Prognostic Values of Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios in Patients with Gynecologic Carcinosarcomas MAGYCS Study from the French TMRG Network
by Jacobs M1*, Romeo C2, Fauvet R3, Lequesne J1, Grellard JM1, Brachet PE1, Weiswald LB1, Coquan E1, Cherifi F1, Ray-Coquard I2,4,5, Joly F4,6
1Medical Oncology Department, Centre François Baclesse, 14000 Caen, France.
2Medical Oncology Department, Centre Léon Bérard, 69008 Lyon, France.
3Gynecologic Department, CHU Caen, 14000 Caen, France.
4GINECO Group, 75008 Paris, France.
5University Claude Bernard Lyon 1, 69100 Villeurbanne, France.
6Medical Oncology Department, Centre François Baclesse, 14000 Caen, France.
*Corresponding author: Jacobs M, Medical Oncology Department, Centre François Baclesse, 14000 Caen, France.
Received Date: 15 July, 2025
Accepted Date: 28 July, 2025
Published Date: 30 July, 2025.
Citation: Jacobs M, Romeo C, Fauvet R, Lequesne J, Grellard JM, et al. (2025) Prognostic Values of Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios in Patients with Gynecologic Carcinosarcomas MAGYCS Study from the French TMRG Network. J Oncol Res Ther 10: 10297. https://doi.org/10.29011/2574-710X.10297.
Abstract
Background: Gynecological carcinosarcomas (GC) are rare but aggressive tumors. After surgery, there is no consensus on the optimal adjuvant therapy and new prognostic factors may help in decision-making. The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are robust indicators of inflammation often observed in GC. Objective: To assess the prognostic value of NLR and PLR on overall survival (OS) and progression-free survival (PFS) at 2 years from diagnosis in women with GC. Methods: Patients followed up for ovarian or uterine GC diagnosed between January 2011 and December 2018 were included in this multicenter retrospective study conducted within the French TMGR network. NRL and PLR outcomes were collected at diagnosis. Survival analysis according to PLR and NLR respectively were performed with univariate and multivariable Cox models including identified prognostic factors. Results: 148 patients were included. At diagnosis, the median age was 69 years, 30% had an ovarian and 70% a uterine primary localization, 61% had a FIGO stage III/IV. The 2-year OS rate was 55.7% and 2-year PFS was 40.2%. A relevant cut-off of 4 for NLR and 277 for PLR were defined by ROC curves. In univariate analysis, OS and PFS were significantly worse in patients with NLR>4 (p=0.0027 and p=0.017), and in patients with PLR>277 (p=0.019 and p= 0.006). In the multivariable analysis, NLR>4, and PLR>277 remained independent prognostic factors for OS in addition to age, uterine primary, and stage III/ IV. Conclusion: PLR and NLR at diagnosis are important independent prognostic factors in the management of GCs.
Keywords: Carcinosarcoma; Inflammation; Neutrophils; Lymphocytes; Platelets; Prognostic;
Introduction
Carcinosarcomas are rare malignancies (2-5% and 1-2% of endometrial and ovarian malignancies) [1]. They contain malignant sarcomatous and carcinomatous elements. They are very aggressive and carry a poor prognosis with an overall survival at 5 years ranging from 6 to 30 % [1, 2].
Various prognostic factors in carcinosarcomas have been reported such as the International Federation of Gynecology and Obstetrics (FIGO) stage, complete surgery (R0), age, histological grade (including sarcomatous component) and the use or not of adjuvant chemotherapy [3]. However, biological parameters have received little attention to date, despite a growing body of evidence demonstrating the importance of inflammation in cancer development [4].
The inflammatory response is a key factor in the initiation and development of cancer [4]. Inflammation is responsible for immunosuppression, creating an environment that promotes tumoral development [5-7].
Tumor-associated neutrophils have recently been presented as a cornerstone of tumor initiation, angiogenesis, tumor growth and the metastatic process [8, 9].
Platelets also produce several growth factors including vascular endothelial growth factor (VEGF), which plays an important role in blood vessel production and endothelial proliferation and thus promotes tumor growth and angiogenesis [10-12].
High levels of neutrophils and/or platelets at diagnosis have been found to be poor prognostic factors in gynecological carcinosarcomas [13-15]. Several studies have shown that lymphopenia is associated with a poor prognosis in many cancers [16]. However, the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) could even be stronger and more robust indicator of inflammation [17].
The NLR is a systemic inflammation index. An elevated NLR reflects a relative depletion of lymphocytes, impairing the proper functioning of the antitumor immune response and an increase in circulating neutrophils. An elevated NLR has already been identified as an indicator of poor prognosis in a variety of cancers [18-21]. In a meta-analysis compiling 26 studies with gynecological cancer patients, Ethier et al. reported a higher correlation between a high NLR (greater than 2.95) and both unfavorable overall survival (OS) and shorter event-free survival [18].
The PLR is considered as a predictor of thrombotic and inflammatory states and is therefore an interesting biomarker of inflammation. An elevated PLR has been recognized as an indicator of poor prognosis in various cancers [22]. A correlation was found between high PLR and OS and progression-free survival (PFS) in ovarian and cervical cancers [22].
The present study assessed the prognostic value of NLR and PLR on OS and PFS in patients with the rare and aggressive gynecologic carcinosarcomas subtype in the French national gynecological rare tumor network (TMRG).
