Prevalence of Dengue Serotypes and its Association with Hematological Parameters in Dengue Fever Patients
by Mahesh Chandra1*, Ansar Ahmad Paray2, Upasana Bhumbla3
1,2Demonstrator, Department of Microbiology, Adesh Institute of Medical Sciences and Research, Adesh University Bathinda, Punjab, India
3Professor and Head, Department of Microbiology, Adesh Institute of Medical Sciences and Research, Adesh University Bathinda, Punjab, India.
*Corresponding author: Mahesh Chandra, Demonstrator, Department of Microbiology, Adesh Institute of Medical Sciences and Research, Adesh University Bathinda, Punjab, India. E-mail: mahesh0231997@yahoo.com
Received Date: 15 December 2025
Accepted Date: 23 December 2025
Published Date: 26 December 2025
Citation: Chandra M, Paray AA, Bhumbla U (2025) Prevalence of Dengue Serotypes and its Association with Hematological Parameters in Dengue Fever Patients. Rep GlobHealth Res 8: 222. https://doi.org/10.29011/2690-9480.100222
Abstract
Introduction: Dengue is an emerging arboviral threat and a major public health issue in tropical and subtropical regions worldwide, where large populations are at risk and the disease presents with varying clinical severity. This study aimed to analyze the clinical and laboratory patterns linked to the four dengue virus serotypes. Materials and Methods: This cross-sectional study was conducted in the Department of Microbiology, Adesh Institute of Medical Sciences and Research, Adesh University Bathinda, Punjab, India. Blood samples from all clinically dengue suspected patients were received from various department of AIMSR hospital, between the period of September 2024 to August 2025. Samples were tested for NS1Ag, IgM and IgG by ELISA method. Dengue serotypes were detected by using RT-PCR at BSL-III laboratory of AIMSR hospital. Result: During the study period, 378 samples were tested among which 39 were positive for one or more dengue parameters by ELISA method. 18 samples were positive for NS1Ag, 4 were positive for IgM, 1 was positive for IgG only and among 16 samples were positive for more than one parameter. Prevalence of Dengue was found to be 10.31%. Among the confirmed dengue cases, DENV-2 was the predominant serotype, comprising 51.28% of infections. The remaining serotypes were far less common, with DENV-1 and DENV-3 each detected in 7.69% of cases and DENV-4 in 2.56%. Co-infections were also recorded: 10.25% of patients were infected with both DENV-2 and DENV-3, while 5.12% had combined infections of DENV-1 + DENV-2 and DENV-1 + DENV-3. Additionally, a single case (2.56% each) showed co-infection with DENV-3 + DENV-4. Conclusion: Most dengue cases in our study were residents of Malwa region of Punjab. Our study revealed DENV-2 as the predominant serotype with the highest severity, also findings revealed infection was more common in females compared to males.
Keywords: Dengue; Dengue fever; Dengue shock syndrome; Platelet; Liver enzymes
Introduction
The dengue virus belongs to the Flavivirus group under the Flaviviridae family. Dengue and other mosquito-borne illnesses have become a major global health concern, especially in tropical and subtropical regions where outbreaks frequently occur [1]. In many places, millions of cases of these infections are seen every year [2]. Dengue fever, often called “breakbone fever,” is a viral disease spread mainly by Aedes mosquitoes (Aedes aegypti). It is also one of the neglected tropical diseases (NTDs) [3]. There are four dengue virus types—DENV-1, DENV-2, DENV-3, and DENV-4, each capable of causing the full range of illness. Severe forms like dengue hemorrhagic fever usually happen when a person who has already had one dengue infection becomes infected again with a different dengue serotype [4].
About 2.5 billion people live in areas where dengue is common, and nearly 400 million infections occur every year [5, 6]. In some regions, the disease can have a fatality rate of more than 5–20% [6]. Dengue is now reported from over 100 countries, including nations in Europe and the United States [7]. In India, the earliest record of a dengue-like illness dates back to 1780 in Madras, and the first confirmed Dengue Fever (DF) outbreak occurred in Kolkata and parts of eastern India during 1963–1964 [8]. The disease shows a wide range of symptoms from no asymptomatic illness to classic dengue fever and, in severe cases, dengue hemorrhagic fever or Dengue Shock Syndrome (DSS) [4].
