Preventive Oral Iron Supplementation with Sucrosomial® Iron to Maintain Hemoglobin Level During Chemotherapy in Cancer Patients: A Prospective Observational Study
by Fausto Petrelli1, Raffaele Ardito2, Veronica Lonati1, Sandro Barni1*
1Medical Oncology ASST BG Ovest Treviglio Hospital, Treviglio (BG), Italy.
2Medical Oncology CROB, Rionero in Vulture (PZ), Italy.
*Corresponding author: Barni S, Medical Oncology ASST BG Ovest Treviglio Hospital, Treviglio (BG), Italy.
Received Date: 24 September, 2025
Accepted Date: 01 October, 2025
Published Date: 06 October, 2025
Citation: Petrelli F, Ardito R, Lonati V, Barni S (2025) Preventive Oral Iron Supplementation with Sucrosomial® Iron to Maintain Hemoglobin Level During Chemotherapy in Cancer Patients: A Prospective Observational Study. J Oncol Res Ther 10: 10307. https://doi.org/10.29011/2574-710X.10307
Abstract
Introduction: Anemia is common among cancer patients, often worsened by chemotherapy and the cancer itself. This leads to fatigue, weakness, and reduced physical functioning, which can limit patients’ ability to continue treatment. Conventional iron therapies are critical for managing anemia, but oral iron supplements often cause gastrointestinal side effects, reducing patient compliance. Intravenous iron, though effective, carries risks like infusion reactions and requires hospital visits. Sucrosomial® Iron (SI) provides an alternative approach, encapsulating iron in a phospholipid plus sucrose esters of fatty acids matrix (sucrosome) for better absorption and scarce gastrointestinal side effects. This study evaluates the efficacy and tolerability of SI in cancer patients undergoing chemotherapy with mild iron deficiency anemia (IDA), aiming to maintain hemoglobin (Hb) levels and improve iron status without needing red blood cell transfusions or erythropoietin-stimulating agents (ESAs). Materials and Methods:This prospective, multicenter observational study enrolled 26 patients with solid tumors, hemoglobin levels between 10-12 g/dL, and transferrin saturation (TSAT) between 15-50% before starting chemotherapy. Patients received Sideral Forte® (30 mg elemental iron) daily for 12 weeks during chemotherapy. Blood samples were taken at baseline, 6 and 12 weeks. The primary outcome was hemoglobin maintenance, with secondary outcomes including iron status. Results: After 12 weeks, hemoglobin levels remained stable, with ferritin increasing by 49% and TSAT by 58%. No patients required transfusions or ESAs. SI was well-tolerated with minor chemotherapy-related side effects. Conclusions: SI is an effective and well-tolerated option for managing mild IDA in cancer patients, improving iron absorption and reducing the need for transfusions or ESAs, with consequent improvement of quality of life.
Key words: Oral Iron; Anemia Prevention; Chemotherapy; Safety;
Introduction
Iron Deficiency Anemia (IDA) is a common condition among cancer patients, often caused by the cancer itself or as a side effect of treatments such as chemotherapy. Characterized by a reduction in red blood cells and hemoglobin (Hb), IDA leads to symptoms like fatigue, weakness, and shortness of breath, which significantly affect patients’ quality of life and their capacity to undergo cancer treatment. Various factors, including malignancy, chemotherapy, radiation therapy, and nutritional deficiencies, can cause cancer-related IDA. More than just a minor inconvenience, IDA can intensify cancer symptoms, decrease patients’ physical functioning, and potentially worsen outcomes by limiting the effectiveness of cancer treatments [1].
Traditionally, iron supplementation has been a fundamental aspect of IDA management. However, conventional iron supplements are often linked to gastrointestinal side effects and limited absorption, which challenge effective treatment [2]. The introduction of Sucrosomial® Iron (SI) supplementation marks a potentially revolutionary advancement in managing cancer-related IDA.