Methods
This study is an ancillary study of the national MAGYCS study” Therapeutic Challenges in Patients with Gynecologic Carcinosarcomas: Analysis of a Multicenter National Cohort Study from the French Prospective TMRG Network “, coordinated by the Léon Bérard Center in Lyon and recently published [2].
The French TMRG network is a national prospective network allowing a systematic double pathology review by an expert in gynecological malignancies and providing multidisciplinary expert advice.
The MAGYC multicenter cohort was set up within the network. The MAGYCS study included 425 patients with a gynecological carcinosarcoma diagnosed between January 2011 and December 2018 in 12 centers belonging to the TMRG. It highlighted the positive impact of adjuvant chemotherapy on survival in all localized stages (including FIGO IA uterine carcinosarcomas) [2].
In the present ancillary study, we included patients from the MAGYCS study whom a pre-treatment blood cell count was reported in the database.
Demographic data (age at diagnosis, personal history, comorbidities, family history of cancer), tumor characteristics (date of initial diagnosis, histopathology types of the first sample, FIGO stage), and treatments (initial surgery, chemotherapeutic agents, radiotherapy, vaginal brachytherapy) were collected from the medical records.
Blood cell count at diagnosis was also collected, including neutrophil, lymphocyte and platelet values. The NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count, and the PLR was defined as the absolute platelet count divided by the absolute lymphocyte count.
The primary outcome was the 2-year OS rate, which was defined as the time from the date of diagnosis to the date of cancer-specific death, with alive patients censored at the date of the latest news.
The secondary endpoint was 2-year PFS, which was defined as the time from the date of diagnosis to the first event of interest, defined as relapse, progressive tumor or death from any cause, and censored at the date of the latest news for event-free patients.
Statistical Analysis
The statistical analysis was conducted in 148 patients who had a baseline blood count.
Demographic data were summarized using descriptive statistics. Survival curves with associated log-rank tests were generated using the Kaplan Meier method. A p value lower than 5% was considered statistically significant for all tests.
Univariate and multivariable Cox proportional hazards models were performed to identify potential prognostic factors. Only sufficiently informative variables (less than 10% of missing data) with p < 0.10 on the univariate analysis were included in the multivariable model. Distinct models were computed to assess separately the prognostic value of NLR and PLR on survival outcomes. Multivariable models were adjusted for location (ovarian and uterine), owing to the heterogeneity of these populations in terms of characteristics and prognosis.
All statistical analyses were performed using R software (version 4.1.2).
Since no standard cut-off values have been reported for gynecological carcinosarcomas, optimal cut-offs of NLR and PLR were defined by receiver operating characteristic curve (ROC) analysis as the values which simultaneously maximize sensitivity and specificity in the prediction of death 2 years after diagnosis. Accordingly, patients were divided into “high” and “low” groups according to their initial NLR and PLR.
Ethics
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, the Good Clinical Practices of the International Conference on Harmonization (ICH-E6, 17/07/96), the applicable regulatory requirements and in accordance with the French General Data Protection Regulation (GPDR).
Data was collected from hospital databases, after requesting for the consent of the patients still alive by sending them an information note and an opposition form.