Aims and Objectives
The aim and objective of this study was to determine the prevalence of different dengue serotypes and assess the severity of infection associated with each type. The study also sought to evaluate the correlation between specific dengue serotypes and the degree of thrombocytopenia as well as total leukocyte count.
Material and Method
Study location
The study was conducted in the Department of Microbiology, and Central laboratory of Adesh Institute of Medical Science and Research (AIMSR), Adesh University Punjab. Molecular testing (RT-PCR) was done at BSL-III laboratory AIMSR. The study was carried out after getting the approval letter from AIMSR Research Committee with Ref. No. AIMSR/MC/Estt/620 and Ethics Committee for Biomedical and Health Research, Adesh University, Bathinda with Ref. No AU/EC_BHR/2k24/66.
Duration of study
A one-year time duration cross-sectional study was conducted from September 2024 to August 2025. During the study period 378 dengue suspected blood samples received from various departments of Adesh Institute of Medical Sciences and Research (AIMSR) hospital.
Study design
A cross-sectional study was conducted on 39 patients with dengue confirmed (Positive) by Enzyme-Linked Immunosorbent Assay (ELISA) against dengue-specific non-structural protein 1 (NS1Ag) antigen, immunoglobulin M (IgM), and immunoglobulin G (IgG).
Selection of patient
DDengue suspected samples were received from all age group. ELISA test was performed and those found to be positive for any parameter (NS1Ag/IgM/IgG) were further tested by RT-PCR. Clinical history of patient was taken and recorded on a Performa.
Inclusion criteria
All freshly collected blood samples labelled properly received from various departments into Serum Separator Tube (SST) were included in the study.
Exclusion Criteria
Samples which were inadequate and leaked, without labelled, other vials were excluded. Samples received from patients taking any antiviral therapy were also excluded.
Methodology
All suspected blood samples were received in yellow colour Serum Separation Tube (SST). The samples were properly checked and those sample falling in inclusion criteria were included in the study. Then samples were allowed to clotted after which they were centrifuged at 3000 RPM for 10 min to obtain the serum for Dengue ELISA (NS1Ag, IgM and IgG). Serum samples found positive by ELISA were stored and RT-PCR was doe to detection of dengue serotypes. Complete Blood cells (CBC) and biochemical analysis was done at hematology and biochemistry department of central laboratory AIMSR hospital.
Laboratory evaluation required
Laboratory tests including Total Leukocyte Count (TLC), Differential Leukocyte Count (DLC), platelet count, blood urea, serum creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and serum albumin were recorded. Dengue serotypes were identified using RT-PCR. Viral RNA was extracted using a QIAGEN kit, and amplification was performed on a Bio-Rad RT-PCR thermocycler with a Genes2Me Pvt. Ltd.
PCR kit.
Hematological and biochemical profiling
To keep track of how the illness developed, hematological and biochemical tests were conducted multiple times during the hospital stay. These evaluations took place three to four days after symptoms began and were carried out again as needed based on clinical assessments.
Hematological assessments were performed using Beckman Coulter analysis system (unicel DxH 800). Standard quality control protocols were adhered according to the manufacturer’s instructions. Biochemical analyses were performed on fresh serum samples using a fully automated biochemistry analyzer. The metabolic panel included liver function tests (ALT, AST, total protein, and bilirubin) and renal function tests (serum creatinine, blood urea, and uric acid).
Statistical analysis
Statistical analyses were conducted using IBPM SPSS statics software version 30.0.0.0 (172) and MS excel. Descriptive statistics like means, stranded deviation (SD), Ranges were calculated for demographic variables, clinical characteristics, hematological and biochemical parameters stratified by dengue serotype. One-way analysis of variance (ANOVA) for continuous variables. Statistical significance was set at P<0.05.
Results
Characteristics and Demographics of the Study Population
During the study period from August 2024 to July 2025 a total of 378 dengue suspected samples were received from various department of AIMSR hospital. 194 (56.55%) samples were received from males and 149 (43.44%) from female patients. Out of these 378, 39 (10.31%) were dengue positive and 339 (89.69%) were dengue negative. Among 39 dengue-positive subjects, 18 (46.15%) were positive only for NS1, 04 (10.25%) were positive for IgM-only, 01 (2.56%) was positive only for IgG, 06 (15.38%) were positive for both NS1 + IgM, 03 (7.69%) were IgM + IgG positive and 07 (17.94%) were NS1Ag + IgM + IgG positive. Out of 39 Dengue positive samples, 19 (48.71%) were of male patients and 20 (51.28%) were of female patients. In the study, highest prevalence was observed in age groups 10-20 years (23.07%) followed by 31-40 years (15.38%), 0-10 and 61-70 years (12.82%). Figure 1: showing age wise distribution of dengue positive cases.