While traditional iron therapies provide benefits, they also have notable drawbacks. Oral iron supplementation frequently causes gastrointestinal discomfort, constipation, and nausea, leading to poor patient compliance. Intravenous iron formulations, although effective at circumventing gastrointestinal issues, pose their own challenges, including the risk of infusion reactions and the requirement for hospital visits for administration. Otherwise, severe IDA requires transfusions with related or matched donors to stabilize hemoglobin levels and alleviate symptoms [3].
Sucrosomial® Iron introduces an innovative delivery system for iron supplementation, aiming to overcome the limitations of traditional oral iron treatments. SI technology is represented by ferric pyrophosphate conveyed into a phospholipid plus sucrose esters of fatty acids matrix known as sucrosome, allowing for direct absorption of iron into the bloodstream from the intestine. A representative figure of the Sucrosomial® Iron structure was shown in Figure 1.
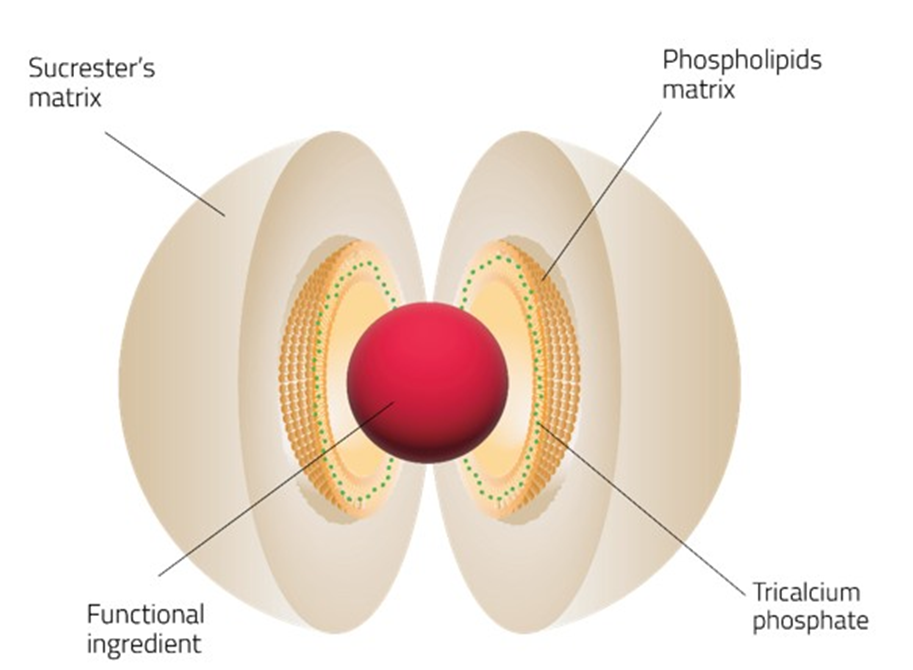
Figure 1: Graphical representation of Sucrosomial® Iron structure.
By bypassing the typical absorption pathway, which is often impaired in patients with cancer-related anemia, SI significantly reduces the gastrointestinal side effects associated with oral iron supplements. This innovative method of delivery may provide a potential alternative for populations with chronic conditions such as kidney disease or cancer, who often suffer from IDA and may have limited treatment options due to side effects or absorption issues [4].
SI supplementation in cancer patients demonstrated to improve hemoglobin level if administrated with ESA, ensuring tolerability and adherence to treatment [5]. This can significantly impact anemia management, enabling patients to sustain their strength and continue their cancer treatment regimens.
Limited reports using intravenous or oral iron before starting chemotherapy showed significant improvement of hemoglobin levels and reduction of blood transfusions in cancer patients. Our research investigated prospectively the efficacy of SI supplementation in cancer patients before starting chemotherapy in limiting hemoglobin drop in mild (grade [G]1) cancer-related anemia.
