Prediction of Anastomotic Leakage after Right Colectomy Using Ph, Lactate and Glucose from Peritoneal Drain
by Kornaropoulos Michail1*, Chatzipetrou Maria1, Zevlas Andreas2, Politopoulos Christos1, Stavrou Effrosyni1, Tzanakis Nickolaos1, Arkadopoulos Nikolaos3, Danias Nikolaos3, Vassiliu Panteleimon3
12nd Surgical Department, Asklepieio General Hospital Voula, Voula, Greece
21st Surgical Department, Gennimatas General Hospital, Athens, Greece
34th Surgical Department, Attikon University Hospital, Athens, Greece
*Corresponding author: Kornaropoulos Michail, 2nd Surgical Department, Asklepieio General Hospital Voula, Voula, Greece
Received Date: 19 July 2024
Accepted Date: 23 July 2024
Published Date: 25 July 2024
Citation: Kornaropoulos M, Chatzipetrou M, Zevlas A, Politopoulos C, Stavrou E, et al. (2024) Prediction of Anastomotic Leakage after Right Colectomy Using Ph, Lactate and Glucose from Peritoneal Drain. J Surg 9: 11094 https://doi.org/10.29011/2575-9760.11094
Abstract
Introduction: Anastomotic leak (AL) is one of the most dreadful complications in general surgery. Various studies have explored peritoneal fluid biomarkers for early detection of AL. Our study investigates the diagnostic accuracy of pH, lactate, and glucose levels from drain fluid to predict AL following right hemicolectomy.
Methods: This prospective study was conducted in three hospitals in Athens between 2021 and 2022. Patients undergoing right hemicolectomy with a primary anastomosis for any pathology in the right colon, either elective or emergency were enrolled. All patients were assigned to one of two groups according to the presence or absence of AL: with AL (Group AL), without AL (Group n-AL) and were compared according to peritoneal pH, lactate and glucose levels on first 5 postoperative days.
Results: A total of 58 patients underwent right hemicolectomy during the study period were included. Diagnosis of AL (confirmed with CT) occurred in 6 patients (10.3%). It was clear that in the group AL the mean pH values demonstrated a consistent tendency to decrease, whereas the n-AL group mean pH values showed a stable and rising pattern. Mean values of lactate in the non-AL group showed a decreasing trend, compared with the AL group, which exhibited an increasing pattern and remained consistently above 10.
Discussion: Our study is the first to evaluate pH, lactate and glucose from peritoneal drain in every right colectomy, regardless of the underlying reason and method. Given our results, pH from the peritoneal drain fluid corelates with AL earlier than its clinical presentation, even from 2nd post-operative day.
Conclusion: Ischemia-related biomarkers such as pH, glucose and lactate from peritoneal drain fluid may offer a quick, easy, and inexpensive alternative for early detection of AL
Keywords: Anastomotic Leakage; Glucose; Lactate; Peritoneal Drain; Ph; Right Colectomy
Introduction
Anastomotic Leak (AL) is one of the most dreadful complications in general surgery, with occurrence rates between 2% and 14% and higher tendency in left-sided colon or rectal operations [1,2]. Nevertheless, ileocolic anastomoses in right -sided colectomies also show a significant leakage rate, ranging from 3.4% to 8% [3]. AL is not only related with high morbidity, but can also adversely affect oncological outcomes. Delayed detection of anastomotic leakage following colorectal surgery is linked to higher mortality rates [4]. Clinical signs of AL are diverse and often nonspecific, presenting either with fever,lack of bowel movement, abdominal pain,or more specific like peritonitis, localized fluid collections, surgical wound infection or even subclinical leaks detected only through contrast radiology. Typically, these signs become evident between the fourth and seventh postoperative days [5]. In this regard, biomarkers such as White Blood Cells (WBC), C-Reactive Protein (CRP), and Procalcitonin (PRL) are employed as diagnostic or predictive tools for Anastomotic Leaks (AL) following colorectal surgeries [6,7]. Nevertheless, these biomarkers have limited positive predictive value and are often influenced also by other complications in the postoperative period. Currently, abdominal CT scans are the standard diagnostic method for detecting AL, but they often offer low sensitivity, which can delay both diagnosis and appropriate treatment [8]. There is a critical need for accurate early-stage diagnostic markers for AL after colorectal surgery to reduce diagnostic delays and their negative consequences. Various studies have explored peritoneal fluid biomarkers for early detection of AL, with interesting results. (28) These markers from peritoneal fluid are typically categorized into four groups: ischemia markers (pH and lactate), inflammation markers (TNFa,IL), microbiological parameters (lipopolysaccharides) and tissue repair markers (MMPs), all suggested as potential early indicators of processes that hinder anastomotic healing [9]. This prospective study investigates the diagnostic accuracy of pH, lactate, and glucose levels from drain fluid to predict anastomotic leakage following right hemicolectomy.
Materials and Methods
Study Design
This prospective study was conducted in three hospitals in Athens (Asklepieio Voula, Attikon Hospital Athens and G. Gennimatas General Hospital Athens). Patients were enrolled between November 2021 and November 2022. The study was approved by the Ethics Committee of each participating medical center, and informed consent was obtained from all patients. For the current study, all patients undergoing right hemicolectomy with a primary anastomosis for any pathology in the right colon, either elective or emergency were considered eligible and were analyzed. Exclusion criteria were operations requiring temporary ileostomy and right colectomies without placement of peritoneal drains. Collected data included patient demographics, symptoms at admission, TNM score, serum albumin, CEA, ASA score, BMI, comorbidities, indication for surgery, operative time, estimated blood loss, use of inotropes, postoperative day of bowel movement, postoperative day of feeding, WBC and CRP on POD1 to POD5 were obtained. Postoperative course was documented in detail, including the occurrence of fever, bowel function restoration by means of flatus, bleeding, prolonged hospital stay, readmissions and Anastomotic Leakage (AL).
The choice to drain, the type and placement of the drain tube were on surgeon’s discretion. Type of drainage used included Penrose drain (corrugated silicone silastic drain), and Jackson-Pratt drain (silicone flat drain connecting to a vacuum ball). In our study, the drain was placed in the subhepatic space as near to the anastomosis as possible. Drain fluid was collected every day after the ward round respecting rules of sterility with a syringe. The contents of the first 24h (referred as POD 0) were evacuated (but not analyzed). POD 1 was considered the drain fluid obtained 24 h after surgery and this specimen included in the analysis. Similarly, drain fluid was collected and marked for the following days. Peritoneal lactate, pH and glucose from the abdominal drain were analyzed immediately after collection using an ABL700 blood gas analyzer (Radiometer, Copenhagen, Denmark). The outcome of interest was AL within 30 days postoperatively. In our study AL was defined with clinical (gas, pus or fecal discharge from the drain, fecal discharge from the operative wound, peritonitis) or radiologic criteria (pelvic abscess, peri-anastomotic fistula, extravasation of contrast and peri-anastomotic liquid and air on CT scan). As AL were only considered cases confirmed either with CT scan or reoperation. Cases with minimal clinical presentation that were confirmed by CT scan were included.
All the patients were assigned to one of two groups according to the presence or absence of AL: with AL (Group AL), without AL (Group n-AL). The two groups were compared according to peritoneal pH, lactate and glucose levels on POD 1-5. AL was classified according to the system proposed by the International Study Group of Rectal Cancer (ISREC): Grade A can be left untreated; grade B requires medical management or minimally invasive therapeutic intervention (radiological drainage or other drainage) and grade C requiring revision surgery.