|
Parameters |
Case, dengue positive (n =39) Median (Q3–Q1) |
Control, dengue negative (n =39) Median (Q3–Q1) |
p value |
|
Age (years) |
31.0 (57.0–15.0) |
40 (60-24) |
0.330 |
|
Hemoglobin (gm/dl) |
12.9 (14–10.6) |
11.6 (13.1-9.9) |
0.283 |
|
RBC (X 1012/L) |
4.49 (4.87–3.81) |
4.17 (4.87-3.7) |
0.310 |
|
TLC (×10^3/µL) |
5.80 (1.10–3.40) |
8.80 (1.09-6.50) |
-0.058 |
|
Neutrophil (%) |
61.8 (72.5–52.6) |
71.8 (80.1-56.6) |
0.241 |
|
Lymphocyte (%) |
27.5 (35–18.3) |
19 (33-11.2) |
0.060 |
|
Monocyte (%) |
8 (9.2–6.2) |
7.1 (9.4-4.9) |
0.072 |
|
Eosinophil (%) |
0.7 (1.6–0) |
0.4 (1.3-0.1) |
0.104 |
|
Basophils (%) |
0.4 (0.8-0) |
0.4 (0.5-0.1) |
0.063 |
|
Platelets (×10^3/µL) |
100 (150–36) |
142 (244-525) |
-0.191 |
|
Urea (mg/dl) |
28.5 (45–21) |
26 (58-19) |
-0.141 |
|
Creatinine (mg/dl) |
0.92 (1.17–0.76) |
0.7 (1.7-0.5) |
-0.103 |
|
Uric acid (mg/dl) |
4.65 (6.7-3.6) |
3.9 (7.7-3.4) |
-0.097 |
|
Total bilirubin (mg/dl) |
0.6 (1.4-0.4) |
0.6 (0.9-0.4) |
0.017 |
|
Total protein (gm/dl) |
6.2 (7.2–5.5) |
6.3 (6.9-5.8) |
-0.390 |
|
AST (U/L) |
104 (231–48) |
49 (137-24) |
-0.103 |
|
ALT (U/L) |
75 (292–34) |
45 (75-21) |
-0.077 |
|
RBC: Red Blood Cell, TLC: Total Leukocyte Count; AST: Aspartate Aminotransferase, ALT: Alanine Aminotransferase; ns: Nonsignificant. |
|||
Table 1: Correlation between dengue positive and negative participants via Mann–Whitney U test.
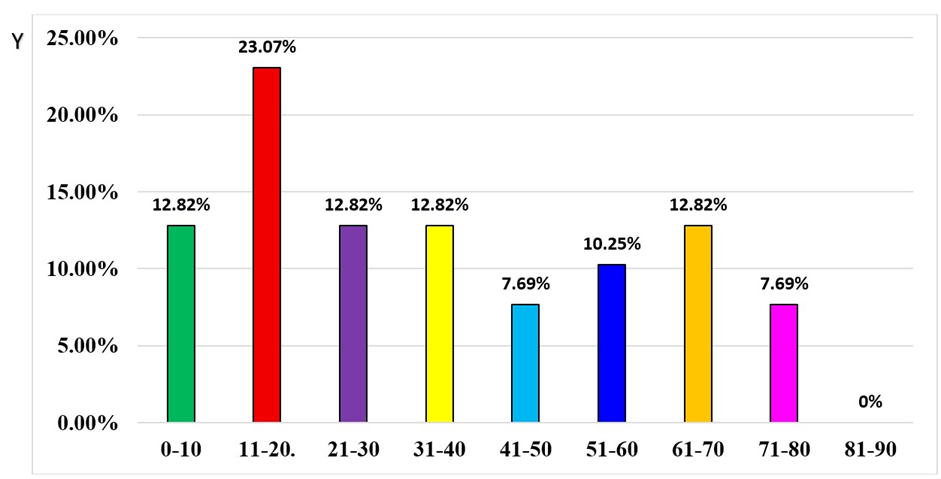
Figure 1: Age wise distribution of dengue positive cases.
