce new unrelated cancer development, including sarcomas and lung cancer.
Aqueous zinc chloride, like intraoperative radiation, treats perioperative tumor growth by immediately killing remaining tumorous cells that can exist beyond the narrow margins of a lumpectomy wound before these malignant cells have a chance to proliferate. Zinc chloride is simpler to apply than intraoperative radiation is to administer and has no radiation hazards or systemic toxicity.
The technique of applying a strong 50% aqueous zinc chloride solution for the treatment of a melanoma excision wound has been reported [42]. Experimentation with a weak 20% concentration of aqueous zinc chloride has shown that a weak concentration kills and preserves malignant tissue immediately after application. The depth of penetration of zinc chloride is determined by the concentration and the amount applied. A weak 20% concentration of aqueous zinc chloride solution is advantageous as an alternative to intraoperative radiation in lumpectomy surgery.
Research has been done on selected elderly patients with a low risk of tumor recurrence to improve lumpectomy surgery by using a breast conserving surgery without radiation. The surgical technique uses repeated slice biopsies (5 mm interval slices and 5 mm margin free). The authors stated the benefit of avoiding radiation: “radiotherapy is time-consuming, and costly, and can cause severe treatment-related adverse events.” In breast conserving surgery the excision margin may be larger than a lumpectomy but is still a narrow margin [43].
Zinc chloride, without the severe treatment-related adverse events of radiation, assists with both a lumpectomy and a breast conserving surgery to protect against perioperative tumor growth because occult malignant disease can exist beyond the narrow margins of either surgical technique. The selected population of elderly patients with a low risk of tumor recurrence in the research of breast conserving surgery to avoid radiation is the same population ideal for research of aqueous zinc chloride solution as an alternative to radiation immediately following a lumpectomy or a breast conserving surgery.
Declarations
The author declares no conflict of interest. There are no financial interests, funding, or competing interests. Photographic consents, human, and animal welfare are not applicable. The manuscript was written by the author. This paper complies with relevant ethical guidelines.
Acknowledgment
Jeffrey Gornbein, DrPH, Department of Computational Medicine, David Geffen School of Medicine at UCLA
References
- Mohs FE (1978) Chemosurgery Charles C. Thomas: Springfield. 3-29, 153, 156, 175, 181, 207-209, 217, 225-248.
- Kalish RS, Siegel DM, Brooks NA, et al. (1997) Experimental rationale for treatment of high-risk human melanoma with zinc chloride fixative paste. Dermatol Surg 23:1043-6.
- Clark JJ, Woodcock A, Cipriano SD, Hyde MA, Edwards SL, et al. (2016) Community perceptions about the use of black salve. JAAD 74: 1021-1023.
- Douglas-Joness AG, Woods V (2009) Molecular assessment of sentinel lymph node in breast cancer management. Histopathology 55: 107-13.
- Scoggins CR, Ross MI, Reintgen DS, Noyes DR, Goydos JS, et al. (2006) Prospective multi-institutional study of reverse transcriptase polymerase chain reaction for molecular staging of melanoma. J Clin Oncol 24: 2849-57.
- Tohme S, Simmons RL, Tsung A (2017) Surgery for cancer: A trigger for metastases. Cancer Res 77: 1548-1552.
- Chiarella P, Brazzo J, Meiss RP, Ruggiero RA (2012) Concomitant tumor resistance. Cancer letters 324: 133-141.
- Alieva M, van Rheenen J, Broekman MLD (2018) Potential impacts of invasive surgical procedures on primary tumor growth and metastasis. Clin Exp Metastasis 35: 319-331.
- Horowitz M, Neeman E, Sharon E, Ben-Eliyahu S (2015) Exploiting the critical perioperative period to improve long-term cancer outcomes. Nature reviews Clinical oncology 12: 213-226.
- Qadri SSA, Wang JH, Coffey JC, Alam Md, O’Donnell A, et al. Can surgery for cancer accelerate the progression of secondary tumors within residual minimal disease at both local and systemic levels? Ann Thorac Surg 80: 1046-50.
- Smolle J, Soyer HP, Smolle-Juttner FM, Rieger E, Kerl H (1997) Does surgical removal of primary melanoma trigger growth of occult metastases? An analytic epidemiological approach. Dermatol Surg 23: 1043-6.
- Pachmann K (2011) Tumor cell seeding during surgery---Possible contribution to metastasis formations. Cancers (Basel) 3: 2540-2553.
- Van der Bij GJ, Oosterling SJ, Beelen RH, Meijer S, Coffey JC, et (2009) The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Annals of surgery 249: 727-734.
- Yamaguchi K, Takagi Y, Aoki S, Futumura M, Saji S (2000) Significant detection of circulating cancer in the blood by reverse transcriptasepolymerase chain reaction during colorectal cancer resection. Annals of surgery 232: 58-65.
- Warr RP, Zebedee Z, Kenealy J, Rigby H, Kemshead JT (2002) Detection of melanoma seeding during surgical procedures--- an RTPCR based model. Eur J Surg Oncol 28: 832-7.
- Koch M, Kienle P, Hinz U, Antolvic D, Schmidt J, et al. (2005) Detection of hematogenous tumor cell dissemination predicts tumor relapse in patients undergoing surgical resection of colorectal liver metastases. Annals of surgery 241: 199-205.
- Demicheli R, Retsky MW, Hrushesky WJ, Baum M, Dukas ID, et (2008) The effects of surgery on tumor growth: a century of investigations. Annals of oncology: official journal of the European Society for Medical Oncology/ ESMO 19: 1821-1828.
- Fortner JG (1993) Inadvertent spread of cancer at surgery. J Surg Oncol 53: 191-6.
