Polyglucosan Inclusion Myopathy as Gastrointestinal Neuromuscular Disease: A Challenge to Diagnosis and Treatment
by Giulia Santi1*, Benjamin Misselwitz1, Yves Borbely1, Michele Leuenberger1, Johannes Lenglinger1, Pascal Juillerat2, Heather Dawson3, Swantje Engelbrecht4, Bahtiyar Yilmaz1,5, Andrew Macpherson1, Reiner Wiest1
1Gastroenterology, Clinic for Visceral Surgery and Medicine, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
2Intesto - Gastroenterology practice and Crohn-colitis Center, Bern and Fribourg, Switzerland
3Institute of Tissue Medicine and Pathology, University of Bern, Bern, Switzerland
4Department of Nuclear Medicine, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
5Maurice Muller Laboratories, Department for Biomedical Research, University of Bern, 3008 Bern, Switzerland
*Corresponding author: Giulia Santi, Gastroenterology, Clinic for Visceral Surgery and Medicine, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
Received Date: 14 December 2024
Accepted Date: 18 December 2024
Published Date: 20 December 2024
Citation: Santi G, Misselwitz B, Borbely Y, Leuenberger M, Lenglinger J, et al. (2024) Polyglucosan Inclusion Myopathy as Gastrointestinal Neuromuscular Disease: A Challenge to Diagnosis and Treatment. Ann Case Report. 9: 2128. https://doi.org/10.29011/25747754.102128
Abstract
Background: The polyglucosan inclusion myopathy is a very rare condition that belongs to the gastrointestinal neuromuscular diseases. The accumulation of polyglucosan bodies in the smooth muscles of the gastrointestinal tract can cause intestinal motility disorders up to intestinal failure. The diagnostic and therapeutic pathway of this disease is often tortuous and complex.
Case presentation: A 54-year-old caucasian woman presented with a 30-year history of diarrhea, vomiting, malnutrition and intermittent ileus. She had no response to conservative (prucalopride, octreotide, 5-HT agonists) as well as surgical treatment (subtotal colectomy, small bowel resection). Endoscopy revealed a dilated small intestine with duodenal manometry demonstrating gastrointestinal dysmotility. A fecal transplant resulted in temporary improvement only. During laparoscopic placement of a gastric pacemaker full thickness small bowel wall biopsy was performed finally, evidencing the presence of polyglucosan bodies. The gastric pacemaking and the introduction of a Glucagon-like-2 agonist resulted in clinical and nutritional improvement. The latter however, did require discontinuation due to side effects.
Conclusion: After thorough routine diagnostic work-up full thickness biopsy with histopathological analysis should be considered in cases of longstanding gastrointestinal neuromuscular disorders of unclear origin. Polyglucosan inclusion myopathy is one of the potential rare etiopathogenesis unmasked by this approach. Treatment of polyglucosan inclusion myopathy is extremely difficult and refractory to common prokinetic therapeutics. Experimental utilization of FMT or off-label use of GLP-2-analogs can be considered but did not deliver long-term benefit in the presented case.
Keywords: Gastrointestinal Neuromuscular Diseases (GINMD); Polyglucosans; Malnutrition.
Abbreviations: BMI: body mass index; CIF: chronic intestinal failure; FMT: to be added as fecal microbial transplantation; FTRD : Full Thickness Resection Device; GINMD: Gastrointestinal neuromuscular diseases; GLP2: glucagon-like peptide-2; 5-HT: 5-hydroxytryptaminide receptors or serotonin receptors ; PICC: Peripherally Inserted Central Catheter; MSA: muscle-specific actin; MEN: multiple endocrine neoplasia; PAS: periodic acid– Schiff; PEG:percutaneous endoscopic gastrostomy; PICA: posterior inferior cerebellar artery; PTA: transluminal angioplasty; CIF: chronic intestinal failure; SBS: Short bowel syndrome.
Introduction
Gastrointestinal neuromuscular diseases (GINMDs) comprise heterogeneous disorders presenting with abnormal intestinal motility due to a dysfunction of the enteric neuromuscular system. Myenteric ganglia, Cajal cells and/ or smooth muscle cells can be affected [1]. GINMDs comprise highly heterogeneous disorders including Hirschsprung’s disease, achalasia, gastroparesis, intestinal pseudo-obstruction, mitochondrial disorders, slow-transit constipation or idiopathic megarectum/megacolon. For most of these primary/ idiopathic conditions the molecular mechanism has not been clarified. A minority of GINMDs is secondary to systemic diseases such as muscular dystrophy, neurofibromatosis, multiple endocrine neoplasia (MEN) 2B, neoplasia`s, diabetes mellitus, or systemic sclerosis [2,3].