Clinical features
This study evaluated the clinical profiles of 39 individuals who tested positive for dengue infection (Figure 2). Fever was the most common symptom in all 38 cases (97.43%) followed by headache 32 (82.05%), myalgia 28 (71.79%), chills 26 (66.66%), and nausea and vomiting 22 (56.41%). Abdominal pain was reported in 18 (46.1%) of the cases, whereas diarrhea was observed in 10 (25.64%) of the patients. Mucosal bleeding was present in 05 (12.80%) cases. Less frequent symptoms included rash 05 (12.870%) and eye pain 03 (7.69%).
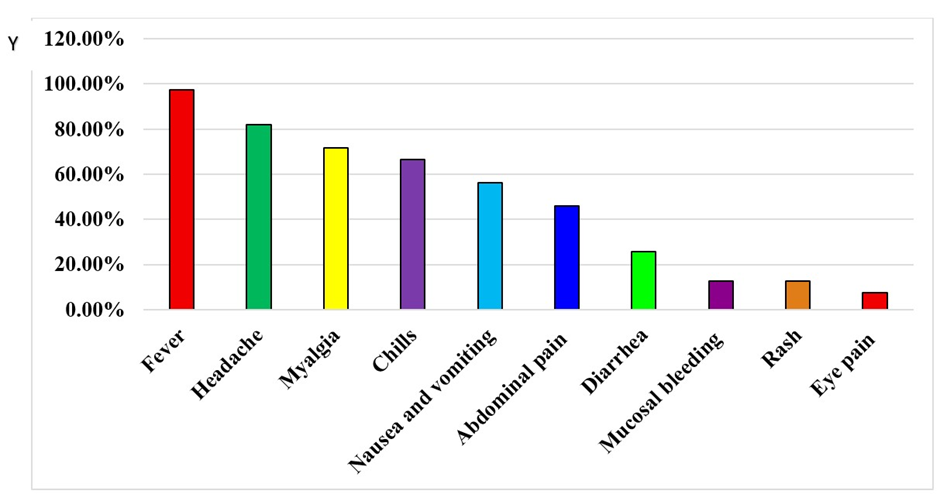
Figure 2: Clinical features of dengue infections.
Hematological profiling
Hematological findings showed that anemia was relatively common, affecting 42.2% of male and 33.33% female patients. Leukopenia (reduced WBC count), was seen in 14.61% of cases. Neutropenia was identified in 21.91% of the patients, while most lymphocyte counts were normal or low, with only 8.43% showing possible lymphocytosis. Elevated hematocrit values were noted in 29.36% of males and 18.84% of females. Thrombocytopenia was prominent, with 67.98% of patients. Thrombocytopenia was observed, in 67.98% of the patients.
Biochemical profiling
In this study, most patients (84.83%) had normal total serum bilirubin levels, while 15.16% demonstrated elevated values. Increased ALT levels were detected in 75.84% of patients (normal limit of 42 IU/L) and 60.11% showed elevated AST levels. Kidney Function Test (KFT) markers elevated only in 10.11% and 15.38% of patients. Majority (74.71%) participants had total serum protein levels at or above the normal minimum value of 6.4 mg/dL. Table 2: showing Comparison of hematological and biochemical findings in NS1, IgM, and NS1 + IgM & Ns1Ag + IgM + IgG positive dengue patients. The mean platelet count was 105 ± 077 × 10³/µL, with a range from 028-182 × 10³/µL. The mean Hemoglobin was 12.26 ± 2.65gm/dl, ranging from 9.61-14.91 gm/dl. The mean WBC count was 8.40 ± 7.08 × 10³/µL, with a range from 1.32-15.48 × 10³/µL given in Table 3.
|
Parameter |
Mean ± SD |
Range |
|
Platelet Count (×10^3/µL) |
105± 077 |
0.28-182 ×10^3/µL |
|
White Blood Cell Count (×10^3/µL) |
8.40 ± 7.08 |
1.32-15.48 ×10^3/µL |
|
Hemoglobin (gm/dl) |
12.26 ± 2.65 |
9.61-14.91 gm/dl |
|
Neutrophil (%) |
61 ± 14 |
47-75% |
|
Lymphocyte (%) |
28.11 ± 13.69 |
14.4-41.8% |
|
Monocyte (%) |
7.67 ± 2.86 |
4.81-10.53% |
|
Eosinophil (%) |
1.24 ± 1.79 |
0-3% |
Table 2: Hematological Parameters in Dengue Patients.
