Posterior Lumbar Interbody Fusion Results With Supercritical CO Processed Bone Allografts in a 2 Series of 85 Consecutive Patients
by Rainer Giacomelli*
Hôpital Privé De La Miotte, 15 av Miotte, 90000 Belfort, France
*Corresponding author: Rainer Giacomelli, Hôpital Privé De La Miotte, 15 av Miotte, 90000 Belfort, France
Received Date: 16 December 2024
Accepted Date: 19 December 2024
Published Date: 23 December 2024
Citation: Giacomelli R (2024) Posterior Lumbar Interbody Fusion Results With Supercritical CO2 Processed Bone Allografts in a Series of 85 Consecutive Patients. Ann Case Report. 9: 2131. https://doi.org/10.29011/2574-7754.102131
Abstract
Purpose: The aim of this study was to report our experience in lumbar fusion cases using the Supercritical CO2 processed bone allografts. Materials and Methods: 85 patients underwent 1 or 2 level posterior lumbar fusion using bone allografts processed by Supercritical CO2 extraction combined with chemical viral inactivation. Radiographic evaluation was performed at immediate postoperative, 3 months, 6 months and at a mean 12.4 months post-surgery combined with a CT including fine coronal tomography. Results: No patient had surgery-related spinal nerve injury, one dural laceration was stitched without clinical consequences. The radiological evaluation of the 92 operated levels at last follow-up showed that 88 of these levels (95.7%) were fused. Four of the operated levels (4.3%) were non-fused and considered to have complications. Conclusion: Within the limitations of this study, the use of supercritical CO2 processed bone allograft resulted in good clinical outcome results and fusion rate in lumbar surgeries.
Introduction
Lumbar and cervical arthrodesis are often the best option for various degenerative spinal conditions that do not respond to conservative treatment. Historically, the gold standard for spinal arthrodesis was the use of autogenous iliac crest bone graft (ICBG) [1,2]. However, the use of ICBG has several drawbacks, including donor site pain and morbidity, increased operating time and variable quality of the autograft [3-6].
Alternatives to ICBG for spinal arthrodesis include the use of allografts, graft extensions and osteobiologic materials to increase fusion rates [7,8].
BIOBank offers an allogeneic bone graft in the form of bone powder inactivated by the Supercrit® process, which has been meeting the need for bone filling in dental and orthopedic surgery since 2003 [9-17].
The aim of this study was to present our experience in posterior lumbar spine surgery using bone allograft processed with the Supercrit® technology.
Materials and Methods
Between February 2020 and October 2023, eighty-five (85) patients received the BIOBank supercritical CO2 processed bone allografts for lumbar fusion:
Patients’ demographic information is detailed in Table n°1 below:
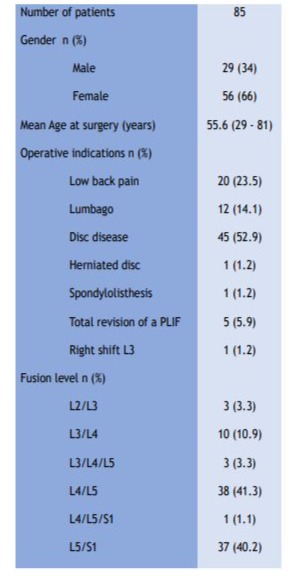
Table 1: Baseline characteristics of the patients.
This study was conducted in accordance with all applicable regulations including the French Data Protection Authority (the CNIL) Reference Methodology MR003 and with the principles of the Declaration of Helsinki.
The graft material used was the BIOBank cancellous bone allograft granules processed by the Supercrit® technology (Figure 1). The allografts were prepared from living donor femoral heads treated by the supercritical CO2 process through degreasing steps and a gentle chemical oxidation of the residual proteins with preserved bone architecture. Before using for fusion, the bone allograft powder and granules drawn from the cleaned femoral head and packed in syringe or vial were hydrated with saline or blood.
Figure 1: Cancellous bone powder prepared from living donor femoral head treated by Supercrit® process.
All the surgical procedures were performed by a senior neurosurgeon.
Lumbar arthrodesis surgery was performed by posterior approach: the procedure included a complete laminectomy, facetectomy, discectomy, interbody fusion by two cages followed by instrumented posterolateral fusion using pedicle screw systems (Romeo®, Juliet® cage Spineart or Instinct® Java®, Zimmer). Positioning of implants was controlled by conventional perioperative radiography. After realizing total bilateral discectomy vertebral plates were scraped and bone allograft was inserted in the interbody space and around the cages (2x 2ml).