Material and Methods
Study Design and Patient Population
This prospective, multicenter, academic observational study was conducted across two centers in Italy: ASST Bergamo Ovest in Treviglio (BG) and Centro di Riferimento Oncologico della Basilicata (CROB), Rionero in Vulture (PZ). The primary objective is the effectiveness of Sucrosomial® Iron (SI) in limiting hemoglobin drop in patients who had a hemoglobin (Hb) level below the upper normal limit but above 10 g/dL. This is the threshold that might typically need more aggressive interventions, such as blood transfusions or ESA [6]. The secondary objective is to assess other iron parameters, compliance, and tolerability of treatment. The inclusion criteria were cancer patients aged over 18 years with a confirmed diagnosis of solid tumors; Hb levels between 10 and 12 g/dL for women and between 10 and 13 g/ dL for men; and transferrin saturation (TSAT) between 15% and 50%. Individuals who received other oral iron, intravenous iron, erythropoiesis-stimulating agents (ESAs), blood transfusions within 30 days prior to enrollment, or who were undergoing previous CT or RT in the last 3 months were excluded. All participants were provided with written and verbal information about the study and gave their signed written informed consent. The study received approval from the local Ethics Committees and was conducted in accordance with the Declaration of Helsinki.
Treatment Plan and Assessments
The oral iron supplementation consists in taking one capsule per day containing 30 mg of elemental iron from Sucrosomial® Iron plus 70 mg of Vitamin C (Sideral Forte®, Phrmanutra Spa, Italy) for 12 weeks. At the same time as the start of supplementation, patients began chemotherapy cycles as per normal clinical practice. Patients attended three visits: at baseline (V1), at week 6 (V2), and at week 12 (V3). Blood samples were collected at each timepoint. During the first visit, demographic characteristics, medical history, and previous and current drug history were documented for each patient. A routine clinical examination, including a complete physical examination as well as blood tests and relevant laboratory analyses (measuring Hb, hematocrit, mean corpuscular volume [MCV], mean corpuscular hemoglobin [MCH], erythrocytes, ferritin, TSAT, sideremia, transferrin, vitamin B12 and folate), was performed upon enrollment.
After 6 weeks (V2) patients underwent the same blood tests and laboratory analyses, physical examination; tolerability of the study oral iron, recording all adverse events occurring during the study period and correlating or not with the experimental product, and adherence to iron supplementation. After 12 weeks (V3), the same evaluations of V2 were conducted. Red blood cell transfusions or ESAs agents were recorded during the study period (0-12 weeks).
Outcomes
The primary outcome was the maintenance of Hb over 10 g/dL during the study period.
Secondary outcomes included effects of supplementation on TSAT and ferritin levels, transfusions and ESAs requirements, adherence to study treatment and occurrence of adverse events.
Statistical Analysis
All safety assessments were performed on the full-set population, which included all included patients who received at least one dose of the study product. All effectiveness analyses were conducted on the intent-to-treat (ITT) population, consisting of patients who completed 6 weeks of treatment and underwent blood tests and laboratory analyses at week 6.
Based on both the primary objective and the secondary objectives, relying on the epidemiological data reported in the literature considered for the rationale of the study in question, considering a standard deviation (SD) of 1.5, setting a Type I error (α) of 0.05, and starting from a test power (β) of 0.9 (90%), the estimate of the sample size is a total of 95 patients.
Demographic and disease-related features were summarized descriptively using means, SD, minimum and maximum values, and absolute and relative frequencies, as appropriate. For all statistical analyses, a p-value of <0.05 was considered significant. The p-value for change from baseline was calculated using
Student’s t-test.
Results
This study included n=26 patients meeting inclusion criteria. The study was interrupted in 2020 due to the Covid-19 pandemic. After the emergency period, the study has been reactivated but it was not possible to continue enrollment due to changes in the hospital departments involved in the study. For this reason, after 3 years of reactivation, the investigators decided to stop the study without reaching the 95 planned patients.