|
Parameters |
NS1Ag (n=18) |
NS1Ag + IgM (n= 6) |
NS1Ag + IgM + IgG (n=7) |
|
Median (Q3–Q1) |
Median (Q3–Q1) |
Median (Q3–Q1) |
|
|
Hemoglobin (gm/dl) |
12.9 (14.1-8.0) |
12.9 (14.1–12.0) |
14.0(16.1–12.1) |
|
RBC (X 1012/L) |
4.45 (4.78–3.77) |
4.72 (5.01–4.46) |
4.76 (5.02–4.18) |
|
TLC (×10^3/µL) |
4.50 (7.40–3.20) |
4.60 (3.40–1.70) |
9.70 (2.2–6.10) |
|
Neutrophil (%) |
62 (72-52) |
63.2 (82.2–42.9) |
59.3 (68–37) |
|
Lymphocyte (%) |
27.9 (29.4–16.6) |
28.95 (50.5–9.5) |
30 (53–22) |
|
Monocyte (%) |
9.2 (9.6–6.9) |
6.45 (7.4–5.0) |
8(9–6.7) |
|
Eosinophil (%) |
0 (0.5–0) |
0.85 (1.6–0.5) |
1.2 (2–0.2) |
|
Platelets (×10^3/µL) |
140 (176–500) |
112 (150–360) |
350 (700–220) |
|
Urea (mg/dl) |
22 (30–18) |
29 (55.5-21.9) |
33.5 (58–26) |
|
Creatinine (mg/dl) |
0.7 (0.85–0.55) |
0.8 (0.95–0.45) |
1.15 (1.6–0.9) |
|
Uric acid |
5.6 (7.15-3.15) |
6.1(7.1-5.1) |
5 (7.6-4) |
|
Total bilirubin (mg/dl) |
0.6 (0.9–0.5) |
0.45 (1.2–0.4) |
1.1 (2.0–0.5) |
|
Total protein (gm/dl) |
6.5 (7.65–5.7) |
7.24 (7.56–6.89) |
6.3 (6.8-5.6) |
|
AST (U/L) |
90.5 (190–44.5) |
170 (2000–129) |
543.5 (1687–73) |
|
ALT (U/L) |
50 (123–29.5) |
151.5 (977–103) |
708 (934-197) |
Table 3: Comparison of hematological and biochemical findings in NS1Ag, IgM, and NS1 + IgM & Ns1Ag + IgM + IgG positive dengue patients.
In the present study, 51.20% patients were positive for Dengue IgM antibodies indicating recent infections. The presence of NS1 antigen in 38.46% of patients supports the acute phase of dengue and dengue IgG antibodies indicating past infections. The mean values of these markers suggest a predominance of recent dengue infections shown in table 4.
|
Marker |
Total Positive (%) |
Mean ± SD |
Range |
|
NS1 Antigen |
15(38.46%) |
4.38 ± 2.04 |
2.34-6.42 |
|
Dengue IgM Antibody (single + multiple) |
20 (51.20%) |
2.45 ± 1.33 |
1.12-3.78 |
|
Dengue IgG Antibody (single + multiple) |
12 (30.76%) |
2.59 ± 1.40 |
1.19–3.99 |
Table 4: Serological Markers in Dengue Patients.
Distribution of dengue Serotype
In the present study DENV-2 emerged as the most prevalent serotype, representing 51.28% cases (21/39 (Table 5). The remaining serotypes were much less frequent, with DENV-1 and DENV-3 each detected in 3 patients (7.69%) and DENV-4 identified in only 1 case (2.56%). Co-infections with more than one dengue serotype were observed in a small number of patients., 4 cases (10.25%) showed dual infection with DENV-2 and DENV-3, two cases (5.12%) involved co-infection with DENV-1 + DENV-2 and DENV-1 + DENV-3, respectively. Single cases (2.56% each) demonstrated co-infection with DENV-3 + DENV-4 and with all four serotypes simultaneously. Though co-infection was uncommon, DENV-3 appeared consistently across the mixed-serotype infections identified. In two denguepositive cases, none of the four known serotypes could be detected. These findings indicate that although co-infections are relatively rare, they do occur, with DENV-3 being involved in both types of co-infections detected in this study.