Results
Patient Characteristics
A total of 425 patients were included in the MAGYCS study. Biological analysis with blood counts was collected for 148 patients, which constituted the cohort of this ancillary study.
Clinical and biological characteristics are outlined in Tables 1 and S1 and were similar to those of the MAGYCS cohort [2].
|
Criteria |
Total |
NLR >4 |
NLR<4 |
p |
PLR>277 |
PLR<277 |
p |
|
n = 148 (%) |
n = 69 |
n = 79 |
n = 48 |
n = 100 |
|||
|
|
(%) |
(%) |
|
|
|||
|
Age (median) |
69 |
69 |
69 |
0.67 |
66 |
69.5 |
0.037 |
|
Localization |
n = 148 |
n = 69 |
n = 79 |
0.002 |
n = 48 |
n = 100 |
<0.001 |
|
Ovarian |
45 (30) |
30 (44) |
15 (19) |
|
24 (50) |
21 (21) |
|
|
Uterine |
103 (70) |
39 (56) |
64 (81) |
|
24 (50) |
79 (79) |
|
|
History of cancer |
n = 145 |
n = 67 |
n = 78 |
1 |
n = 47 |
n = 98 |
1 |
|
34 (23) |
16 (24) |
18 (23) |
11 (23) |
23 (24) |
|||
|
Tamoxifen exposure |
n = 145 |
n = 67 |
N = 78 |
0.66 |
n = 47 |
n = 98 |
0.33 |
|
5 (3) |
3 (5) |
2(3) |
3 (6) |
2 (2) |
|||
|
History of pelvic irradiation |
n = 146 |
n = 67 |
N = 79 |
0.37 |
n = 47 |
n = 99 |
1 |
|
5 (3) |
1 (2) |
4 (5) |
1 (2) |
4 (4) |
|||
|
Hormone replacement therapy |
n = 120 |
n = 56 |
n = 64 |
|
n = 40 |
n = 80 |
0.77 |
|
15 (13) |
5 (9) |
10 (16) |
0.41 |
4 (10) |
11 (13.8) |
||
|
Stage |
n = 145 |
n = 68 |
n = 77 |
< 0.001 |
n =47 |
n = 98 |
<0.001 |
|
I |
47 (33) |
11 (16) |
36 (47) |
|
5 (11) |
42 (43) |
|
|
II |
10 (7) |
2 (3) |
8 (10) |
|
2 (4) |
8 (8) |
|
|
III |
63 (43) |
37 (54) |
26 (34) |
|
29 (62) |
34 (35) |
|
|
IV |
25 (17) |
18 (27) |
7 (9) |
|
11 (23) |
14 (14) |
|
|
Majority contingent |
n = 94 |
n = 44 |
n = 50 |
0.24 |
n = 27 |
n = 67 |
0.034 |
|
50/50 |
3 (3) |
3 (7) |
0 |
|
3 (11) |
0 |
|
|
Epithelial |
54 (57) |
24 (54) |
30 (60) |
|
15 (56) |
39 (58) |
|
|
Sarcomatous |
37 (40) |
17 (39) |
20 (40) |
|
9 (33) |
28 (42) |
|
|
Sarcomatous type |
n = 118 |
n = 55 |
n = 66 |
0.87 |
n = 36 |
n =82 |
0.68 |
|
Homologous |
41 (35) |
19 (36) |
22 (33) |
|
14 (39) |
27 (33) |
|
|
Heterologous |
77 (65) |
33 (64) |
44 (67) |
|
22 (61) |
55 (67) |
|
|
Epithelial type |
n = 99 |
n = 47 |
n = 52 |
0.066 |
n = 28 |
n = 71 |
0.003 |
|
Serous |
45 (45) |
24 (51) |
21 (40) |
|
15 (53) |
30 (42) |
|
|
Clear cell |
3 (3) |
3 (7) |
0 |
|
3 (11) |
0 |
|
|
Endometrioid |
33 (33) |
10 (21) |
23 (44) |
|
5 (18) |
28 (39) |
|
|
Mucinous |
1 (1) |
1 (2) |
0 |
|
1 (4) |
0 |
|
|
Undifferenciated |
2 (2) |
1 (2) |
1 (2) |
|
1 (4) |
1 (1) |
|
|
Other |
14 (15) |
8 (17) |
7 (13) |
|
3 (11) |
12 (17) |
|
Table 1: Clinical Characteristics.
Median age at diagnosis was 69 [63-76] years. Forty-five patients (30%) were followed for ovarian carcinosarcoma and 103 (70%) for uterine carcinosarcoma. 40% of patients were stages I-II, 43% were stage III and only 17% were stage IV. The epithelial component was predominant in 57% of tumors and the sarcomatous contingents in 40%. Among sarcomatous tumors, 35% had homologous and 65% heterologous components. Most patients underwent surgery (n = 129, 87%) with R0 resection (surgery was considered as macroscopically complete) in 87% of patients (S2). One hundred and three patients received initial chemotherapy with a complete response in 60% of patients (S2). Eighty-eight patients (62%) relapsed after primary treatment (S2).
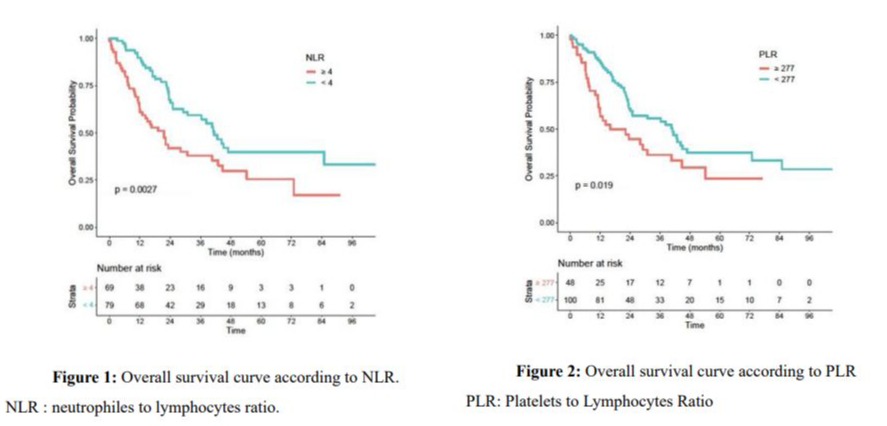
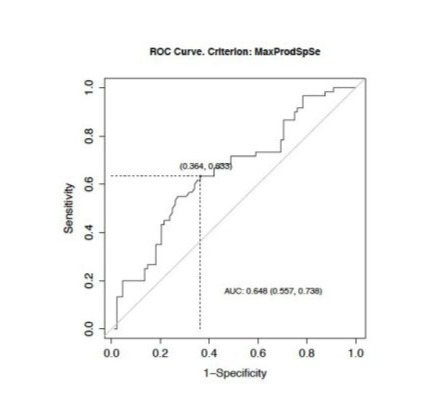
An NLR value higher than 4 better predicted the death rate 2 years after diagnosis, with a sensitivity of 63.3%, specificity of 63.6% and area under the curve of 0.648 (S3). A PLR value higher than 277 was better predicted the death rate 2 years after diagnosis, with a sensitivity of 43.3%, specificity of 73.9% and area under the curve of 0.551 (S4).