Statistical Analysis
Data for pH, Lactate and Glucose values were analyzed within the methodological frame of General Mixed Models using the ANOVA method (36) according to the model that includes the effects (main and interaction) of one between subjects’ factor with two levels (patients with an without AL) and one factor within subjects with five levels (the 5 post-operative days, treated as repeated measures). The ANOVA method was performed mainly for estimating the correct standard errors of the mean differences used for the clinically interesting comparisons of mean pH values. The mean values were compared with the protected Least Significant Difference (LSD) criterion. The model’s assumptions relative to sphericity and homogeneity of error variances were fulfilled. All statistical analyses were accomplished with the IBM SPSS Statistics v.23.0 software. In all hypothesis testing procedures, the significance level was predetermined at a=0.05 (P≤0.05)
Results
A total of 58 patients underwent right hemicolectomy during the study period (31 male patients, 27 female patients) were included. Median age was 71 (range 31-91) years. Most cases (66%, n=38) underwent elective surgery, whereas 20 (34%) underwent emergency surgery. Surgery was performed with a laparoscopic approach in 20 patients (34%). The diagnosis of AL (confirmed with CT) occurred in 6 patients (10.3%) becoming clinically evident after a mean time of 8 days (6-12) from surgery. Of these patients, 66.6% (n=4) developed grade B AL, whereas 33.3% (n=2) developed grade C AL. The incident of AL occurred in 4 out of 20 emergency cases and 2 out of 38 elective cases, whereas one out of laparoscopic 20 cases had AL and 5 out of 38 open cases had AL. (Table 1).
|
Operation Type |
Total number of patients |
No- Anastomotic Leakage |
Anastomotic Leakage |
|
Right Colectomy |
58 |
52 |
6 |
|
Laparoscopic |
20 |
19 |
1 |
|
Open |
38 |
33 |
5 |
|
Emergency |
20 |
16 |
4 |
|
Elective |
38 |
36 |
2 |
|
Malignancy |
43 |
39 |
4 |
|
Benign |
15 |
13 |
2 |
Table 1: Type of Operations and Distribution of Anastomotic Leakage.
Patients developing AL showed a significantly lower pH value even from POD1 with mean pH value 6.93 whereas the n-AL group had mean pH=7.46 and were consistent every postoperative day (POD2 AL 6.85 vs n-AL 7.55, POD3 6.84 vs 7.58, POD4 AL 6.91 vs 7.61 and POD5 AL 6.85 vs n-AL 7.67) (Table 2). In the postoperative period, it was clear that in the group AL the mean pH values demonstrated a consistent tendency to decrease, whereas the n-AL group mean pH values showed a stable and rising pattern (Figure 1).
|
Presence or Absence of AL |
||||||
|
n-AL (n=52) |
AL (n=6) |
|||||
|
Days |
Lower Bound of 95% CI |
Mean |
Upper Bound of 95% CI |
Lower Bound of 95% CI |
Mean |
Upper Bound of 95% CI |
|
1st |
7.380 |
7.466 a |
7.552 |
6.684 |
6.938 b |
7.192 |
|
2nd |
7.465 |
7.557 a |
7.648 |
6.586 |
6.854 b |
7.123 |
|
3rd |
7.502 |
7.588 a |
7.675 |
6.587 |
6.842 b |
7.098 |
|
4th |
7.530 |
7.618 a |
7.705 |
6.655 |
6.913 b |
7.170 |
|
5th |
7.598 |
7.671 a |
7.744 |
6.638 |
6.853 b |
7.067 |
Table 2: Comparisons of mean pH values along with the corresponding 95% Confidence Intervals (CI) between patients with (AL) and without (n-AL) Anastomotic Leakage (AL) within the five Post-Operative Days. For each Post-Operative Day mean pH values followed by different letter are statistically significant different at significance level a=0.05 according to the results of the LSD criterion. In all comparisons the corresponding p-values were <0.001.
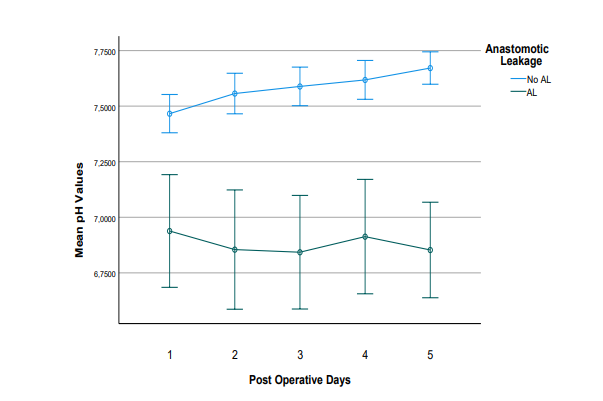
Figure 1: Evolution of mean pH values in the postoperative period in patients with (n=6) and without (n=52) Anastomotic Leakage (AL). The error bars correspond to the 95% Confidence Intervals (CI) around the mean values.
Regarding lactate from the abdominal drain as a predictor for anastomotic failure in right colectomy, the mean values in the non-AL group showed a decreasing trend throughout the postoperative period. In contrast, the mean lactate values in the AL group exhibited an increasing pattern during the postoperative days and remained consistently above 10. From POD 3 onwards, there was a significant difference between the mean values of the AL and non-AL groups (Table 3, Figure 2).
|
Presence of AL |
||||||
|
n-AL |
AL |
|||||
|
Days |
Lower Bound |
Mean |
Upper Bound |
Lower Bound |
Mean |
Upper Bound |
|
1 |
9.937 |
10.873 a |
11.808 |
9.097 |
11.850 a |
14.603 |
|
2 |
9.480 |
10.607 a |
11.735 |
10.131 |
13.450 a |
16.769 |
|
3 |
7.668 |
8.977 a |
10.286 |
10.163 |
14.017 b |
17.870 |
|
4 |
6.804 |
7.918 a |
9.033 |
8.819 |
12.100 b |
15.381 |
|
5 |
5.863 |
6.932 a |
8.001 |
9.469 |
12.617 b |
15.764 |
Table 3: Comparisons of mean lactate values between patients with (AL) and without (n-AL) Anastomotic Leakage (AL) within the five Post-Operative Days. For each Post-Operative Day mean Lactate values followed by different letter are statistically significant different at significance level a=0.05 according to the results of the LSD criterion. The p-value for the comparison for the 3rd day was 0.016, the p-value for the comparison for the 4th day was 0.019 and the p-value for the comparison for the 5th day was 0.001.
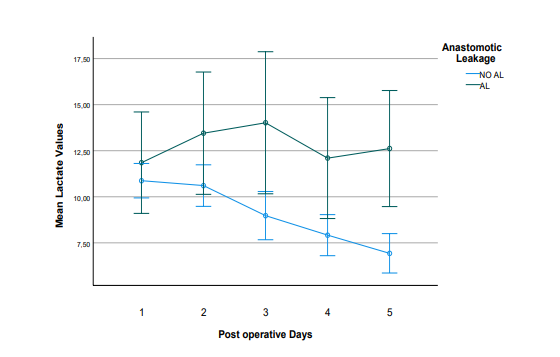
Figure 2: Evolution of mean Lactate values in the postoperative period in patients with (n=6) and without (n=52) Anastomotic Leakage (AL). The error bars correspond to the 95% Confidence Intervals (CI) around the mean values.