|
Sr No. |
Types of serotypes |
Total detected (n= 37) |
Percentage (%) |
|
1 |
DENV 1 |
03 |
7.69% |
|
2 |
DENV 2 |
20 |
51.28% |
|
3 |
DENV 3 |
03 |
7.69% |
|
4 |
DENV 4 |
01 |
2.56% |
|
5 |
DENV 1+2 |
02 |
5.12% |
|
6 |
DENV 2+3 |
04 |
10.25% |
|
7 |
DENV 1+3 |
02 |
5.12% |
|
8 |
DENV 3+4 |
01 |
2.56% |
|
9 |
DENV 1+2+3+4 |
01 |
2.56% |
Table 5: Prevalence of dengue serotypes.
Comparison of dengue serotypes with hematological parameters
The findings of present study showed less statistically significant difference in mean platelet counts across the groups (p = 0.999), and similarly, no significant difference was observed in mean leukocyte counts (p = 0.990). However, a statistically significant variation was noted in mean neutrophil and lymphocyte percentages among the groups (p = 0.0106) (Table 6).
|
Serotype |
Platelets (Mean±SD) |
TLC (×10^3/µL) (Mean±SD) |
Neutrophils (%) (Mean±SD) |
Lymphocytes (%) (Mean±SD) |
Monocyte (%) (Mean±SD) |
Eosinophils (%) (Mean±SD) |
|
DENV 1 |
105±1.66 |
8.70±7.0 |
67.4±12.40 |
22.9±11.66 |
8.1±1.66 |
0.9±0.25 |
|
DENV 2 |
107±0.77 |
6.5±5.0 |
57.43±16.49 |
29.96±16.79 |
7.26±3.63 |
1.88±2.31 |
|
DENV 3 |
103±0.89 |
9.1±5.0 |
57.8±13.11 |
32.7±12.85 |
9.2±1.05 |
0.7±1.1 |
|
DENV 1+2 |
0.90±1.1 |
10.6±4.5 |
64.6±10.32 |
24.75±9.82 |
9.55±0.35 |
0.65±0.35 |
|
DENV 2+3 |
100±046 |
9.8±8.5 |
67.7±6.03 |
23.75±4.15 |
7.52±2.12 |
0.57±0.43 |
|
DENV 1+3 |
0.87±0.83 |
11.8±8.3 |
63.2±5.5 |
27.05±5.8 |
8.95±0.63 |
0.1±0.1 |
Table 6: Comparison of mean with hematological parameters based on dengue serotypes.
Discussion
Dengue is a significant mosquito-borne virus that has undergone considerable changes in its spread over the past quarter-century, posing a serious health issue globally, particularly in tropical and subtropical countries [9-12].
In the present study, prevalence of dengue remained 10.31% (39/378), which was lower than the prevalence (62.26%) reported by Pachori et al., 2021 [13-15]. Deshkar et al., 2017 reported 24.49% prevalence [16,17], 31.3% prevalence was reported by Ukey et al.,2010 in central India [18], Saini et al.,2013 reported 30.6% prevalence in western Maharashtra [19], 18.99% prevalence was reported in Rajasthan, India [20] and 17.7% prevalence was reported by Rao et al., 2013 in Andra Pradesh, India [21]. Singh et al., 2023, reported less prevalence of dengue (3.25%) [16].
In our study, among 39 dengue-positive patients, 23.07% belonged to the 11–20-year age group, followed by 12.82% each in the 0–10, 21–30, 31–40, and 61–70-year age ranges. Similar results had also been reported in previous studies. Rao et al. (2013) from Andhra Pradesh observed the highest seropositivity (35.84%) among children aged 0–10 years, followed by 22.66% in the 11–20-year group [22]. Ukey et al. (2010) in central India reported the highest positivity (43.90%) in the 0–10-year age group, with 31.71% in the 15–30-year group [9]. Likewise, Deshkar et al., (2017) found 40.50% of cases in children aged 0–10 years and 26.71% in those aged 11–20 years [8]. Overall, dengue affects individuals across all age groups; however, younger populations tend to be more susceptible. This increased vulnerability in children and adolescents is thought to be due to higher vascular permeability and relatively less mature immune defenses compared to adults.