Patients were significantly younger in the high PLR group (66 years versus 69.5 p = 0.037, see Table 1). There were significantly more patients with ovarian carcinosarcomas in the high NLR and PLR patient groups (respectively p = 0.002, p < 0.001). There were significantly more patients with advanced stages in both the high NLR and PLR patient groups (p < 0.001).
Overall Survival
Median OS was 30.9 months (95% CI: 23.4-44.4). The 2-year OS rate for patients was 55.7% (95% CI: 0.478-0.649). The median OS was 43 months (95% CI: 29.2-not reached) for ovarian carcinosarcomas and 25 months (95% CI: 22.5-41.4) for uterine carcinosarcomas (p = 0.12).
In the univariate analysis, patients with an NLR > 4 and with a PLR > 277 had significantly worse OS, HR = 1.93 (95% CI: 1.252.94) (p = 0.003) and HR = 1.70 (95% CI: 1.09-2.63) (p = 0.02), respectively (Figure 1 and 2). The median OS in the high NLR group was 21.6 months versus 41.4 months in the low NLR group. The median OS in the high PLR group was 16.1 months versus 41 months in the low PLR group.
Patients with a platelet-to-lymphocyte ratio > 277 had significantly worse overall survival, hazard ratio = 1.70 (95% CI: 1.09-2.63) (p = 0.02)
Patients with an neutrophil-to-lymphocyte ratio > 4 had significantly worse overall survival, hazard ratio = 1.93 (95% CI: 1.25-2.94) (p = 0.003)
Age > 69 years (p = 0.02), majority of sarcomatous contingent (p < 0.001), stage III/IV disease (p = 0.01), and incomplete response after chemotherapy (p < 0.001) were also poor prognostic factors for OS (Table 2).
|
Variable |
n |
overall survival HR (95%IC) |
p |
Progression free disease HR (95%IC) |
p |
|
Age |
148 |
||||
|
Age < 69 |
71 |
reference |
reference |
||
|
Age > 69 |
77 |
1.7 (1.09-2.63) |
0.02 |
1.03 (0.68-1.57) |
0.88 |
|
Localization |
148 |
||||
|
Ovarian |
45 |
reference |
reference |
||
|
Uterine |
103 |
1.50 (0.90-2.50) |
0.12 |
1.05 (0.67-1.65) |
0.84 |
|
Majority Contingent |
145 |
||||
|
Epithelial |
54 |
reference |
reference |
||
|
Sarcomatous |
37 |
2.37 (1.34-4.21) |
<0.001 |
2.07 (1.23-3.48) |
0.01 |
|
Stage |
145 |
||||
|
I-II |
57 |
Reference |
reference |
||
|
III-IV |
88 |
1.88 (1.17-3.0) |
0.01 |
2.07 (1,31-3,26) |
0.001 |
|
Resection quality |
102 |
||||
|
R0 (complete surgery) |
89 |
reference |
reference |
||
|
R+ |
13 |
1.83 (0.88-3.80) |
0.1 |
2.16 (1.12-4.18) |
0.02 |
|
Chemotherapy response |
80 |
||||
|
Complete |
48 |
reference |
reference |
||
|
Partial or progression |
32 |
8.25 (4.15-16.38) |
<0.001 |
5.91 (3.33-10.48) |
<0.001 |
|
Hemoglobin |
148 |
|
|
||
|
>12 |
90 |
reference |
|
reference |
|
|
<12 |
58 |
1.51 (0.97-2.33) |
0.06 |
1.32 (0.87-2.02) |
0.19 |
|
NLR |
148 |
|
|
||
|
NLR < 4 |
79 |
reference |
|
reference |
|
|
NLR> 4 |
69 |
1.93 (1.25-2.94) |
0.003 |
1.66 (1.09-2.5) |
0.02 |
|
PLR |
148 |
|
|
||
|
PLR < 277 |
100 |
reference |
|
reference |
|
Table 2: Univariate analysis of prognostic factors of overall anf progression-free survival.
After adjustment for age, FIGO stage and location of the primary, high NLR and PLR were still associated with worse OS, with respectively HR=1.91 (95% CI: 1.16-3.13) (p = 0.01) and HR=2.24 (95% CI: 1.30-3.85) (p = 0.004) (Table 3).
|
Variable |
n (%) |
Overall Survival HR (95%IC) |
p |
Progression Free Disease HR (95%IC) |
p |
|
Age |
|||||
|
Age < 69 |
71 |
reference |
reference |
||
|
Age > 69 |
77 |
1.77 (1.11-2.78) |
0,02 |
1.10 (0.71-1.69) |
0.68 |
|
Localization |
|||||
|
Ovarian |
45 |
reference |
reference |
||
|
Uterine |
103 |
2.07 (1.17-3.64) |
0,01 |
1.43 (0.88-2.34) |
0.15 |
|
Stage |
|||||
|
I-II |
57 |
reference |
reference |
||
|
III-IV |
88 |
1,99(1.17-3.380) |
0,01 |
1.99 (1.19-3.36) |
0.01 |
|
NLR |
148 |
|
|
|
|
|
NLR < 4 |
79 |
reference |
|
reference |
|
|
NLR> 4 |
69 |
1.91 (1.16-3.13) |
0,01 |
1.43 (0.88-2.27) |
0.14 |
Table 3A: Prognostic factors in multivariate analysis taking NLR into account.