Out of the 26 patients, n=15 had advanced-stage disease while n=11 had early-stage disease. The median age was 68 (range 46-88). Among chemotherapy regimens n=18 out of 26 were platinum-based. The most represented type of tumor was gastrointestinal (n=13) (Table 1). All patients were supplemented with Sucrosomial® Iron for a period of 12 weeks.
|
Characteristics |
Number (%) |
|
Patients |
26 |
|
Sex |
Male=13 (50%) |
|
Female=13 (50%) |
|
|
Type |
Lung=5 |
|
Breast=3 |
|
|
Gynecological=3 |
|
|
Gastrointestinal=13 |
|
|
Pancreatic=2 |
|
|
Stage |
Advanced=15 (58%) |
|
Localized=11 (42%) |
|
|
Chemotherapy |
Platinum-based=18 (69%) |
|
Non-platinum-based=8 (31%) |
|
|
Age |
Median=68 years (range 46-88) |
|
Ethnicity |
Caucasian=26 (100%) |
Table 1: Characteristics of patients included.
Efficacy Outcomes
Hemoglobin (Hb), Ferritin, Transferrin saturation and Serum iron mean values are reported in Table 2. Average changes in values and changes in percentage are also reported in Table 2. Hb mean levels were maintained at 12 weeks, without significative difference between V1 and V3 (p = 0.78). Only 4 patients out of 26 (15.38%) had a decrease in Hb levels below 10 g/dL (9.8, 9.7, 9.6 and 9 g/ dL respectively). Ferritin has a variable trend; at the end of the study (V3) an increase is observed although not significant. Transferrin saturation and Serum iron increased significatively between V1 and V3 (Table 2). Mostly important, no patients required red blood cell transfusions or ESAs.
|
Values |
Baseline (V1) |
After 6 weeks (V2) |
After 12 weeks (V3) |
Change (V3-V1) |
% Change (V3- V1) |
p (V3-V1) |
|
Hemoglobin (g/dL) |
11.07 ± 0.5 |
11.0 ± 0.4 |
11.03 ± 0.45 |
-0.04 |
-0.36% |
0.78 |
|
Ferritin (mcg/L) |
220.9 ± 50 |
401.4 ± 70 |
329.7 ± 60 |
108.8 |
49.20% |
0.28 |
|
Transferrin saturation |
12.8 ± 3.0 |
22.3 ± 4.2 |
20.2 ± 3.8 |
7.4 |
57.80% |
<0.01 |
|
Serum iron (mcg/ dL) |
39.7 ± 10 |
80 ± 15 |
79 ± 12 |
39.3 |
98.90% |
<0.01 |
Table 2: Efficacy outcomes at 6 and 12 weeks. Data showed as mean ± standard deviation.
Safety and adherence to the study treatment
Six patients reported grade 2 events, namely nausea, vomiting, and diarrhea, attributed to chemotherapy rather than to oral iron supplementation. Only one patient experienced chemotherapyrelated grade 3 nausea, not leading to the discontinuation of Sucrosomial® iron treatment.
Discussion
The relationship between cancer, iron metabolism, and IDA is complex. Cancer can disturb the balance of iron in the body, leading to both absolute and functional iron deficiency. Absolute iron deficiency occurs when there is a true lack of iron, whereas functional iron deficiency occurs when there is enough iron in the body, but it is not adequately available for Hb synthesis due to inflammatory processes [7].
Our study focuses on the pre-emptive use of oral SI in cancerassociated IDA patients who have adequate iron levels, with the aim of maintaining Hb levels above the threshold that would require red blood cells transfusions, thus enabling uninterrupted chemotherapy. In this way we can prevent the reduction of hemoglobin and limit the related symptoms such as fatigue, weakness, and impaired cognitive function, ultimately enhancing overall quality of life for patients [8].
Furthermore, by correcting iron deficiency and improving anemia, oral iron supplementation can help reduce the need for red blood cell transfusions [9]. This is particularly beneficial considering the risks associated with transfusions, including infections, allergic reactions, and iron overload with repeated administration [10].
Oral iron supplements are easily accessible and can be conveniently taken at home, making them a practical option for cancer patients. They also tend to be more cost-effective than intravenous iron formulations, offering a viable alternative for managing IDA in these patients.