In the suspected cases most, common clinical feature was fever (97.43%) followed by headache (82.05%), myalgia (71.79%), chills (66.66%), and nausea and vomiting 22 (56.41%). Abdominal pain (46.1%) diarrhea (25.64%). Mucosal bleeding (12.80%). Less frequent symptoms included rash 12.870% and eye pain in
7.69% cases. Deshkar et al., (2017) documented fever in all cases (100%), followed by myalgia in 74.64%, arthralgia in 65.76%, and headache in 60.92% of patients [8]. Similar observations were made by Turbadkar et al., (2012), who noted fever as the most common symptom, with icterus and myalgia each present in 26% of cases, and headache in 14% of suspected dengue patients [16]. Mandal et al., (2013) also reported universal fever among patients, followed by headache in 62.16%, rash in 37.84%, and bleeding manifestations in 13.51% of cases [17].
In our study, mean TLC (Table 1) was within the normal limits in all serotypes of infection. But the mean neutrophil and lymphocyte percentages were significantly different between the groups. Thrombo-cytopenia and platelet dysfunction was frequently seen in dengue; both strongly associated to the result of the treatment. The WHO recommendations in 2009 were reaffirmed that there is a sudden drop or that the platelet count is less than 150,000/ Clinical dengue is indicated by several signs, one of which is blood discharge deteriorating.
The NS1Ag was the only component studied by Kulkarni et al.,2011 [18]. IgM was positively correlated with thrombocytopenia. The present study showed a link between dengue and thrombocytopenia and Sero-markers. This could be attributed to the following factors. The amount of NS1Ag is determined by the viral burden, because the duration of illness rises, as the quantity of NS1Ag lowers. When antibodies begin to develop. The NS1Ag antigen is sequestered into immune cells when it appears complicated [19].
Among these 39 individuals, the largest group, accounting for 51. 28%, was infected with the second serotype of the dengue virus (DENV 2), as shown in table 5. There were also 7. 69% of patients each infected with DENV 1 and DENV 3, while 2. 56% had DENV 4. These findings align with earlier research by Afreen et al., 2014, Mishra et al., 2012, and Shastri et al., 2012, conducted in Delhi, Uttar Pradesh, and Mumbai respectively [9-11].
In the present study, 8 patients (n=39) were identified as having co-infections with DENV 2 serotype, 4 patients (10. 25%) had co-infections with serotypes 2 and 3, while 2 patients (5. 12%) were found to possess both serotype 1 and 2, and one patient with each of serotypes 1 and 3. A prior study by Afreen et al., 2014 from Delhi highlighted the first observed cases of simultaneous infections by varying dengue serotypes [9].
Our findings are similar with the work of Saxena, 2024. The highest incidence of severe dengue cases was seen with DENV 2 (20. 6%) and DENV 4 (20%). Racherla et al., 2018 determined that DENV 2 was the most common serotype, while DENV 3 and DENV 4 had increased rates of infection [23,24].
Conclusion
This research offers important insights into the demographic profiles, circulating serotypes of the dengue virus, and how clinical characteristics relate to laboratory results among patients with dengue at a specialized hospital in Bathinda, Punjab, India. The outcomes of this research highlight the essential importance of public health initiatives in areas where DENV-2 infection rates are high. Our findings reveal the significance of clinical indicators, including alterations in blood and chemical markers, which are vital for the management and forecasting of dengue fever’s progression. As the intensity of dengue fever increased, significant shifts in these indicators were noted, including lower levels of hemoglobin and platelet counts, in addition to higher levels of liver enzymes, bilirubin, and kidney function indicators. These findings can inform treatment strategies and public health actions, ultimately aiming to alleviate the impact of the disease in the affected areas.
Early serotyping in dengue patients is valuable for tracking epidemiological patterns and understanding the clinical and laboratory characteristics associated with different serotypes. Besides early serotyping, timely diagnosis and treatment, continuous surveillance, and effective vector control measures are essential to control the spread of dengue, particularly in regions like Punjab.
Acknowledgment
The authors thank the Department of Microbiology and included patients of Adesh Institute of Medical Sciences and Research, Bathinda, Punjab, for their support and cooperation during the study.
Financial support and sponsorship: Nil.
Conflicts of interest: Nil.
Use of Artificial Intelligence (AI)-Assisted Technology for Manuscript Preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Kilpatrick AM, Randolph SE (2012) Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet 380: 1946-1955.
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, et al. (2013) The global distribution and burden of dengue. Nature 496: 504-507.