|
Variable |
n (%) |
Overall survival HR (95%IC) |
p |
Progression Free Disease HR (95%IC) |
p |
|
Age |
|||||
|
Age < 69 |
71 |
reference |
reference |
||
|
Age > 69 |
77 |
2.15 (1.33-3.45) |
0.002 |
1.21 (0.77-1.92) |
0.4 |
|
Localization |
|||||
|
Ovarian |
45 |
reference |
reference |
||
|
Uterine |
103 |
2.37 (1.32-4.26) |
0.004 |
1.65 (0.98-2.79) |
0.06 |
|
Stage |
|||||
|
I-II |
57 |
reference |
reference |
||
|
III-IV |
88 |
2.18 (1.31-3.65) |
0.003 |
2.06 (1.25-3.39) |
0.004 |
|
PLR |
148 |
|
|
|
|
|
PLR <277 |
100 |
reference |
|
reference |
|
|
PLR >277 |
48 |
2.24 (1.30-3.85) |
0.004 |
1.73 (1.20-2.94) |
0.04 |
Table 3B: Prognostic factors in multivariate analysis taking PLR into account.
Progression Free Survival
Median PFS was 16.4 months (95% CI: 13.5 – 23.7). The 2-year PFS rate was 40.2%. Median PFS was 20.9 months (95% CI: 15.134) for ovarian carcinosarcomas and 14.8 (95% CI: 12.3-23.3) months for uterine carcinosarcomas (p = 0.84).
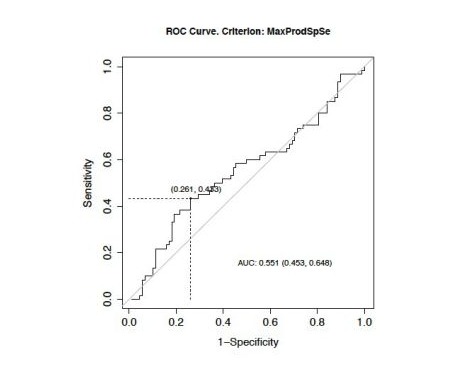
In univariate analysis, patients with an NLR > 4 and a PLR > 277 had significantly worse PFS, with HR = 1.66 (95% CI: 0.400.92) (p=0.017) and HR =1.81 (95% CI: 0.36-0.85) (p = 0.01) respectively (Figures S5 and S6). Median PFS in the high NLR group was 13.5 months versus 23.3 months in the low NLR group. Median PFS in the high PLR group was 12.8 months versus 21.6 months in the low PLR group.
Sarcomatous contingent, FIGO stage, surgical resection, and response to chemotherapy were also poor prognostic factors for PFS (Table 2).
After adjustment for age, stage and location of the primary, only high PLR was significantly associated with worse PFS, with HR=1.73 (1.20-2.94) (p = 0.04), in addition to stage (Table 3).
Prognostics Scores
Two prognostics scores were constructed, one for NLR and one for PLR, according to the presence of one or more independant predictive factors of poor survival identified in multivariable analysis, namely : age > 69 years, uterine primary, stage 3/4, and NLR > 4 or PLR > 277 (Figures S7 and S8). Patients were in the good prognosis group if they had at most 1 risk factor, in the intermediate prognosis group if they had 2 risk factors and in the poor prognosis group if they had 3 or 4 risk factors. As defined, patients in the good NLR prognosis group did not reached median overall survival, whereas median overall survival was of 41 months in the intermediate NLR prognosis group and 14.2 months in the poor NLR prognosis group. Similarly, median overall survival was 85 months in the good PLR prognosis group, 41 months in the intermediate PLR prognosis group and 12.9 months in the poor PLR prognosis group.
Discussion
We found that high NLR and PLR are strong independent prognostic factors in patients with gynecological carcinosarcomas.
Since gynecological carcinosarcomas are rare, data on their prognostic factors are sparse. Furthermore, they are most often extrapolated from endometrial or ovarian epithelial tumors or from studies with a low level of evidence. To our knowledge, there are very few studies evaluating the PLR and NLR as prognostic factors specifically in gynecological carcinosarcomas.
Findings concerning median age, stage at diagnosis, and histological components are consistent with data already published, [23-27]. Thus, our series of gynecological carcinosarcomas is similar to previous ones. The median OS of 30.9 months (95% CI: 23.4 – 44.4) and median PFS of 16.4 (95% CI: 13.5 – 23.7) observed in this study are consistent with the total MAGYCS series reported by Romeo et al. They observed 37.1 months of OS for ovarian location, 30.6 months of OS for uterine location, 15.1 of PFS for ovarian location, and 14.8 of PFS for uterine location [2].
The finding that high NLR and high PLR as independent poor prognostic factors for OS (with HR = 1.91 (95% CI: 1.16-3.13) and HR = 2.24 (95% CI: 1.30-3.85), respectively), is consistent with those in the literature for endometrial and ovarian cancer although our HR was high compared to those in studies of endometrial or ovarian epithelial tumors 18, [28-32]. This is probably due the aggressiveness and inflammatory environment of carcinosarcoma compared to other histologies.
Sakurai et al., study of uterine carcinosracoma and uterine sarcoma, did not find that high NLR or PLR were prognostic factors for overall survival (p = 0.361 and 0.103, respectively) [33].