As far as glucose from peritoneal drain is concerned, our study demonstrated a stable pattern in both groups, with a difference in mean glucose values between the two groups, beginning from POD2 (Table 4).
|
Presence of AL |
||||||
|
n-AL |
AL |
|||||
|
Days |
Lower Bound |
Mean |
Upper Bound |
Lower Bound |
Mean |
Upper Bound |
|
1 |
40.975 |
55.451 a |
69.927 |
10.629 |
52.833 a |
95.037 |
|
2 |
31.852 |
41.490 a |
51.129 |
0.00 |
15.167 a |
43.267 |
|
3 |
37.393 |
45.667 a |
53.940 |
0 |
11.000 b |
35.121 |
|
4 |
38.593 |
49.396 a |
60.199 |
0 |
14.500 b |
45.995 |
|
5 |
40.344 |
50.822 a |
61.299 |
0 |
15.167 b |
45.714 |
Table 4: Comparisons of mean glucose values between patients with (AL) and without (n-AL) Anastomotic Leakage (AL) within the five Post-Operative Days. For each Post-Operative Day mean Glucose values followed by different letter are statistically significant different at significance level a=0.05 according to the results of the LSD criterion. The p-value for the comparison for the 3rd day was 0.009, the p-value for the comparison for the 4th day was 0.040 and the p-value for the comparison for the 5th day was 0.031.
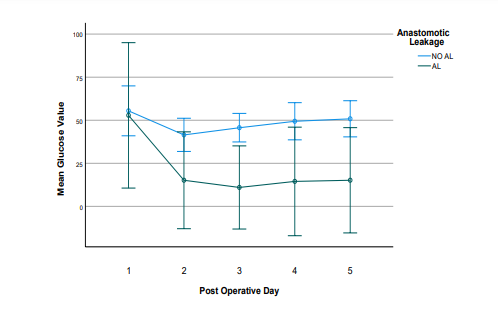
Figure 3: Evolution of mean Glucose values in the postoperative period in patients with (n=6) and without (n=52) Anastomotic Leakage (AL). The error bars correspond to the 95% Confidence Intervals (CI) around the mean values. After the 2nd Post-Operative Day, the lower bounds of the 95% Confidence Intervals had negative values, because the corresponding standard errors were very high relative to the mean values. In Table 4 for clinical reasons the lower bounds of these Confidence Intervals were set to zero.
Discussion
Right hemicolectomy is one of the most commonly performed colorectal operations, primarily used to treat right-sided colon cancer. Other indications for right hemicolectomy are Crohn’s disease and its complications, right-sided colon bleeding, diverticulitis, bowel obstruction, complicated appendicitis involving the appendix base or cecum, ischemic colitis, noniatrogenic trauma, and iatrogenic trauma [10]. This procedure involves the removal of the cecum, ascending colon, hepatic flexure, the first third of the transverse colon, and part of the terminal ileum, along with associated fat and lymph nodes. Since Reybard performed the first successful open right hemicolectomy in 1832, the technique has been refined multiple times [11]. In the 1990s, Jacobs et al. introduced laparoscopic right hemicolectomy, which in time has become the standard treatment for rightsided colon cancer due to its better short-term outcomes and comparable effectiveness and safety to open surgery [12]. In 2009, Hohenberger et al. proposed the concept of Complete Mesocolic Excision (CME) with high arterial ligation. [13,14]. Today, right hemicolectomy can be performed using open, laparoscopic, and robotic approaches. In addition to the various methods used for right colectomy, different approaches have been described within the same method. These include the medial-to-lateral approach, the lateral-to-medial approach, the caudal-to-cranial approach by Li H et al., [15] the duodenal window first approach by Alban et al. [16], and the uncinate process first approach (UFA) by Benz S et al [17].
Despite these advancements, complications following right hemicolectomy remain common, occurring in approximately 30% of cases, with a postoperative mortality rate of around 3%. Anastomotic leakage (AL) is one of the most severe postoperative complications in modern colorectal cancer surgery [18]. The incidence of AL in all colorectal operations ranges from 2% to 19%, and it is more frequent in patients undergoing surgery for leftsided colon or rectal cancer. However, ileocolic anastomosis leaks in right-sided colon surgery are also prevalent, with rates between 3.4% and 8%. AL is not only potentially fatal but also negatively affects oncological outcomes [18,19]. Despite the identification of several pre-operative, intra-operative and postoperative risk factors, Anastomotic Leakage (AL) remains challenging to predict and diagnose early after surgery. Early clinical presentations of AL are heterogenous and often nonspecific. It can manifest as peritonitis, localized fluid collections, or through nonspecific symptoms such as fever, lack of bowel movement, and diarrhea, or even symptoms mimic cardiovascular conditions, which typically become noticeable between postoperative days (PODs) 4 and 7 [20]. What is more, during the early postoperative period, distinguishing intra-abdominal sepsis from the normal systemic inflammatory response to surgery can be difficult. Patients discharged shortly after surgery have the risk of being readmitted with AL or severe sepsis. Abdominal CT scans, the current gold standard for diagnosing AL, may have low sensitivity, potentially delaying diagnosis and appropriate treatment [21,22]. If AL is diagnosed late, it can lead to severe sepsis, multiple organ dysfunction, and death. Delayed diagnosis and subsequent delays in administering antibiotics during septic shock onset are associated with a 7.6% decrease in survival per hour. Long-term consequences of significant AL may include an increased risk of colorectal cancer recurrence, reduced quality of life, and decreased long-term survival [23,24].
The management of an Anastomotic Leakage (AL) after right hemicolectomy depends on factors such as the patient’s overall condition, the presence of peritonitis, and existing comorbidities. Treatment options include oversewing the leak, performing a redo anastomosis, or creating a terminal stoma, depending on the patient’s condition and the intraoperative findings. Creating a terminal stoma is more likely in seriously ill patients, such as those with septic peritonitis, single or multiple organ failure, and significant comorbidities [25]. Therefore, timely diagnosis of Anastomotic Leakage (AL) before clinical symptoms appear is crucial. The need for accurate early-stage diagnostic markers for AL after colorectal surgery, to reduce diagnostic delays and their negative consequences, has led to the investigation of various biomarkers such as C-reactive protein (CRP), and procalcitonin (PCT), neutrophil to lymphocyte ratio (NLR), derived neutrophil to lymphocyte ratio (dNLR), Lymphocyte to Monocyte Ratio (LMR), and Platelet To Lymphocyte (PLR) ratio, which have shown relatively good results [26,27]. Unfortunately, these markers are nonspecific, as elevated levels can result from various postoperative complications, including chest, urinary, and surgical site infections. Given these limitations, new strategies for detecting AL are necessary. One such strategy involves measuring local biomarkers in the immediate environment of the anastomosis.
Potential biomarkers can be classified into four categories: immune parameters (cytokines such as interleukin (IL)-1, IL-6, IL-10, and tumor necrosis factor-alpha (TNF-alpha)), tissue-repair markers (matrix metalloproteinases (MMPs)), ischemia-related parameters (pH, lactate), and microbiological parameters (lipopolysaccharides, a marker of bacteria). [28] Although promising, most of these biomarkers are costly, labor-intensive, and require technically challenging, sophisticated methods for measurement. In contrast, measuring ischemia-related pH and lactate from peritoneal drain fluid may offer a quick, easy, and inexpensive alternative. The principle behind this approach is that inadequate blood supply to the anastomosis increases the risk of a leak and raises the acidity in the area around the anastomosis.