However, the bioavailability is about 10% to 15% for ferrous iron preparations (sulfate, gluconate, fumarate, etc.), and the absorption of these formulations is negatively affected by proton pump inhibitors or antacids, or meals, or presence of an inflammatory status. In addition, a large number of patients reported gastrointestinal side effects which may lead to reduced tolerance and adherence to iron treatment [7].
Athibovonsuk et al. (2013) investigated the opportunity to give oral or intravenous iron prior to start chemotherapy in gynecologic cancer patients. The results showed that both intravenous and oral iron administration did not limit the Hb decline; a smaller median number of transfused patients was reported in the intravenous group (28,1%), respect to oral iron group, with 56.3% of transfused patients (transfusion criterion under 10 g/dL of Hb) [11].
Dangsuwan et al. (2010) obtained similar results in their study in gynecologic cancer patients with 22.7% of transfused patients in IV iron group and 63.6% in oral iron group [12].
In our study, after three months of Sucrosomial oral iron supplementation, we observed no significative drop in Hb levels, and ferritin, TSAT, and serum iron increased by 49%, 58%, and 99% respectively. Only 4 patients experienced a Hb levels below 10 g/dL during the study period. However, the decrease was limited and not less than 9 g/dl. For this reason, none of the patients required red blood cell transfusions or ESAs treatments.
Looking at the Athibovonsuk study, the results given are not comparable with ours because the transfusion threshold is different (Hb < 10 g/dL in Athibovonsuk study and Hb < 9 g/dL in our study). Anyway, if we would consider a Hb cut-off of 10 g/dL, we would obtain that in our study only 15.4% of the patients fell below this threshold.
We can try explaining why the result obtained with SI was better than that obtained with conventional iron. Historically, the use of oral iron has been problematic in cancer patients for various reasons. Factors such as inflammation, gastrointestinal side effects of cancer treatments, and interactions with other medications can affect the absorption of oral iron. Conventional iron can cause gastrointestinal side effects, including constipation, nausea, abdominal pain, and the discoloration of stools. Additionally, the side effects associated with oral iron supplements, along with the need for prolonged treatment to replenish iron stores, can impact patient adherence. The unique structure of SI, which is different from other oral formulations, protects iron from the acid environment of the stomach, increases its absorption across the intestinal epithelium, and ensures low gastrointestinal toxicity as demonstrated in multiple studies, even in patients with inflammatory bowel diseases for example [4, 13, 14].
Moreover, with traditional oral iron, elevated hepcidin levels can significantly hinder the effectiveness of iron supplementation. When hepcidin is high, it reduces the expression of ferroportin, the iron transporter protein on the basal surface of intestinal cells, effectively blocking the release of absorbed iron into the bloodstream. This leads to poor iron absorption and circulation and limits the efficacy of conventional oral iron supplements, particularly in patients with chronic disease and chronic inflammation [3].
Sucrosomial® iron offers a novel approach that may help mitigate the limitations imposed by hepcidin. With conventional iron, in conditions of inflammation, intestinal absorption is limited because the increased levels of hepcidin negatively affect the expression of ferroportin and limit the entry of iron into the blood circulation. The Sucrosome protects the iron in a phospholipid plus sucrose esters of fatty acids matrix, allowing it to be absorbed through the intestinal cells in a way that, at least partially, seems to bypass the usual pathways. In vitro studies have shown that SI absorption can occur mainly through an hepcidin-independent pathway, as it is mostly taken up as a vesicle-like structure by enterocytes and M cells via the paracellular and transcellular routes [4]. In ex-vivo experiments using isolate rat intestine and fluorescein labeled SI, it has been demonstrated that, after passing through M cells, SI was taken up by CD68+ macrophages. These experiments suggest that SI is not released to the portal blood stream, but to the lymphatic circulation (M cells route) and later to the arterial circulation, before reaching the liver [16]. This peculiar delivery system enables iron to be absorbed directly by the intestinal cells and transported across the cell membrane without relying on the traditional pathways explaining its effectiveness even in patients with elevated hepcidin. This makes it a particularly advantageous form of supplementation for individuals suffering from inflammation-related IDA, where traditional iron supplements might fail due to poor absorption [4, 16, 17].