NLR was a prognostic factor for PFS in univariate analysis, but not in multivariable analysis. Like us, Sakurai et al. found that high NLR was not an independent prognostic factor for PFS (p= 0.361) in patients followed for uterine carcinosracoma and uterine sarcoma[33]. However, other authors found that high NLR was an independent prognostic factor for PFS in patients followed for endometrial cancer[18,28]. Data on the administration of corticosteroids, or the presence of an infection during biological workup were not available, so this may have influenced the neutrophil counts, and led to a bias.
However, we found that high PLR was an independent prognostic factor for PFS (with HR = 1.73 (1.20 – 2.94)). This result is consistent with those previously reported in endometrial and ovarian epithelial cancer [28], and in uterine carcinosarcoma and uterine sarcoma [33].
The PLR seems to be a stronger prognostic marker than the NLR and thrombocytosis as reported in various studies in ovarian cancer patients [34, 35]. The preoperative PLR was also greater than other inflammatory markers such as fibrinogen, C-reactive protein, and albumin level in ovarian cancer [35].
The PLR also appeared to be a stronger prognostic marker with higher HRs than the NLR. Since infections, autoimmune diseases, and the use of corticosteroids can increase the neutrophil count, this could bias the NLR but would less affect the PLR.
Age, a predominantly sarcomatous contingent, advanced stage, and incomplete response after treatment were also prognostic factors in univariate analysis, thus confirming results of other published studies.
There is still non consensus regarding NLR and PLR cut-offs used to predict patient outcomes. Reported cut-offs to predict survival ranged from 150 to 300 for PLR and from 2 to 5 for NLR, [29, 33, 36]. Some of the cut-offs were based on normal laboratory values; others were based on the ROC curve, and others still were based on median values from preliminary studies. Further studies are needed to establish the most consensual cut-offs. However, in general, the higher the NLR or PLR is, the worse the prognosis.
To our knowledge, this is one of the few studies to evaluate the relationship between PLR and NLR and survival in patients with gynecologic carcinosarcoma. Our results can be used in routine clinical practice as a biomarker for disease prognosis. While surgery is widely recognized for its role in treating gynecological carcinosarcomas, the impact of optimal chemotherapy is less certain and there is still no consensus on the impact of adjuvant treatment. Intraoperative and postoperative pathological findings are usually used for decision-making. In the early stages, i.e. uterine carcinosarcoma FIGO stage IA, adjuvant chemotherapy seems to improve both PFS and OS as demonstrated in the global MAGYCS series[2]. Our results may reinforce the indication of adjuvant chemotherapy for this group of patients with high NLR/ PLR. Two prognostic scores have been constructed but need to be validated in a larger prospective population to help us in the therapeutic management of patients followed for gynecological carcinosarcoma.
The main limitation of our study is its retrospective design, which may have led to missing data, as we had neither the general status of the patients, nor other inflammatory markers such as CRP, and fibrinogen level. In addition, blood counts at diagnosis were missing in a group of patients from the MAGYCS cohort and could not be included in our analysis.
In conclusion, the NLR and PLR are strong independent prognostic factors in patients with gynecologic carcinosarcomas. The prognostic value of the PLR seems to be stronger than that of the NLR. These findings can help to guide the adjuvant treatment of patients with the rare and aggressive subtype of gynecological carcinosarcomas particularly in the localized stage.
Declarations
Acknowledgments : Many thanks to Dr Alison Johnson for her assistance in English.
Ethics approval ans consent to participate : The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, the Good Clinical Practices of the International Conference on Harmonization (ICH-E6, 17/07/96), the applicable regulatory requirements and in accordance with the French General Data Protection Regulation (GPDR).
Conflict of interest : The authors declare that there are no financial or personal relationships with other people or organizations that could inappropriately influence the work reported or the conclusions, implications, or opinions stated.
Supplementary Material
|
Criteria |
Total |
NLR >4 |
NLR<4 |
PLR>277 |
PLR<277 |
|
n = 148 |
n = 69 |
n = 79 |
n = 48 |
n= 100 |
|
|
Leukocyte (G/L) |
8.84 |
10.65 |
7.24 |
10.59 |
8.02 |
|
Lymphocytes (G/L) |
1.52 |
1.16 |
1.83 |
1.03 |
1.75 |
|
Neutrophils (G/L) |
6.47 |
8.59 |
4.62 |
8.48 |
5.51 |
|
Eosinophiles (G/L) |
0.15 |
0.12 |
0.18 |
0.14 |
0.15 |
|
Basophiles (G/L) |
0.05 |
0.04 |
0.06 |
0.04 |
0.05 |
|
Monocytes (G/L) |
0.6 |
0.68 |
0.53 |
0.7 |
0.55 |
|
Hemoglobin (g/dL) |
12.12 |
11.29 |
12.84 |
10.72 |
12.79 |
|
Platelets(G/L) |
334.97 |
371.32 |
303.23 |
459.38 |
275.26 |
|
NLR ratio |
5.89 |
9.59 |
2.66 |
11.14 |
3.38 |
|
PLR ratio |
277.9 |
392.52 |
177.8 |
507.58 |
167.66 |
S1: Biological characteristics (mean values).
|
Criteria |
Total |
|
n (%) |
|
|
Initial Surgery |
129 (87) |
|
Resection quality |
|
|
R0 |
89 (87) |
|
R1 |
7 (7) |
|
R2 |
5 (5) |
|
R3 |
1 (1) |
|
Initial chemotherapy |
103 (70) |
|
Response to chemotherapy |
|
|
Complete |
48 (60) |
|
Partial |
14 (18) |
|
Stable |
2 (3) |
|
Progression |
16 (20) |
|
Adjuvant Radiotherapy |
58 (39) |
|
Response to radiotherapy |
|
|
Complete |
37 (86) |
|
Partial |
2 (5) |
|
Stable |
1 (2) |
|
Progression |
3 (7) |
|
Brachytherapy |
41 (28) |
|
Relapse |
88 (62) |
S2: Treatments characteristics.