In the literature, three studies have examined pH and lactate levels in peritoneal drain samples as predictors of Anastomotic Leakage (AL). In 2013, Yang et al. conducted a retrospective study of 753 patients after low anterior resection and found a significant decrease in pH among patients who experienced leakage (pH <6.978 on POD3), with excellent sensitivity (98.7%) and specificity (94.7%). Similarly, in 2014, Bini et al. assessed lactate levels in peritoneal drain fluid in 88 patients after abdominal surgery, concluding that a peritoneal/serum lactate level >4.5 or a peritoneal lactate level >9.1 were associated with postoperative complications requiring intervention, including AL. Molinary et al. evaluated drain fluid pH in a study of 173 elective colorectal operations and reported excellent results, with pH <7.53 on POD1 and pH <7.21 on POD3 showing 93.75% sensitivity and 97% specificity, respectively [2931]. Our study is the first to evaluate pH, Lactate and Glucose in every right colectomy, regardless of the underlying reason and method. Given our results, pH from the peritoneal drain fluid corelates with AL earlier than its clinical presentation. The mean peritoneal pH values in the AL group were significant lower when compared to those in the n-AL group. Even from POD2 pH assessment performed well in ruling out AL, whereas on POD3 and POD4 performed even safer.
Peritoneal lactate as a predictor of AL demonstrated a decreasing pattern in the group of n-AL, whereas in AL group ther was a raising tendency up to POD3 and stabilization in high values afterwards. The lactate in AL group had almost constantly values higher than 10, meanwhile in the n-AL group from POD3 and the following days the lactate values were always under 10.Regarding glucose levels from the peritoneal drain, our study demonstrated a stable pattern in both groups. However, there was a difference in the mean glucose values between the AL and non-AL groups. (Table 3). Major limitation of the study is the trend to avoid placement of drains in the current surgical practice, as the benefit of drains has been challenged [32]. In the current study the decision, the position, and the type of the drain was exclusively surgeon’s preference, which may influence the composition of the drained fluid. It is possible that certain patients in n-AL group might have had a subclinical anastomotic leakage. On the other hand, AL group cases were confirmed with a CT or reoperation, a very austere definition that could impact study results. Finally, larger-scale studies involving a substantial number of patients are necessary in order to clarify the stability of these factors in peritoneal fluid and conduct meaningful comparisons of sensitivity and specificity across different studies. As already mentioned, early AL diagnosis is essential to reduce patient morbidity and mortality.
Conclusion
The goal of early detection of AL is not necessarily to completely prevent it, but to ensure a safe outcome. Measures such as delaying the initiation of feeding, avoiding early discharge, adjusting antibiotic use, and, most importantly, being vigilant about the potential need for re-operation can help reduce further complications. Additionally, ruling out AL allows for the identification of candidates for Enhanced Recovery After Surgery (ERAS). (32) This approach, which has become increasingly popular in the management of colorectal surgery patients, reduces morbidity and shortens hospital stays.
References
- Stormark K, Krarup P, Sjövall A, Søreide K, Kvaløy JT, et al. (2020) Anastomotic leak after surgery for colon cancer and effect on longterm survival. Colorectal. Dis. Off. J. Assoc. Coloproctology. G B Irel 22: 1108-1118.
- Kryzauskas M, Bausys A, Degutyte AE, Abeciunas V, Poskus E, et al. (2020) Risk factors for anastomotic leakage and its impact on longterm survival in left-sided colorectal cancer surgery. World J. Surg. Oncol 205.
- (2015) European Society of Coloproctology Collaborating Group. The relationship between method of anastomosis and anastomotic failure after right hemicolectomy and ileo-caecal resection: An international snapshot audit. Colorectal. Dis. Off. J. Assoc. Coloproctology G B Irel 2017.
- Golub R, Golub RW, Cantu R Jr, et al. (1997) A multivariate analysis of factors contributing to leakage of intestinal anastomoses. J Am Coll Surg 184: 364e372.
- Alves A, Panis Y, Trancart D, et al. (2002) Factors associated with clinically significant anastomotic leakage after large bowel resection:multivariate analysis of 707 patients. World J Surg 26: 499e502.
- Reynolds IS, Boland MR, Reilly (2017) C-reactive protein as a predictor of anastomotic leak in the first week after anterior resection for rectal cancer. Colorectal Dis 19: 812-818.
- Warschkow R, Beutner U, Steffen T (2012) Safe and early discharge after colorectal surgery due to C-reactive protein:a diagnostic metaanalysis of 1832 patients. Ann Surg 256: 245-250.
- Singh PP, Zeng IS, Srinivasa S, Lemanu DP, Connolly AB, et al. (2014) Systematic review and meta-analysis of use of serum C-reactive protein levels to predict anastomotic leak after colorectal surgery. Br J Surg 101: 339-346.
- Gray M, Marland J, Murray A, Argyle D, Potter M (2021) Predictive and Diagnostic Biomarkers of Anastomotic Leakage: A Precision Medicine Approach for Colorectal Cancer Patients. J. Pers. Med 11: 471.
- Jessen M, Nerstrøm M, Wilbek TE, Roepstorff S, Rasmussen MS, et al. (2016) Risk factors for clinical anastomotic leakage after right hemicolectomy. Int. J. Colorectal. Dis 31: 1619-1624.
- Cotlar AM (2002) Historical landmarks in operations on the colon-surgeons courageous. Curr Surg 59: 91-95.
- Jacobs M, Verdeja JC, Goldstein HS (1991) Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 191: 144150.
- Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S (2009) Standardized surgery for colonic cancer: complete mesocolic excision and central ligation-technical notes and outcome. Colorectal Dis 11: 354-365.
- Zhang X, Zhang J, Ma P (2022) Tunnel versus medial approach in laparoscopic radical right hemicolectomy for right colon cancer: a retrospective cohort study. BMC Surg 22.
- Li H, He Y, Lin Z, Xiong W, Diao D, et al. (2016) Laparoscopic caudal-to-cranial approach for radical lymph node dissection in right hemicolectomy. Langenbeck’s Archives of Surgery 401: 741.
- Zarzavadjian Le Bian A, Cesaretti M, Smadja C, Costi R (2016) Total laparoscopic right colectomy: The duodenal window first approach. Surgical Oncology 25: 117.
- Benz S, Tam Y, Tannapfel A, Stricker I (2016) The uncinate process first approach: a novel technique for laparoscopic right hemicolectomy with complete mesocolic excision. Surgical Endoscopy 30: 1930.
- Dulskas A, Kuliavas J, Sirvys A, Bausys A, Kryzauskas M, et al. (2022) Anastomotic Leak Impact on Long-Term Survival after Right Colectomy for Cancer: A Propensity-Score-Matched Analysis. J. Clin. Med 4375.
- Jessen M, Nerstrøm M, Wilbek TE, Roepstorff S, Rasmussen MS, et al. (2016) Risk factors for clinical anastomotic leakage after right hemicolectomy. Int. J. Colorectal. Dis 31: 1619-1624.
- Alves A, Panis Y, Trancart D (2002) Factors associated with clinically significant anastomotic leakage after large bowel resection:multivariate analysis of 707 patients. World J Surg 26: 499e502.
- Khoury W, Ben-Yehuda A (2009) Abdom inal computed tomography for diagnosing postoperative lower gastrointestinal tract leaks. J Gastrointest Surg 13: 1454-1458.