- Murthy SM, Goldschmidt RA, Rao LN, Ammirati M, Bachmann T, et al. (1989) The influence of surgical trauma on experimental metastasis. Cancer 64: 2035-2044.
- Gresham E, Don Parsa F (2020) Iatrogenic implantation of cancer cells during surgery. Hawaii J Health Soc Welf 79: 4-6.
- The Ludwig Breast Cancer Study Group (1989) Prolonged diseasefree survival after one course of perioperative adjuvant chemotherapy for node-negative breast cancer. N Engl J Med 320: 491-496.
- Van der Hage JA, Van de Velde CJ, Julien JP, Floira JL, Delozier, et al. (2001) Improved survival after one course of perioperative chemotherapy in the early breast cancer patients. Long-term results from the European Organization for Research and Treatment of Cancer (EORTC) Trial 10854. European journal of cancer 37: 2184
- Nissen-Meyer R, Kjellgren K, Malmio K, Mansson B, Norin T, et al. (1978) Surgical adjuvant chemotherapy: results with one short course with cyclophosphamide after mastectomy for breast cancer. Cancer 41: 2088-2098.
- Sertoli MR, Bruzzi P, Pronzato O, Queirolo P, Amoroso D, et al. (1995) Randomized cooperative study of perioperative chemotherapy in breast cancer. J Clin Oncol 13: 2712-2721.
- Coffey JC, Smith MJF, Wang JH, Bouchier-Hayes D, Cotter TG, et al. (2006) Cancer surgery: risks and opportunities. BioEssays: news and reviews in molecular, cellular and developmental biology 28: 433-437.
- Brooks NA (1992) Fixed-tissue micrographic surgery in the treatment of cutaneous melanoma. An overlooked cancer treatment strategy. J Dermatol Surg Oncol 18: 999-1000.
- Mohs FE (1977) Chemosurgery for melanoma. Arch Dermatol 113: 285-91.
- Clark WH, From L, Bernardino EA, Mihm MC (1969) The histogenesis and biologic behavior of primary human malignant melanomas of the Cancer Res 29: 705-27.
- Snow SN, Mohs FE, Oriba HA, Dudley CM, Leverson G, et al. (1997) Cutaneous malignant melanoma treated by Mohs surgery: review of the treatment results of 179 cases from the Mohs melanoma registry. Dermatol Surg 23: 1055-1060.
- Brooks NA (2018) Mohs melanoma chemosurgery simplified to a single brief caustic application: possible vaccine effect. Dermatologic Surgery 44: 311-313.
- Tsukada T, Nakano T, Matoba M, Matsui D, Sasaki S, et al. (2012) Locally advanced breast cancer made amenable to radical surgery after a combination of systemic therapy and Mohs paste: two case J Med Case Rep 6: 360.
- Takeuchi M, Katsuki T, Yoshida K, Onada M, Iwamura M, et al. (2021) Successful pre-operative local control of skin invasion of breast cancer using a combination of systemic chemotherapy and Mohs paste. J Breast Cancer 24: 481-490.
- Tsubota Y, Yamamoto D, Ishizuka M, Yoshikawa K, Sueoka N, et al. (2018) A successful treatment of locally advanced breast cancer with using Mohs’ paste and chemotherapy- A case report. Gan To Kagaku Cancer & Chemotherapy 45: 725-727.
- Nishiya S, Doi M, Oikawa A, Kawaura A, Mihara K, et al. (2016) Local control of advanced breast cancer with Mohs paste and systemic Gan To Kagaku Ryoho. Cancer & Chemotherapy 43: 443-5.
- Inoue T, Nishi T, Yoshida T, Yayoi E, Sawai Y, et al. (2011) Local control of advanced breast cancer with Mohs’ paste. Gan To Kagaku Ryoho. Cancer & Chemotherapy. 38: 2008-2010.
- Hirai C, Tada M, Yanai H, Hasua H, Sakuma H, et al. (2019) Locally advanced breast cancer treated with Mohs’ paste and neoadjuvant chemotherapy-A case report. Gan To Kagaku Ryoho. Cancer & Chemotherapy 46: 2131-2133.
- Miyazawa K, Oeda Y, Yoshioka S, Shiobara M, Wakatsuki K, et al. (2012) A case of advanced breast cancer in which quality of life was improved by Mohs’ paste and hormone therapy. Gan To Kagaku Cancer & Chemotherapy. November 39: 2051-3.
- Kakimoto M, Tokita H, Okamura T, Yoshino K (2010) A chemical hemostatic technique for bleeding from malignant wounds. J Palliat Med 13: 11-13.
- Arima M, Saito K, Maeda T, Fukushima H, Iwata, et al. (2021) Clinical usefulness of a modified Mohs’ technique and topical application of zinc oxide powder for treating skin infiltration caused by unrespectable malignant tumors. Palliative Medicine Reports 2: 164-174.
- Dickler A, Ivanov O, Francescatti D (2009) Intraopertive radiation therapy in the treatment of early-stage breast cancer utilizing xoft axxent electronic brachytherapy. World J Surg Oncol 7: 24.
- Njeh C F, Saunders M W, Langton C M (2010) Accelerated Partial Breast Irradiation (APBI): A review of available techniques. Radiat Oncol 5: 90.
- Brooks NA (2020) Treatment of melanoma excision wound with 50% zinc chloride solution astringent – Mohs melanoma surgery without the J Clin Aesthet Dermatol 13: 15-16.
- Ohsumi S, Nishimura R, Masuda N, Akashi-Tanaka S, Suemasu K, et (2023) A prospective analysis of two studies that used the 5-mm interval slices and 5-mm margin-free method for ipsilateral breast tumor recurrence after breast-conserving surgery without radiotherapy. Breast Cancer 30: 131-138.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.