Compared to intravenous iron, oral iron is generally considered less rapid in increasing hemoglobin levels and replenishing iron stores, especially for patients with cancer-related anemia and during ESA treatments. This is due to the direct delivery of intravenous iron into the bloodstream, bypassing absorption issues related to the gastrointestinal tract [18].
The introduction of SI into clinical practice represents a significant advancement in the management of cancer-related anemia and other trials demonstrate the efficacy of SI.
In a multicenter, open-label, phase III clinical trial conducted in patients with chemotherapy-related anemia, ESAs were administered with or without SI for 12 weeks. The study found that a higher proportion of patients supplemented with SI achieved the desired response (hemoglobin increase of >2 g/dL from baseline, without prior red blood cell transfusions in the past 28 days, or achieving a hemoglobin level of ≥12 g/dL) compared to the control group (52% vs. 31%).There were no significant differences in safety or the need for transfusions between the two groups [19].
In another study, Mafodda et al. (2017) randomized 64 patients with chemotherapy-related functional anemia (hemoglobin levels >8 g/dL and <10 g/dL, without absolute or functional iron deficiency) to receive chemotherapy and darbepoetin with either SI or intravenous iron. The study found no difference in the hemoglobin response rate between the two treatment arms, nor any differences in red blood cell transfusion requirements or changes in quality of life. However, SI was better tolerated compared to intravenous iron [20].
Conclusions
In conclusion, to our knowledge, this is the first observational study using SI in preventing Hb drop in cancer patients. This study had some limitations: the small number of patients and the absence of control group do not allow us to draw solid conclusions, but only show a trend, to be confirmed with more complete clinical trials. Supplementation with Sucrosomial® Iron seems to offer a promising new approach for managing mild cancer-related IDA in patients who do not require transfusions or ESA treatment. This addresses the critical need for more effective and tolerable iron treatments. The unique delivery system of SI, which minimizes side effects and maximizes absorption, represents a significant advancement in supporting cancer patients throughout their treatment journey [2]. As research continues to progress, SI supplementation may become a standard of care to recover iron deficiency or avoid large drop in Hb in oncology, alleviating the burden of IDA for cancer patients worldwide.
Data Availability Statement
Data supporting the findings of this study are included within the article, further inquiries can be directed to the corresponding author.
Ethics Statement
The study was reviewed and approved by Treviglio Hospital Ethics Committees (Approval No. 5/2013). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
FP, RA, VL, and SB: conceptualization, methodology, resources, supervision, and project administration. FP, RA: investigation and data curation. FP and SB: statistical analysis. FP, VL and SB: writing-original draft preparation, conception and coordination of the study, full access to all data in the study, and responsibility for the integrity of the data and the accuracy of the data analysis. FP, VL and SB: writing-reviewing and editing. All authors have read and approved the final version of the manuscript for submission.
Acknowledgments
The authors would like to thank the participants of this study, and PharmaNutra S.p.A., Pisa, Italy for providing the Sucrosomial® iron supplement (Sideral® Forte). We would also like to thank Germano Tarantino and Maria Sole Rossato for their help with editing.
Funding: PharmaNutra S.p.A., Pisa, Italy will covered Article Processing Fee.
Conflict of interest
Authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Birgegård G, Aapro MS, Bokemeyer C, Dicato M, Drings P, et al. (2005) Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology 68:3-11.
- Barni S, Gascòn P, Petrelli F, García-Erce JA, Pedrazzoli P, et al. (2017) Position paper on management of iron deficiency in adult cancer patients. Expert Rev Hematol 10:685-695.
- Rodgers GM (2024) Update on iron supplementation in patients with cancer-related anemia. Expert Rev Hematol 17:505-514.