- Doeksen A, Tanis PJ, Wüst AF (2008) Radiological evaluation of colorectal anastomoses. Int J Colorectal Dis 23: 863-868.
- Kulu Y, Ulrich A, Bruckner T, Contin P, Welsch T, et al. (2013) Validation of the International Study Group of Rectal Cancer definition and severity grading of anastomotic leakage. Surgery 153: 753-761.
- Hyman N, Manchester TL, Osler T, Burns B, Cataldo PA (2007) Anastomotic Leaks after Intestinal Anastomosis: It’s Later than You Think. Ann. Surg 245: 254-258.
- Schuster S, Aigner C, Raab S, Shamiyeh A (2024) Anastomotic Leakage after Right Hemicolectomy – A Retrospective Analysis of Complication Management and Outcome. Clin Surg 9: 3696
- Paliogiannis P, Deidda S, Maslyankov S (2020) Blood cell count indexes as predictors of anastomotic leakage in elective colorectal surgery: a multicenter study on 1432 patients. World J Surg Oncol 18: 89.
- Reynolds IS, Boland MR, Reilly (2017) C-reactive protein as a predictor of anastomotic leak in the first week after anterior resection for rectal cancer. Colorectal Dis 19: 812-818.
- Wright EC, Connolly P, Vella M, Moug S (2017) Peritoneal fluid biomarkers in the detection of colorectal anastomotic leaks: a systematic review. Int J Colorectal Dis 32: 935-945.
- Yang L, Huang X-E, Xu L, Zhou X (2013) Acidic pelvic drainage as a predictive factor for anastomotic leakage after surgery for patients with rectal cancer. Asian Pac J Cancer Prev 14: 5441-5447.
- Bini R, Ferrari G, Apra F, Viora T (2014) Peritoneal lactate as a potential biomarker for predicting the need for reintervention after abdominal surgery. J Trauma Acute Care Surg 77: 376-380.
- Molinari E, Giuliani T (2019) Drain fluid’s pH predicts anastomotic leak in colorectal surgery: results of a prospective analysis of 173 patients. Minerva Chir 75: 30-36.
- Kisialeuski M, Pedziwiatr M, Matlok M, Major P (2015) Enhanced recovery after colorectal surgery in elderly patients. Wideochir Inne Tech Maloinwazyjne 10: 30-36.
Introduction
Anastomotic Leak (AL) is one of the most dreadful complications in general surgery, with occurrence rates between 2% and 14% and higher tendency in left-sided colon or rectal operations [1,2]. Nevertheless, ileocolic anastomoses in right -sided colectomies also show a significant leakage rate, ranging from 3.4% to 8% [3]. AL is not only related with high morbidity, but can also adversely affect oncological outcomes. Delayed detection of anastomotic leakage following colorectal surgery is linked to higher mortality rates [4]. Clinical signs of AL are diverse and often nonspecific, presenting either with fever,lack of bowel movement, abdominal pain,or more specific like peritonitis, localized fluid collections, surgical wound infection or even subclinical leaks detected only through contrast radiology. Typically, these signs become evident between the fourth and seventh postoperative days [5]. In this regard, biomarkers such as White Blood Cells (WBC), C-Reactive Protein (CRP), and Procalcitonin (PRL) are employed as diagnostic or predictive tools for Anastomotic Leaks (AL) following colorectal surgeries [6,7]. Nevertheless, these biomarkers have limited positive predictive value and are often influenced also by other complications in the postoperative period. Currently, abdominal CT scans are the standard diagnostic method for detecting AL, but they often offer low sensitivity, which can delay both diagnosis and appropriate treatment [8]. There is a critical need for accurate early-stage diagnostic markers for AL after colorectal surgery to reduce diagnostic delays and their negative consequences. Various studies have explored peritoneal fluid biomarkers for early detection of AL, with interesting results. (28) These markers from peritoneal fluid are typically categorized into four groups: ischemia markers (pH and lactate), inflammation markers (TNFa,IL), microbiological parameters (lipopolysaccharides) and tissue repair markers (MMPs), all suggested as potential early indicators of processes that hinder anastomotic healing [9]. This prospective study investigates the diagnostic accuracy of pH, lactate, and glucose levels from drain fluid to predict anastomotic leakage following right hemicolectomy.
Materials and Methods
Study Design
This prospective study was conducted in three hospitals in Athens (Asklepieio Voula, Attikon Hospital Athens and G. Gennimatas General Hospital Athens). Patients were enrolled between November 2021 and November 2022. The study was approved by the Ethics Committee of each participating medical center, and informed consent was obtained from all patients. For the current study, all patients undergoing right hemicolectomy with a primary anastomosis for any pathology in the right colon, either elective or emergency were considered eligible and were analyzed. Exclusion criteria were operations requiring temporary ileostomy and right colectomies without placement of peritoneal drains. Collected data included patient demographics, symptoms at admission, TNM score, serum albumin, CEA, ASA score, BMI, comorbidities, indication for surgery, operative time, estimated blood loss, use of inotropes, postoperative day of bowel movement, postoperative day of feeding, WBC and CRP on POD1 to POD5 were obtained. Postoperative course was documented in detail, including the occurrence of fever, bowel function restoration by means of flatus, bleeding, prolonged hospital stay, readmissions and Anastomotic Leakage (AL).
The choice to drain, the type and placement of the drain tube were on surgeon’s discretion. Type of drainage used included Penrose drain (corrugated silicone silastic drain), and Jackson-Pratt drain (silicone flat drain connecting to a vacuum ball). In our study, the drain was placed in the subhepatic space as near to the anastomosis as possible. Drain fluid was collected every day after the ward round respecting rules of sterility with a syringe. The contents of the first 24h (referred as POD 0) were evacuated (but not analyzed). POD 1 was considered the drain fluid obtained 24 h after surgery and this specimen included in the analysis. Similarly, drain fluid was collected and marked for the following days. Peritoneal lactate, pH and glucose from the abdominal drain were analyzed immediately after collection using an ABL700 blood gas analyzer (Radiometer, Copenhagen, Denmark). The outcome of interest was AL within 30 days postoperatively. In our study AL was defined with clinical (gas, pus or fecal discharge from the drain, fecal discharge from the operative wound, peritonitis) or radiologic criteria (pelvic abscess, peri-anastomotic fistula, extravasation of contrast and peri-anastomotic liquid and air on CT scan). As AL were only considered cases confirmed either with CT scan or reoperation. Cases with minimal clinical presentation that were confirmed by CT scan were included.
All the patients were assigned to one of two groups according to the presence or absence of AL: with AL (Group AL), without AL (Group n-AL). The two groups were compared according to peritoneal pH, lactate and glucose levels on POD 1-5. AL was classified according to the system proposed by the International Study Group of Rectal Cancer (ISREC): Grade A can be left untreated; grade B requires medical management or minimally invasive therapeutic intervention (radiological drainage or other drainage) and grade C requiring revision surgery.