S3: ROC curve to define NLR cut-off.
AUC : Area Under Curve.
S4: ROC curve to define PLR cut-off.
AUC : Area Under Curve
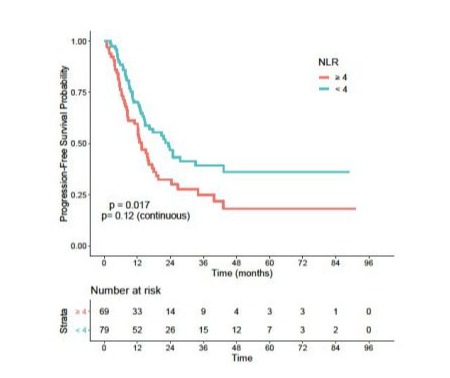
S5: Progression-free survival according to NLR.
NLR: Neutrophiles To Lymphocytes Ratio.
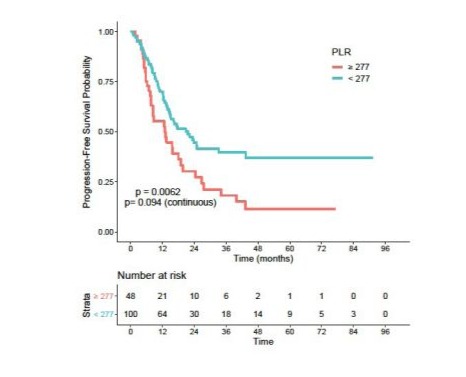
S6: Progression-free survival according to PLR
PLR: Platelets To Lymphocytes Ratio.
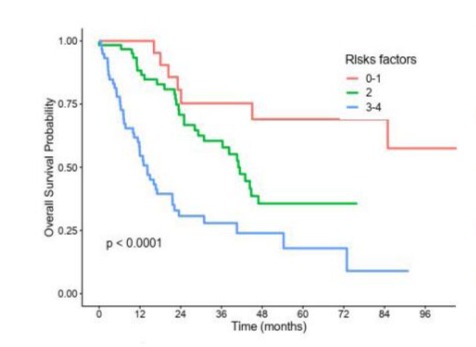
S7: Pronostic score according to NLR.
NLR: Neutrophiles To Lymphocytes Ratio.
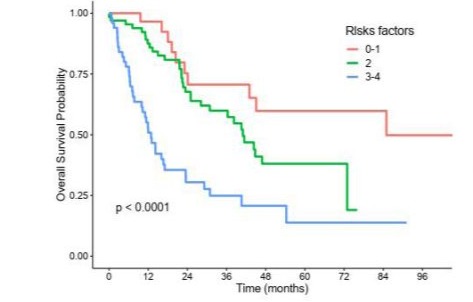
S8: Pronostic score according to PLR.
PLR: Platelets to Lymphocytes Ratio.
References
- Berton-Rigaud D, Devouassoux-Shisheboran M, Ledermann JA, Leitao MM, Powell MA, et al. (2014) Gynecologic Cancer InterGroup (GCIG) consensus review for uterine and ovarian carcinosarcoma. International journal of gynecological cancer 24:S55-60.
- Romeo C, Saux OL, Jacobs M, Joly F, Ferron G, et al. (2022) Therapeutic Challenges in Patients with Gynecologic Carcinosarcomas: Analysis of a Multicenter National Cohort Study from the French Prospective TMRG Network. Cancers 14:354.
- Garg G, Shah JP, Kumar S, Bryant CS, Munkarah A, et al. (2010) Ovarian and uterine carcinosarcomas: a comparative analysis of prognostic variables and survival outcomes. International journal of gynecological cancer 20:888-94.
- Mantovani A, Romero P, Palucka AK, Marincola FM (2008) Tumour immunity: effector response to tumour and role of the microenvironment. Lancet 371:771-83.
- Nicolas-Ávila JA, Adrover JM, Hidalgo A (2017) Neutrophils in Homeostasis, Immunity, and Cancer. Immunity 46:15-28.
- Todoric J, Antonucci L, Karin M (2016) Targeting Inflammation in Cancer Prevention and Therapy. Cancer prevention research 9:895905.
- Schetter AJ, Heeaard NHH, Harris CC (2010) Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis 31:37-49.
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, et al. (2009) Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer cell 16:183-94.
- Liang W, Ferrara N (2016) The Complex Role of Neutrophils in Tumor Angiogenesis and Metastasis. Cancer immunology research 4:83-91.
- Fridman WH, Galon J, Pagès F, Tartour E, Sautès-Fridman C, et al. (2011) Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer research 71:5601-5.
