Primary Cardiac Lymphoma: A Rare Case of Classical Hodgkin Lymphoma
by Cristina Muzi1*, Vittorio Ruggero Zilioli1, Erika Meli1, Emanuele Ravano1, Erika Ravelli1, Silvia Ferrari1, Laura Bandiera2, Emma Gay3, Emanuela Bonoldi2, Roberto Cairoli1
1Division of Hematology, ASST Grande Ospedale Metropolitano Niguarda, Milano, Italy
2Pathology Unit, Hematology & Oncology Department, Niguarda Cancer Center ASST Grande Ospedale Metropolitano Niguarda, Milano, Italy
3Department of Nuclear Medicine, ASST Grande Ospedale Metropolitano Niguarda, Milano, Italy
*Corresponding Author: Cristina Muzi, Division of Hematology, ASST Grande Ospedale Metropolitano Niguarda, Milano Piazza Ospedale Maggiore 3, 20162, Milano, Italy
Received Date: 07 February 2025
Accepted Date: 11 February 2026
Published Date: 13 February 2026
Citation: Muzi C, Zilioli VR, Meli E, Ravano E, Ravelli E, et al. (2026). Primary Cardiac Lymphoma: A Rare Case of Classical Hodgkin Lymphoma. Ann Case Report. 11: 2529. https://doi.org/10.29011/2574-7754.102529
Abstract
Background: Primary cardiac lymphoma (PCL) is a rare extranodal non Hodgkin Lymphoma (NHL), characterized by a primary lesion occuring in the heart and/or in the pericardium. The majority of PCLs are non-Hodgkin diffuse large B cell lymphoma (DLBCL), even if other NHL histologies have been described. Patients with PCL experience heterogeneous clinical signs or symptoms (i.e. dyspnea, thoracic pain, electrocardiographic (EKG) abnormalities, and heart failure), often-delaying correct diagnosis and treatment. The prognosis of PCLs is poor and median overall survival is less than 12 months. We report a case of particular histology for a PCL, a classical Hodgkin Lymphoma.
Case Report: In June 2021, a 62-year-old woman experienced rapid onset dyspnea and edema of legs. Echocardiogram and Cardiac Magnetic Resonance showed a reduction of LVEF (25%) with an obstruction in right ventricle, and then a Positron Emission Tomography showed an abnormal uptake in the heart. A mediastinal biopsy wasper formed with a diagnosis of classical HL, Ann Arbor IIEA stage. A systematic approach with a pre-phase and a subsequent sequential chemotherapy administration was chosen for the first cycle of chemotherapy, then from the second cycle standard AVD was administered. During the therapy, the patient was closely monitoring for risk of arrhytmia or heart failure. The EoT PET-CTshowed a complete remission of cHL and the Ecochardiogram a progressive recovery of EF.
Keywords: Hodgkin Lymphoma; Primary Cardiac Lymphoma; Heart Failure.
Introduction
Primary cardiac lymphoma (PCL) is a rare extranodal non Hodgkin Lymphoma (NHL), characterized by a primary lesion occuring in the heart and/or in the pericardium, being right atrium and right ventricle the most frequent localizations [1]. The most common PCLs histological-type is Diffuse Large B-Cell Lymphoma (DLBCL), while other lymphoma histologies are less frequently observed [2].
We herein report a case of classical Hodgkin-PCL, an entity that at our knowledge has not been previously described in literature, who presented with a cardiac mass in the right ventricular chamber and multiple mediastinal lymphnodes.
Case Presentation
In June 2021 a 62 years-old woman was referred to our hospital with a heart failure (NYHA class 2-3). Her medical history included: a previous breast cancer treated with surgery, hormonal therapy and radiotherapy in October 2020; paroxysmal atrial fibrillation from 2011, a mitral annuloplastic in 2000 and an initial cardiac disfunction from 2015 with an Left Ventricular Ejection Fraction values (LVEF) of 52%. In June 2021 the patient experienced rapid onset dyspnea and edema of legs. An echocardiogram showed a reduction of LVEF (25%) with an obstruction in right ventricle. A Computerized Tomography (CT) scan and a Cardiac Magnetic Resonance (CMR) revelead the presence of pathologic tissue in the anterolateral part of right ventricule (7 cm x 3 cm) causing pulmonary trunk obstruction. The CT and CMR scans also described a right atrial dilation and multiple mediastinal lymphnodes. A Positron Emission Tomography (PET)-CT scan confirmed an abnormal 18-FluoroDeoxyGlucose (FDG)-uptake localized at the right ventricule and mediastinal lymphonodes. A mediastinal biopsy of a para-aortic lymphonode was performed and the histological examination report described a few Hodgkin and Reed-Sternberg (HRS) cells in an inflammatory background; HRS cells tested positive for PAX5, CD30 and CD15 and tested negative for CD20 (Figure 1). The final diagnosis was classical HL, Ann Arbor IIEA stage.