- Gómez-Ramírez S, Brilli E, Tarantino G, Girelli D, Muñoz M (2023) Sucrosomial® Iron: An Updated Review of Its Clinical Efficacy for the Treatment of Iron Deficiency. Pharmaceuticals 16:847.
- Crawford J, Cella D, Cleeland CS, et al. (2002) Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer 95:888895.
- Sbrana A, Antonuzzo A, Brunello A, Petrelli F, Pronzato P, et al. (2020) Management of anemia in patients with cancer: 2019 Italian Association of Medical Oncology (AIOM) guidelines. Tumori 106:337345.
- Antonuzzo A, Cuomo O, Sbrana A, Danova M (2022) Clinical Safety And Efficacy of Iron Supplementation in Cancer Patients. Journal of Oncology Research and Therapy 7:10131.
- Gluszak C, de Vries-Brilland M, Seegers V, Baroin C, Kieffer H, et al. (2022) Impact of Iron-Deficiency Management on Quality of Life in Patients with Cancer: A Prospective Cohort Study (CAMARA Study). The Oncologist 27:328–333.
- Rizzo JD, Brouwers M, Hurley P, et al. (2010) American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood 116:4045–4059.
- Woei-A-Jin F.J.S.H, Zheng S.Z, Kiliçsoy I, Hudig F, Luelmo S.A.C, et al. (2019) Lifetime Transfusion Burden and Transfusion-Related Iron Overload in Adult Survivors of Solid Malignancies. The Oncologist 25:e341-e350.
- Athibovonsuk P, Manchana T, Sirisabya N (2013) Prevention of blood transfusion with intravenous iron in gynecologic cancer patients receiving platinum-based chemotherapy. Gynecologic Oncology 131:679–682.
- Dangsuwan P, Manchana T (2009) Blood transfusion reduction with intravenous iron in gynecologic cancer patients receiving chemotherapy. Gynecologic Oncology 116:522–525.
- Bertani L, Tricò D, Zanzi F, Baiano Svizzero G, Coppini F, et al. (2021) Oral Sucrosomial Iron Is as Effective as Intravenous Ferric CarboxyMaltose in Treating Anemia in Patients with Ulcerative Colitis. Nutrients 13:608.
- Bastida G, Herrera-de Guise C, Algaba A, Ber Nieto Y, Soares JM, et al. (2021) Sucrosomial Iron Supplementation for the Treatment of Iron Deficiency Anemia in Inflammatory Bowel Disease Patients Refractory to Oral Iron Treatment. Nutrients 13:1770.
- Girelli D, Ugolini S, Busti F, Marchi G, Castagna A (2018) Modern iron replacement therapy: clinical and pathophysiological insights. Int J Hematol 107:16-30.
- Fabiano A, Brilli E, Mattii L, Testai L, Moscato S, et al. (2018) Ex Vivo and in Vivo Study of Sucrosomial® Iron Intestinal Absorption and Bioavailability. Int J Mol Sci 19:2722.
- Asperti M, Brilli E, Denardo A, Gryzik M, Pagani F, et al. (2021) Iron distribution in different tissues of homozygous Mask (msk/msk) mice and the effects of oral iron treatments. Am J Hematol 96:1253-1263.
- Aapro M, Beguin Y, Bokemeyer C, et al. (2018) Management of anaemia and iron deficiency in patients with cancer: Esmo clinical practice guidelines. Ann Oncol 29:iv271.
- Zuccarini A, Cicognini D, Tancredi R, et al. (2022) Randomized trial of sucrosomial iron supplementation in patients with chemotherapyrelated anemia treated with ESA. Support Care Cancer 30:7645-7653.
- Mafodda A, Giuffrida D, Prestifilippo A, et al. (2017) Oral sucrosomial iron versus intravenous iron in anemic cancer patients without iron deficiency receiving darbepoetin alfa: a pilot study. Support Care Cancer 25:2779-2786.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.