- Franco AT, Corken A, Ware J (2015) Platelets at the interface of thrombosis, inflammation, and cancer. Blood 126:582-8.
- Labelle M, Begum S, Hynes RO (2011) Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer cell 20:576-90
- HS Kim, KH Han, HH Chung, Kim JW, Park NH, et al. (2010) Neutrophil to lymphocyte ratio for preoperative diagnosis of uterine sarcomas: a case-matched comparison. European journal of surgical oncology 36:691-8.
- Arend R, Arsdale AV, Gojayev A, Roane BM, Doo D, et al. (2019) Neutrophilia and mortality in women with uterine carcinosarcoma. International journal of gynecological cancer 29:1258-1263.
- Menczer J, Elyashiv O, Ben-Shem E, Peled O, Levy T (2018) The Association of an Elevated Thrombocyte Count with Clinicopathological Prognostic Factors and Survival in Patients with Uterine Carcinosarcoma. The Israel Medical Association journal 20:213-216.
- Disis ML (2010) Immune regulation of cancer. Journal of clinical oncology 28.
- Wang L, Jia J, Lin L, Guo J, Ye X, et al. (2017) Predictive value of hematological markers of systemic inflammation for managing cervical cancer. Oncotarget 8:44824-44832.
- Ethier JL, Desautels DN, Templeton AJ, Oza A, Amir E, et al. (2017) Is the neutrophil-to-lymphocyte ratio prognostic of survival outcomes in gynecologic cancers? A systematic review and meta-analysis. Gynecologic oncology 145:584-594.
- Ceran MU, Tasdemir U, Colak E, Güngör T (2019) Can complete blood count inflammatory parameters in epithelial ovarian cancer contribute to prognosis? - a survival analysis. Journal of ovarian research 12:16.
- Li Z, Hong N, Robertson M, Wang C, Jiang G (2017) Preoperative red cell distribution width and neutrophil-to-lymphocyte ratio predict survival in patients with epithelial ovarian cancer. Scientific reports 7:43001.
- Ittiamornlert P, Ruengkhachorn I (2019) Neutrophil-lymphocyte ratio as a predictor of oncologic outcomes in stage IVB, persistent, or recurrent cervical cancer patients treated by chemotherapy. BMC cancer 19:51.
- Jiang S, Liu J, Chen X, et al. (2019) Platelet-lymphocyte ratio as a potential prognostic factor in gynecologic cancers: a meta-analysis. Archives of gynecology and obstetrics 300:829-839.
- Barnholtz-Sloan JS, Morris R, Malone JM, Munkarah AR (2004) Survival of women diagnosed with malignant, mixed mullerian tumors of the ovary (OMMMT). Gynecologic oncology 93:506-512.
- Rauh-Hain JA, Growdon WB, Rodriguez N, et al. (2011) Carcinosarcoma of the ovary: a case-control study. Gynecologic oncology 121:477-481.
- Harris MA, Delap LM, Sengupta PS, et al. (2003) Carcinosarcoma of the ovary. British journal of cancer 88:654-657.
- Saijo M, Nakamura K, Ida N, et al. (2019) Histologic Appearance and Immunohistochemistry of DNA Mismatch Repair Protein and p53 in Endometrial Carcinosarcoma: Impact on Prognosis and Insights Into Tumorigenesis. The American journal of surgical pathology 43:14931500.
- Artioli G, Waberisch J, Ludwig K, Gardiman MP, Borgato L, et al. (2015) Rare uterine cancer: carcinosarcomas. Review from histology to treatment. Critical reviews in oncology/hematology 94:98-104.
- Ni l, Tao j, Xu J, et al. (2020) Prognostic values of pretreatment neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in endometrial cancer: a systematic review and meta-analysis. Archives of gynecology and obstetrics 301:251-261.
- Haruma T, Nakamura K, Nishida T, et al. (2015) Pre-treatment neutrophil to lymphocyte ratio is a predictor of prognosis in endometrial cancer. Anticancer research 35:337-343.
- Takahashi R, Mabuchi S, Kawano M, et al. (2015) Prognostic significance of systemic neutrophil and leukocyte alterations in surgically treated endometrial cancer patients: a monoinstitutional study. Gynecologic oncology 137:112-118.
- Zhao Z, Zhao X, Lu J, Xue K, Liu P, et al. (2018) Prognostic roles of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in ovarian cancer: a meta-analysis of retrospective studies. Archives of gynecology and obstetrics 297:849-857.
- Asher V, Lee J, Innamaa A, Bali A (2011) Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clinical & translational oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico 13:499-503.
- Sakurai A, Yamaguchi K, Ishida K, et al. (2025) Prognostic significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and monocyte-to-lymphocyte ratio in uterine carcinosarcoma. International Journal of Clinical Oncology 30:570-583.
- Raungkaewmanee S, Tangjtgamol S, Manusirivithaya S, Srijaipracharoen S, Thavaramara T (2012) Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. Journal of gynecologic oncology 23:265-273.
- Zhang WW, Liu KJ, Hu GL, Liang WJ (2015) Preoperative platelet/ lymphocyte ratio is a superior prognostic factor compared to other systemic inflammatory response markers in ovarian cancer patients. Tumour biology 36:8831-7.
- Templeton AJ, McNamara MG, Šeruga B, et al. (2014) Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Journal of the National Cancer Institute 106:dju124.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.