Statistical Analysis
Data for pH, Lactate and Glucose values were analyzed within the methodological frame of General Mixed Models using the ANOVA method (36) according to the model that includes the effects (main and interaction) of one between subjects’ factor with two levels (patients with an without AL) and one factor within subjects with five levels (the 5 post-operative days, treated as repeated measures). The ANOVA method was performed mainly for estimating the correct standard errors of the mean differences used for the clinically interesting comparisons of mean pH values. The mean values were compared with the protected Least Significant Difference (LSD) criterion. The model’s assumptions relative to sphericity and homogeneity of error variances were fulfilled. All statistical analyses were accomplished with the IBM SPSS Statistics v.23.0 software. In all hypothesis testing procedures, the significance level was predetermined at a=0.05 (P≤0.05)
Results
A total of 58 patients underwent right hemicolectomy during the study period (31 male patients, 27 female patients) were included. Median age was 71 (range 31-91) years. Most cases (66%, n=38) underwent elective surgery, whereas 20 (34%) underwent emergency surgery. Surgery was performed with a laparoscopic approach in 20 patients (34%). The diagnosis of AL (confirmed with CT) occurred in 6 patients (10.3%) becoming clinically evident after a mean time of 8 days (6-12) from surgery. Of these patients, 66.6% (n=4) developed grade B AL, whereas 33.3% (n=2) developed grade C AL. The incident of AL occurred in 4 out of 20 emergency cases and 2 out of 38 elective cases, whereas one out of laparoscopic 20 cases had AL and 5 out of 38 open cases had AL. (Table 1).
|
Operation Type |
Total number of patients |
No- Anastomotic Leakage |
Anastomotic Leakage |
|
Right Colectomy |
58 |
52 |
6 |
|
Laparoscopic |
20 |
19 |
1 |
|
Open |
38 |
33 |
5 |
|
Emergency |
20 |
16 |
4 |
|
Elective |
38 |
36 |
2 |
|
Malignancy |
43 |
39 |
4 |
|
Benign |
15 |
13 |
2 |
Table 1: Type of Operations and Distribution of Anastomotic Leakage.
Patients developing AL showed a significantly lower pH value even from POD1 with mean pH value 6.93 whereas the n-AL group had mean pH=7.46 and were consistent every postoperative day (POD2 AL 6.85 vs n-AL 7.55, POD3 6.84 vs 7.58, POD4 AL 6.91 vs 7.61 and POD5 AL 6.85 vs n-AL 7.67) (Table 2). In the postoperative period, it was clear that in the group AL the mean pH values demonstrated a consistent tendency to decrease, whereas the n-AL group mean pH values showed a stable and rising pattern (Figure 1).
|
Presence or Absence of AL |
||||||
|
n-AL (n=52) |
AL (n=6) |
|||||
|
Days |
Lower Bound of 95% CI |
Mean |
Upper Bound of 95% CI |
Lower Bound of 95% CI |
Mean |
Upper Bound of 95% CI |
|
1st |
7.380 |
7.466 a |
7.552 |
6.684 |
6.938 b |
7.192 |
|
2nd |
7.465 |
7.557 a |
7.648 |
6.586 |
6.854 b |
7.123 |
|
3rd |
7.502 |
7.588 a |
7.675 |
6.587 |
6.842 b |
7.098 |
|
4th |
7.530 |
7.618 a |
7.705 |
6.655 |
6.913 b |
7.170 |
|
5th |
7.598 |
7.671 a |
7.744 |
6.638 |
6.853 b |
7.067 |
Table 2: Comparisons of mean pH values along with the corresponding 95% Confidence Intervals (CI) between patients with (AL) and without (n-AL) Anastomotic Leakage (AL) within the five Post-Operative Days. For each Post-Operative Day mean pH values followed by different letter are statistically significant different at significance level a=0.05 according to the results of the LSD criterion. In all comparisons the corresponding p-values were <0.001.

1 2 3 4 5
Post Operative Days
Figure 1: Evolution of mean pH values in the postoperative period in patients with (n=6) and without (n=52) Anastomotic Leakage (AL). The error bars correspond to the 95% Confidence Intervals (CI) around the mean values.
Regarding lactate from the abdominal drain as a predictor for anastomotic failure in right colectomy, the mean values in the non-AL group showed a decreasing trend throughout the postoperative period. In contrast, the mean lactate values in the AL group exhibited an increasing pattern during the postoperative days and remained consistently above 10. From POD 3 onwards, there was a significant difference between the mean values of the AL and non-AL groups (Table 3, Figure 2).
|
Presence of AL |
||||||
|
n-AL |
AL |
|||||
|
Days |
Lower Bound |
Mean |
Upper Bound |
Lower Bound |
Mean |
Upper Bound |
|
1 |
9.937 |
10.873 a |
11.808 |
9.097 |
11.850 a |
14.603 |
|
2 |
9.480 |
10.607 a |
11.735 |
10.131 |
13.450 a |
16.769 |
|
3 |
7.668 |
8.977 a |
10.286 |
10.163 |
14.017 b |
17.870 |
|
4 |
6.804 |
7.918 a |
9.033 |
8.819 |
12.100 b |
15.381 |
|
5 |
5.863 |
6.932 a |
8.001 |
9.469 |
12.617 b |
15.764 |
Table 3: Comparisons of mean lactate values between patients with (AL) and without (n-AL) Anastomotic Leakage (AL) within the five Post-Operative Days. For each Post-Operative Day mean Lactate values followed by different letter are statistically significant different at significance level a=0.05 according to the results of the LSD criterion. The p-value for the comparison for the 3rd day was 0.016, the p-value for the comparison for the 4th day was 0.019 and the p-value for the comparison for the 5th day was 0.001.

1 2 3 4 5
Post operative Days
Figure 2: Evolution of mean Lactate values in the postoperative period in patients with (n=6) and without (n=52) Anastomotic Leakage (AL). The error bars correspond to the 95% Confidence Intervals (CI) around the mean values.
As far as glucose from peritoneal drain is concerned, our study demonstrated a stable pattern in both groups, with a difference in mean glucose values between the two groups, beginning from POD2 (Table 4).
|
Presence of AL |
||||||
|
n-AL |
AL |
|||||
|
Days |
Lower Bound |
Mean |
Upper Bound |
Lower Bound |
Mean |
Upper Bound |
|
1 |
40.975 |
55.451 a |
69.927 |
10.629 |
52.833 a |
95.037 |
|
2 |
31.852 |
41.490 a |
51.129 |
0.00 |
15.167 a |
43.267 |
|
3 |
37.393 |
45.667 a |
53.940 |
0 |
11.000 b |
35.121 |
|
4 |
38.593 |
49.396 a |
60.199 |
0 |
14.500 b |
45.995 |
|
5 |
40.344 |
50.822 a |
61.299 |
0 |
15.167 b |
45.714 |
Table 4: Comparisons of mean glucose values between patients with (AL) and without (n-AL) Anastomotic Leakage (AL) within the five Post-Operative Days. For each Post-Operative Day mean Glucose values followed by different letter are statistically significant different at significance level a=0.05 according to the results of the LSD criterion. The p-value for the comparison for the 3rd day was 0.009, the p-value for the comparison for the 4th day was 0.040 and the p-value for the comparison for the 5th day was 0.031.

Post Operative Day
Figure 3: Evolution of mean Glucose values in the postoperative period in patients with (n=6) and without (n=52) Anastomotic Leakage (AL). The error bars correspond to the 95% Confidence Intervals (CI) around the mean values. After the 2nd Post-Operative Day, the lower bounds of the 95% Confidence Intervals had negative values, because the corresponding standard errors were very high relative to the mean values. In Table 4 for clinical reasons the lower bounds of these Confidence Intervals were set to zero.