- Halstead SB (1988) Pathogenesis of dengue: Challenges to molecular biology. Science 239: 476-481.
- Kurane I (2007) Dengue hemorrhagic fever with special emphasis on immunopathogenesis. Comp Immunol Microbiol Infect Dis 30: 329340.
- Gubler DJ (1998) Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 11: 480-496.
- World Health Organization (WHO) (2009) Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. New ed. Geneva, Switzerland.
- Pachori P, Sharma GK, Pachori S, Pachori G (2022) A Study of Correlation Between Dengue Serological Markers and Platelet Count in Ajmer Region. Ann of Pathol and Lab Med 9: 50-53.
- Deshkar ST, Raut SS, Khadse RK (2017) Dengue infection in central India: a 5 years study at a tertiary care hospital. Int J Res Med Sci 5: 2483-2489.
- Ukey PM, Bondade SA, Paunipagar PV, Powar RM, Akulwar SL (2010) Study of seroprevalence of dengue Fever in central India. Indian J Community Med 35: 517-519.
- Saini S, Kinikar AG, Deorukhkar S, Bhalerao D, Roushani SB (2013) Epidemiology and seropositivity of dengue fever cases in a rural tertiary care hospital of western Maharashtra, India. Int J Bio Med Res 4: 473-477.
- Sood S (2013) A hospital based Sero surveillance study of dengue infection in Jaipur (Rajasthan), India. J Clin Diagn Res 7: 1917-1920.
- Rao MS, Pavani K, Dass M, Kareem MA, Vinaya raj EV (2013) Seroprevalence of dengue virus in a tertiary care hospital, Andhra Pradesh, South India. Int J Res Med Sci 1: 448-450.
- Singh N, Singh AK, Kumar A (2023) Dengue outbreak update in India: 2022. Indian J Public Health 67: 181-183.
- Karoli R, Fatima J, Siddiqi Z, Kazmi K, Sultania A (2012) Clinical profile of dengue in India. J Infect Dev Ctries 6: 551-554.
- Murugananthan K, Kandasamy M, Rajeshkannan N, Noordeen F (2014) Demographic and clinical features of suspected dengue and dengue hemorrhagic fever in the Northern Province of Sri Lanka, a region afflicted by an internal conflict for more than 30 years-a retrospective analysis. Int J Infect Dis 27: 32-36.
- Turbadkar D, Ramchandran A, Mathur M, Gaikwad S (2012) Laboratory and clinical profile of dengue: A study from Mumbai. Ann Trop Med Public Health 5: 20-23.
- Mandal SK, Ganguly J, Sil K, Chatterjee S, Chatterjee K, et al. (2013) Clinical profiles of dengue fever in a teaching hospital of eastern India. Nat J Med Res 3: 173-176.
- Kulkarni RD, Patil SS, Ajantha GS, Upadhya AK, Kalabhavi AS, et al. (2011) Association of platelet count and serological markers of dengue infection importance of NS1 antigen. Indian J Med Microbiol 29: 359362.
- Hang VT, Nguyet NM, Trung DT, Tricou V, Yoksan S, et al. (2009) Diagnostic accuracy of NS1 ELISA and lateral flow rapid tests for dengue sensitivity, specificity, and relationship to viraemia and antibody responses. PLoSNegl Trop Dis 3: e360.
- Afreen N, Deeba F, Naqvi I, Shareef M, Ahmed A, et al. (2014) Molecular investigation of 2013 dengue fever outbreak from Delhi, India. PLoS Curr.
- Mishra G, Jain A, Prakash O, Prakash S, Kumar R, et al. (2015) Molecular characterization of dengue viruses circulating during 20092012 in Uttar Pradesh, India. J Med Virol 87: 68-75.
- Shastri J, Williamson M, Vaidya N, Agrawal S, Shrivastav O (2017) Nine-year trends of dengue virus infection in Mumbai, Western India. J Lab Physicians 9: 296-302.
- Saxena S (2024) The Laboratory Profile and The Prevalence of Dengue Serotypes at A Tertiary Care Hospital. European Journal of Cardiovascular Medicine 4: 1159-1164.
- Racherla RG, Pamireddy ML, Mohan A, Mudhigeti N, Mahalakshmi PA, et al. (2018) Co circulation of four dengue serotypes at Southeastern Andhra Pradesh, India: a prospective study. Indian J Med Microbiol 36: 236-240.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.