All patients were assessed preoperatively to determine their general health status, and the following assessments were performed at 3, 6 and 12 months post grafting visits .
Adequate fusion was defined as the presence of bridging bone across and around the interbody spacer. Postoperative x-ray and CT scans were reviewed at the 1-year follow-up both by independent radiologists and third-party examiners.
Statistical analyses were carried out in IBM SPSS Statistics 26 (SPSS Inc. Chicago, USA). All included cases were reviewed, and the summary statistics were analyzed as means (standard deviations) for continuous variables and percentages for categorical variables. Significance level was set at P < 0.05.
Results
A total of 85 patients underwent lumbar fusion surgery with the bone allograft representing 90 operated levels (Table 1).
There were 3 case of intraoperative complications with no consequence on the outcome which are:
- Two dural lacerations that were sutured and covered with standard neurosurgical techniques.
- One osteoporosis leading to a unilateral osteosynthesis instead of a bilateral fixation
- No patient had surgery-related spinal nerve injury or new neurological deficit.
At a mean follow-up of 12.4 months (ranged 10 to 22 months postsurgery), no patient was lost to follow-up.
Based on radiological assessment, 88 (95.7%) of the 92 operated levels were considered fused (Figures 2, 3 and 4) and 4 levels (4.3%) showed complications:
- 2 cages subsidence.
- 1 severe left convex low back scoliosis with significant lumbar spondylarthrosis.
- 1 retrolisthesis with bulging disc disease.
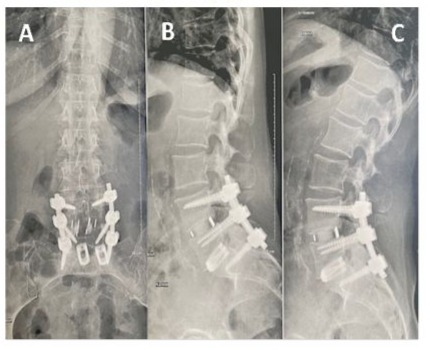
Figure 2: Two-level lumbar fusion using BIOBank allograft on a 54-year-old female at L4-L5 and L5-S1. Antero-posterior (A) and lateral radiographic views in flexion (B) and extension (C) radiographic views obtained at 1 year postoperative showing normal height of vertebral bodies with no significant static anomalies.
Figure 3: One-level lumbar fusion using BIOBank allograft on a 51-year-old male at L3-L4. Antero-posterior (A) and lateral radiographic views in extension (B) CT-Scan views obtained at 1 year postoperative showing bone fusion
Figure 4: One-level lumbar fusion using BIOBank allograft on a 72-year-old female at L4-L5 . Lateral 3D CT-Scan view obtained at 1 year postoperative showing bone fusion
Discussion
For spinal fusion, bone allografts are good alternative to Iliac Crest Bone autograft (ICBA) reported to be associated with an increased risk for donor site-related complications (pain, hematoma local infection etc.) [3-6, 18].
Bone allografts provide a practical and effective alternative to osteogenetic products, offering advantages in terms of availability, reduced morbidity, and versatility. While they may lack inherent osteogenic properties, they can be enhanced with additional biological factors to improve their efficacy in bone regeneration. These benefits make allografts a valuable option in orthopedic and reconstructive surgeries [19]. Allografts are generally more cost-effective and safer compared to some osteogenetic products that may involve complex processing or high costs, such as those involving growth factors like bone morphogenetic proteins [20].
Bone allografts used in PLIF procedures obtain good fusion rates while preserving cost effectiveness and avoiding downsides seen with common osteoconductive or osteogenetic: insertion of the cages in an interbody space filled up with week hydroxyapatite paste may push the latter towards the foramina.
The bone allografts used in our lumbar fusion study were made from living donors’ femoral heads, collected after hip replacement surgery and processed using supercritical CO2 extraction technology. By injecting directly the bone allograft in the disc space before placing the interbody cage, we reported good stability and fusion in 95.7% of our case at a mean 12 months. . There were no procedure nor did graft relate complications. Our results are consistent with literature were fusion rates between 83% to 100% were reported by several authors [21]. Long-term research will be conducted to confirm these encouraging results.