Discussion
Right hemicolectomy is one of the most commonly performed colorectal operations, primarily used to treat right-sided colon cancer. Other indications for right hemicolectomy are Crohn’s disease and its complications, right-sided colon bleeding, diverticulitis, bowel obstruction, complicated appendicitis involving the appendix base or cecum, ischemic colitis, noniatrogenic trauma, and iatrogenic trauma [10]. This procedure involves the removal of the cecum, ascending colon, hepatic flexure, the first third of the transverse colon, and part of the terminal ileum, along with associated fat and lymph nodes. Since Reybard performed the first successful open right hemicolectomy in 1832, the technique has been refined multiple times [11]. In the 1990s, Jacobs et al. introduced laparoscopic right hemicolectomy, which in time has become the standard treatment for rightsided colon cancer due to its better short-term outcomes and comparable effectiveness and safety to open surgery [12]. In 2009, Hohenberger et al. proposed the concept of Complete Mesocolic Excision (CME) with high arterial ligation. [13,14]. Today, right hemicolectomy can be performed using open, laparoscopic, and robotic approaches. In addition to the various methods used for right colectomy, different approaches have been described within the same method. These include the medial-to-lateral approach, the lateral-to-medial approach, the caudal-to-cranial approach by Li H et al., [15] the duodenal window first approach by Alban et al. [16], and the uncinate process first approach (UFA) by Benz S et al [17].
Despite these advancements, complications following right hemicolectomy remain common, occurring in approximately 30% of cases, with a postoperative mortality rate of around 3%. Anastomotic leakage (AL) is one of the most severe postoperative complications in modern colorectal cancer surgery [18]. The incidence of AL in all colorectal operations ranges from 2% to 19%, and it is more frequent in patients undergoing surgery for leftsided colon or rectal cancer. However, ileocolic anastomosis leaks in right-sided colon surgery are also prevalent, with rates between 3.4% and 8%. AL is not only potentially fatal but also negatively affects oncological outcomes [18,19]. Despite the identification of several pre-operative, intra-operative and postoperative risk factors, Anastomotic Leakage (AL) remains challenging to predict and diagnose early after surgery. Early clinical presentations of AL are heterogenous and often nonspecific. It can manifest as peritonitis, localized fluid collections, or through nonspecific symptoms such as fever, lack of bowel movement, and diarrhea, or even symptoms mimic cardiovascular conditions, which typically become noticeable between postoperative days (PODs) 4 and 7 [20]. What is more, during the early postoperative period, distinguishing intra-abdominal sepsis from the normal systemic inflammatory response to surgery can be difficult. Patients discharged shortly after surgery have the risk of being readmitted with AL or severe sepsis. Abdominal CT scans, the current gold standard for diagnosing AL, may have low sensitivity, potentially delaying diagnosis and appropriate treatment [21,22]. If AL is diagnosed late, it can lead to severe sepsis, multiple organ dysfunction, and death. Delayed diagnosis and subsequent delays in administering antibiotics during septic shock onset are associated with a 7.6% decrease in survival per hour. Long-term consequences of significant AL may include an increased risk of colorectal cancer recurrence, reduced quality of life, and decreased long-term survival [23,24].
The management of an Anastomotic Leakage (AL) after right hemicolectomy depends on factors such as the patient’s overall condition, the presence of peritonitis, and existing comorbidities. Treatment options include oversewing the leak, performing a redo anastomosis, or creating a terminal stoma, depending on the patient’s condition and the intraoperative findings. Creating a terminal stoma is more likely in seriously ill patients, such as those with septic peritonitis, single or multiple organ failure, and significant comorbidities [25]. Therefore, timely diagnosis of Anastomotic Leakage (AL) before clinical symptoms appear is crucial. The need for accurate early-stage diagnostic markers for AL after colorectal surgery, to reduce diagnostic delays and their negative consequences, has led to the investigation of various biomarkers such as C-reactive protein (CRP), and procalcitonin (PCT), neutrophil to lymphocyte ratio (NLR), derived neutrophil to lymphocyte ratio (dNLR), Lymphocyte to Monocyte Ratio (LMR), and Platelet To Lymphocyte (PLR) ratio, which have shown relatively good results [26,27]. Unfortunately, these markers are nonspecific, as elevated levels can result from various postoperative complications, including chest, urinary, and surgical site infections. Given these limitations, new strategies for detecting AL are necessary. One such strategy involves measuring local biomarkers in the immediate environment of the anastomosis.
Potential biomarkers can be classified into four categories: immune parameters (cytokines such as interleukin (IL)-1, IL-6, IL-10, and tumor necrosis factor-alpha (TNF-alpha)), tissue-repair markers (matrix metalloproteinases (MMPs)), ischemia-related parameters (pH, lactate), and microbiological parameters (lipopolysaccharides, a marker of bacteria). [28] Although promising, most of these biomarkers are costly, labor-intensive, and require technically challenging, sophisticated methods for measurement. In contrast, measuring ischemia-related pH and lactate from peritoneal drain fluid may offer a quick, easy, and inexpensive alternative. The principle behind this approach is that inadequate blood supply to the anastomosis increases the risk of a leak and raises the acidity in the area around the anastomosis.
In the literature, three studies have examined pH and lactate levels in peritoneal drain samples as predictors of Anastomotic Leakage (AL). In 2013, Yang et al. conducted a retrospective study of 753 patients after low anterior resection and found a significant decrease in pH among patients who experienced leakage (pH <6.978 on POD3), with excellent sensitivity (98.7%) and specificity (94.7%). Similarly, in 2014, Bini et al. assessed lactate levels in peritoneal drain fluid in 88 patients after abdominal surgery, concluding that a peritoneal/serum lactate level >4.5 or a peritoneal lactate level >9.1 were associated with postoperative complications requiring intervention, including AL. Molinary et al. evaluated drain fluid pH in a study of 173 elective colorectal operations and reported excellent results, with pH <7.53 on POD1 and pH <7.21 on POD3 showing 93.75% sensitivity and 97% specificity, respectively [2931]. Our study is the first to evaluate pH, Lactate and Glucose in every right colectomy, regardless of the underlying reason and method. Given our results, pH from the peritoneal drain fluid corelates with AL earlier than its clinical presentation. The mean peritoneal pH values in the AL group were significant lower when compared to those in the n-AL group. Even from POD2 pH assessment performed well in ruling out AL, whereas on POD3 and POD4 performed even safer.
Peritoneal lactate as a predictor of AL demonstrated a decreasing pattern in the group of n-AL, whereas in AL group ther was a raising tendency up to POD3 and stabilization in high values afterwards. The lactate in AL group had almost constantly values higher than 10, meanwhile in the n-AL group from POD3 and the following days the lactate values were always under 10.Regarding glucose levels from the peritoneal drain, our study demonstrated a stable pattern in both groups. However, there was a difference in the mean glucose values between the AL and non-AL groups. (Table 3). Major limitation of the study is the trend to avoid placement of drains in the current surgical practice, as the benefit of drains has been challenged [32]. In the current study the decision, the position, and the type of the drain was exclusively surgeon’s preference, which may influence the composition of the drained fluid. It is possible that certain patients in n-AL group might have had a subclinical anastomotic leakage. On the other hand, AL group cases were confirmed with a CT or reoperation, a very austere definition that could impact study results. Finally, larger-scale studies involving a substantial number of patients are necessary in order to clarify the stability of these factors in peritoneal fluid and conduct meaningful comparisons of sensitivity and specificity across different studies. As already mentioned, early AL diagnosis is essential to reduce patient morbidity and mortality.