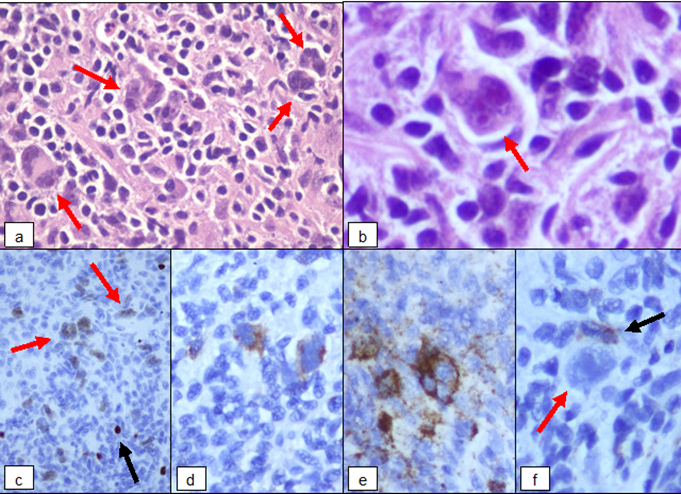
Figure 1: (a) Hodgkin and Reed-Sternberg cells in polymorphic inflammatory background. (b) Binucleated RS diagnostic cell with eosinophilic nucleoli. (c) The characteristic weak positivity for PAX5 in neoplastic cells (red arrows) despite the strong expression on reactive B-lymphocytes (black arrow). (d) The typical selective membrane and perinuclear dot-like staining for CD30. (e) Expression of CD15 observed in diagnostic cells is analogous to CD30. (f) Negativity of CD20 in neoplastic cells (red arrow) and positive control on reactive B-lymphocytes (black arrow).
The widely adopted standard of care for HL is ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) chemotherapy. However, taking into account the cardiac localization and the severe LVEF reduction, a step-by-step approach was chosen with a first pre-phase and a subsequent sequential chemotherapy administration. The pre-phase treatment consisted of prednisone administration at the dose of 50 mg daily from day -5 to day -1. The chemotherapy treatment was delivered as follows: Vinblastine 10 mg on day 1, Doxorubicin 25 mg on day 3, Dacarbazine 5, while Bleomycine was omitted.
The patient underwent strict cardiologic controls during both the steroid prephase, before, and after each chemotherapy administration: in particular, a continuous multi-parameter monitoring, sequential echocardiograms and laboratory test were performed in order to early detect a cardiac damage. The patient clinical conditions rapidly improved after chemotherapy, with a substantial reduction of dyspnea. After 14 days, the first cycle was completed with a second infusion of AVD (cycle 1, day 15) in single day. The patient continued to receive AVD treatment in an inpatient setting up to cycle 2. A PET-CT scan performed after 2 cycles showed complete response (DS3), and echocardiography showed an initial resolution of cardiac involvement. For persistent ECG abnormalities with ventricular ectopic beats, after 2 cycle of therapy a subcutaneous ICD was implanted.
After ICD implant, the patient continued AVD therapy in an outpatient setting completing all the planned 6 cycles.
During the therapy, we observed a progressive increase of LVEF (42%). The echocardiography showed a progressive normalization of ventricle thickness. The EoT PET-CT confirmed the complete remission. At present, after 5 years of follow-up, the patient is still in CR and no other relevant cardiac events have been observed.
Discussion
cHL is a lymphoproliferative B disease characterized by the presence of supradiaphragmatic And/or less frequently subdiaphragmatic lymphoadenopaties, often with a bulky disease. Extranodal HL involvement is unfrequent, being noted in 15%-30% of HL cases 3 and the most common extranodal sites are liver, lungs and bones5. No cases of primary cardiac Hodgkin lymphoma in adult have been reported so far in literature. There is no standard therapy for primary cardiac (both non-Hodgkin and Hodgkin) lymphoma. Chemotherapy represents the mainstay for PCL treatment; anthracycline- based chemotherapy combined to Rituximab (as R-CHOP) is the most common used regimen4 in NHL in particular in patients with a normal LVEF5. Nevertheless, treatment-related mortality is high (10% reported by Petrich et al.) mainly due to severe infections and fatal arrhythmia (Rolla et al. 6).
In our patient in order to prevent fatal cardiac events, we administered steroid, then we started the first cycle of AVD with a sequential infusion of chemotherapy, monitoring cardiac function and clinical parameters. As a result, chemotherapy was never delayed, and patient improved her clinical conditions [3-6].
In conclusion, cardiac involvement of Hodgkin disease is extremely rare, nevertheless should be considered in the differential diagnosis of cardiac lymphoma.
Financial Disclosure Statement: The authors have no financial disclosures, conflicts of interest, or relevant product affiliations to declare.
References
- Burke A, Tavora F. (2016). The 2015 WHO classification of tumors of the heart and pericardium. Journal of Thoracic Oncology 11: 441-452.
- Jonavicius K, Salcius K, Meskaukas R, Valeviciene N, Tarutis V, et al. (2015). Primary cardiac lymphoma: Two cases and a review of literature. Journal of Cardiothoracic Surgery 10.
- Li ZM, Zhu YJ, Xia Y, Huang JJ, Jiang WQ. (2012). Clinical characteristics of the patients with Hodgkin's lymphoma involving extranodal sites. Chinese Journal of Cancer 31: 342-347.
- Petrich A, Cho SI, Billet H. (2011). Primary cardiac lymphoma. An analysis of presentation, treatment, and outcome patterns. Cancer 117: 581-589.
- Sultan I, Aranda-Michel E, Habertheuer A, Kilic A, Arnaoutakis G, et al. (2020). Long-term outcomes of primary cardiac lymphoma. Circulation 142: 2194-2195.
- Rolla G, Bertero MT, Pastena G, Tartaglia N, Corradi F, et al. (2002). Primary lymphoma of the heart. A case report and review of the literature. Leukemia Research 26: 117-120.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.