Within the limitations of this single center study, the use of bone allografts treated with supercritical CO2 resulted in good clinical outcomes and fusion rates, with good safety for lumbar fusion. The use of bone allografts treated with supercritical CO2 appears to be a safe strategy for achieving vertebral fusion while limiting the morbidity associated with autograft harvesting.
References
- Rajaee SS, Bae HW, Kanim LE, Delamarter RB. (2012). Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine. 37: 67-76.
- Marchesi DG. (2000). Spinal fusions: bone and bone substitutes. Eur Spine J. 9: 372-378.
- Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. (1996). Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996: 300-309.
- Tuchman A, Brodke DS, Youssef JA, Meisel HJ, Dettori JR, et al. (2017). Autograft versus Allograft for Cervical Spinal Fusion. Global Spine J. 7: 59-70.
- Gagdag AR, Lane JM, Glaser D, Forster RA. (1995). Alternatives to Autogenous Bone Graft: Efficacy and Indications. J. Am. Acad. Orthop. Surg. 195: 1-8.
- Gruskay JA, Basques BA, Bohl DD, Webb ML, Grauer JN. (2014). Short-Term Adverse Events, Length of Stay, and Readmission after Iliac Crest Bone Graft for Spinal Fusion. Spine. 39: 1718-1724.
- Grabowski G, Cornett CA. (2013). Bone graft and bone graft substitutes in spine surgery: current concepts and controversies. J Am Acad Orthop Surg. 21: 51- 60.
- Damien CJ, Parsons JR. (1991). Bone graft and bone graft substitutes: a review of current technology and applications. J Appl Biomater. 2: 187-208.
- Fages J, Marty A, Delga C, Condoret JS, Combers D, et al. (1994). Use of supercritical CO2 for bone delipidation. Biomaterials. 15: 650656.
- Fages J, Poirier B, Barbier Y, Frayssinet P, Joffret ML, et al. (1998). Viral inactivation of human bone tissue using supercritical fluid extraction. ASAIO J. 44: 289-293.
- Fages J, Jean E, Frayssinet P, Mathon D, Poirier B, et al. (1998). Bone allografts and supercritical processing: effects on osteointegration and viral safety. J. Supercrit. Fluids. 13: 351-356.
- Frayssinet P, Rouquet N, Mathon D, Autefage A, Fages J. (1998). Histological integration of allogeneic cancellous bone tissue treated by supercritical CO2 implanted in sheep bones. Biomaterials. 19: 22472253.
- Mitton D, Rappeneau J, Bardonnet R. (2005). Effect of a supercritical CO2 baed treatment on mechanical properties of human cancellous bone. Eur. J. Orthop. Surg. Traumatol. 15: 264-269.
- Chalard JJ, Edorh G. (2021). Long-Term Clinical and Radiographic Outcome of Extraction Socket Grafting with a Supercritical CO2 ViralInactivated Allogeneic Bone Graft. Biomed J Sci & Tech Res. PP: 35.
- Chalard JJ. (2021). Supercritical CO2 Viral-Inactivated Allogenic Bone Graft in Maxillary Sinus Augmentation Procedures: 10-Year Retrospective Clinical and Radiographic Results. Int J Periodontics Restorative Dent. 41: 433-441.
- Awiłło K. (2022). Supercritical CO2 Processed Bone Allografts in Implantology Treatment. Biomed J Sci & Tech Res. 44: 2022.
- Aurouer N, Guerin P, Cogniet A, Pedram M. (2023). The safe and effective use of supercritical CO2-processed bone allografts for cervical and lumbar interbody fusion: A retrospective Study. Front Surg. 10: 984028.
- Salamanna F, Tschon M, Borsari V, Pagani S, Martini L, et al. (2020). Spinal fusion procedures in the adult and young population: a systematic review on allogenic bone and synthetic grafts when compared to autologous bone. J Mater Sci Mater Med. 31: 1-20.
- Baldwin, P., Li, D., Auston, D., Mir, H., Yoon, R., & Koval, K. (2019). Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. Journal of Orthopaedic Trauma, 33, 203–213.
- Stark, J., Hsieh, J., & Waller, D. (2019). Bone Graft Substitutes in Single- or Double-Level Anterior Cervical Discectomy and Fusion: A Systematic Review. SPINE, 44, E618–E628
- D’Souza M, Macdonald NA, Gendreau JL, Duddleston PJ, Feng AY, et al. (2019).. Graft Materials and Biologics for Spinal Interbody Fusion. Biomedicines. 7: 75.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.