Conclusion
The goal of early detection of AL is not necessarily to completely prevent it, but to ensure a safe outcome. Measures such as delaying the initiation of feeding, avoiding early discharge, adjusting antibiotic use, and, most importantly, being vigilant about the potential need for re-operation can help reduce further complications. Additionally, ruling out AL allows for the identification of candidates for Enhanced Recovery After Surgery (ERAS). (32) This approach, which has become increasingly popular in the management of colorectal surgery patients, reduces morbidity and shortens hospital stays.
References
1. Stormark K, Krarup P, Sjövall A, Søreide K, Kvaløy JT, et al. (2020) Anastomotic leak after surgery for colon cancer and effect on longterm survival. Colorectal. Dis. Off. J. Assoc. Coloproctology. G B Irel 22: 1108-1118.
2. Kryzauskas M, Bausys A, Degutyte AE, Abeciunas V, Poskus E, et al. (2020) Risk factors for anastomotic leakage and its impact on longterm survival in left-sided colorectal cancer surgery. World J. Surg. Oncol 205.
3. (2015) European Society of Coloproctology Collaborating Group. The relationship between method of anastomosis and anastomotic failure after right hemicolectomy and ileo-caecal resection: An international snapshot audit. Colorectal. Dis. Off. J. Assoc. Coloproctology G B Irel 2017.
4. Golub R, Golub RW, Cantu R Jr, et al. (1997) A multivariate analysis of factors contributing to leakage of intestinal anastomoses. J Am Coll Surg 184: 364e372.
5. Alves A, Panis Y, Trancart D, et al. (2002) Factors associated with clinically significant anastomotic leakage after large bowel resection:
multivariate analysis of 707 patients. World J Surg 26: 499e502.
6. Reynolds IS, Boland MR, Reilly (2017) C-reactive protein as a predictor of anastomotic leak in the first week after anterior resection for rectal cancer. Colorectal Dis 19: 812-818.
7. Warschkow R, Beutner U, Steffen T (2012) Safe and early discharge after colorectal surgery due to C-reactive protein:a diagnostic metaanalysis of 1832 patients. Ann Surg 256: 245-250.
8. Singh PP, Zeng IS, Srinivasa S, Lemanu DP, Connolly AB, et al. (2014) Systematic review and meta-analysis of use of serum C-reactive protein levels to predict anastomotic leak after colorectal surgery. Br J Surg 101: 339-346.
9. Gray M, Marland J, Murray A, Argyle D, Potter M (2021) Predictive and Diagnostic Biomarkers of Anastomotic Leakage: A Precision Medicine Approach for Colorectal Cancer Patients. J. Pers. Med 11: 471.
10. Jessen M, Nerstrøm M, Wilbek TE, Roepstorff S, Rasmussen MS, et al. (2016) Risk factors for clinical anastomotic leakage after right hemicolectomy. Int. J. Colorectal. Dis 31: 1619-1624.
11. Cotlar AM (2002) Historical landmarks in operations on the colon-surgeons courageous. Curr Surg 59: 91-95.
12. Jacobs M, Verdeja JC, Goldstein HS (1991) Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 191: 144150.
13. Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S (2009) Standardized surgery for colonic cancer: complete mesocolic excision and central ligation-technical notes and outcome. Colorectal Dis 11: 354-365.
14. Zhang X, Zhang J, Ma P (2022) Tunnel versus medial approach in laparoscopic radical right hemicolectomy for right colon cancer: a retrospective cohort study. BMC Surg 22.
15. Li H, He Y, Lin Z, Xiong W, Diao D, et al. (2016) Laparoscopic caudal-to-cranial approach for radical lymph node dissection in right hemicolectomy. Langenbeck’s Archives of Surgery 401: 741.
16. Zarzavadjian Le Bian A, Cesaretti M, Smadja C, Costi R (2016) Total laparoscopic right colectomy: The duodenal window first approach. Surgical Oncology 25: 117.
17. Benz S, Tam Y, Tannapfel A, Stricker I (2016) The uncinate process first approach: a novel technique for laparoscopic right hemicolectomy with complete mesocolic excision. Surgical Endoscopy 30: 1930.
18. Dulskas A, Kuliavas J, Sirvys A, Bausys A, Kryzauskas M, et al. (2022) Anastomotic Leak Impact on Long-Term Survival after Right Colectomy for Cancer: A Propensity-Score-Matched Analysis. J. Clin. Med 4375.
19. Jessen M, Nerstrøm M, Wilbek TE, Roepstorff S, Rasmussen MS, et al. (2016) Risk factors for clinical anastomotic leakage after right hemicolectomy. Int. J. Colorectal. Dis 31: 1619-1624.
20. Alves A, Panis Y, Trancart D (2002) Factors associated with clinically significant anastomotic leakage after large bowel resection:
multivariate analysis of 707 patients. World J Surg 26: 499e502.
21. Khoury W, Ben-Yehuda A (2009) Abdominal computed tomography for diagnosing postoperative lower gastrointestinal tract leaks. J Gastrointest Surg 13: 1454-1458.
22. Doeksen A, Tanis PJ, Wüst AF (2008) Radiological evaluation of colorectal anastomoses. Int J Colorectal Dis 23: 863-868.
23. Kulu Y, Ulrich A, Bruckner T, Contin P, Welsch T, et al. (2013) Validation of the International Study Group of Rectal Cancer definition and severity grading of anastomotic leakage. Surgery 153: 753-761.
24. Hyman N, Manchester TL, Osler T, Burns B, Cataldo PA (2007) Anastomotic Leaks after Intestinal Anastomosis: It’s Later than You Think. Ann. Surg 245: 254-258.
25. Schuster S, Aigner C, Raab S, Shamiyeh A (2024) Anastomotic Leakage after Right Hemicolectomy – A Retrospective Analysis of Complication Management and Outcome. Clin Surg 9: 3696
26. Paliogiannis P, Deidda S, Maslyankov S (2020) Blood cell count indexes as predictors of anastomotic leakage in elective colorectal surgery: a multicenter study on 1432 patients. World J Surg Oncol 18: 89.
27. Reynolds IS, Boland MR, Reilly (2017) C-reactive protein as a predictor of anastomotic leak in the first week after anterior resection for rectal cancer. Colorectal Dis 19: 812-818.
28. Wright EC, Connolly P, Vella M, Moug S (2017) Peritoneal fluid biomarkers in the detection of colorectal anastomotic leaks: a systematic review. Int J Colorectal Dis 32: 935-945.
29. Yang L, Huang X-E, Xu L, Zhou X (2013) Acidic pelvic drainage as a predictive factor for anastomotic leakage after surgery for patients with rectal cancer. Asian Pac J Cancer Prev 14: 5441-5447.
30. Bini R, Ferrari G, Apra F, Viora T (2014) Peritoneal lactate as a potential biomarker for predicting the need for reintervention after abdominal surgery. J Trauma Acute Care Surg 77: 376-380.
31. Molinari E, Giuliani T (2019) Drain fluid’s pH predicts anastomotic leak in colorectal surgery: results of a prospective analysis of 173 patients. Minerva Chir 75: 30-36.
32. Kisialeuski M, Pedziwiatr M, Matlok M, Major P (2015) Enhanced recovery after colorectal surgery in elderly patients. Wideochir Inne Tech Maloinwazyjne 10: 30-36.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.