Symptoms of GINMDs mostly vary but frequently include abdominal pain, vomiting, intestinal pseudo-obstruction due to slow transit, and intestinal failure with malnutrition [4]. Due to variable symptoms and a slow onset in most patients, the diagnosis of GINMDs is frequently delayed. The diagnosis of GINMDs is hence, usually cumbersome since routine thorough multidisciplinary diagnostic work-up does not unmask the disease. Fundamental for its valid diagnosis is objective proof of neuromuscular pathology necessitating full-thickness biopsies [2].
Therapy of GINMDs is complex and not infrequently frustrating for the patient and the caregiver. A multidisciplinary team of gastroenterologists, surgeons, dieticians/ nutritionists, nephrologists and psychologist is recommended. Failure to clarify the molecular mechanism in most patients has hindered the development of more specific therapies for individual conditions. Intestinal failure is the most severe complication of GINMDs. In these patients, oral and additional enteral nutrition is no longer sufficient to ensure adequate caloric intake. After failure of oral/ enteral nutrition, parenteral nutrition should be attempted [5].
We report here the case of a patient with GINMDs for whom after prolongated diagnostic efforts the diagnosis could be narrowed to polyglucosan inclusion body myopathy, an exceedingly rare condition with only 12 additional cases described in the literature so far to our knowledge [6,7].
Methods
This is a Case Report Study. A signed consent was obtained from the patient. We also conducted an analysis of the fecal microbiota transplantation (FMT). The study included one donor sample and three fecal samples from a recipient of fecal microbiota transplantation (FMT) collected at baseline (t=0), 4 weeks (t=4), and 8 weeks (t=8) post-transplant. DNA was extracted from each sample [8], and 16S rRNA gene sequencing targeting the V5-V6 region was performed using the Ion Torrent S5 platform. Data was then analyzed using QIIME2 and phyloseq pipeline [9,10].
Case Presentation
A 48-year-old female was referred to our outpatient clinic in 2015 for evaluation of chronic diarrhea, intermittent (sub)ileus and recurrent vomiting since 1985. In 2015 the patient had a Campylobacter infection treated with Clarithromycin.
Various bowel resections were carried out in the previous years in other tertiary centers, resulting in reduced length of small bowel and subtotal colectomy with approximately 20 cm residual colon remaining, and entero-colonic anastomosis. The exact length of the remaining small intestine was unknown. The patient presented with severe malnutrition, dehydration, impaired renal function, dyselectrolytemia and severe metabolic acidosis. Body mass index (BMI) was 18 kg/m². Since that time, multiple serum and fecal analyses had already been carried out (markers of celiac disease, microbiological stool analysis including parasites, fecal pancreatic elastase, small intestinal secretion analysis, endocrinological tests) without evidence of underlying disease. Various gastroscopies and colonoscopies including mucosal biopsies done over the years, showed a dilated small intestine, but did not establish a firm diagnosis (Figure 1). The Amyloidosis screening (2021) in a small bowel segment, show no evidence of amyloid deposition in a Congo red stain.
A gastric emptying scintigraphy with [99mTc] Tc-MAA labelled semi-solid test meal (2017) showed significantly reduced halfemptying time of 6.5 min (normal reference value 20 ± 3 min), consistent with early dumping, as well as a severe impaired motility with a pathological contraction frequency pattern. A duodenal manometry (07/02/2019) showed markedly reduced motoneuronal activity: in the small intestine, segmental contractions with an amplitude <20 mmHg and a duration of 10-15 seconds were registered during this measurement period, occurring at intervals of 3 to 5 minutes. Occasionally, amplitudes >50 mmHg (maximum 72 mmHg) were observed. A diagnosis of gastrointestinal motility disorder with intestinal neuronal dysplasia and subsequent chronic intestinal failure was considered.
From a metabolic and renal perspective, the patient developed recurrent metabolic acidosis and chronic renal failure CKD G4bA1. Due to muscle weakness in the legs, neurological involvement was suspected. Functional tests confirmed a mild proximal paraparesis with normal electroneurography. The patient developed multiple extraintestinal complications affecting various organs, along with several spontaneous thromboembolic events (pulmonary embolisms, thrombosis of the lower limbs) or secondary to the presence of venous catheters (thrombosis in the upper limbs, subclavian vein, basilica vein, internal jugular vein, superior vena cava, vertebral vein, cephalic vein, closure of the left vertebral artery as well as the posterior inferior cerebellar artery - PICA with a cerebral stroke), making lifelong anticoagulant therapy necessary. Hematological investigations were conducted to rule out thrombophilia and/or other abnormalities, but no pathological findings were observed.
Apart from parenteral nutrition, care of the patient required substitution of liquids and electrolytes in unusually high amounts (up to 5000 ml NaCl and sodium bicarbonate solutions per day), necessitating various interventions/procedures including: Hickman lines, peripherally inserted central catheters (PICC’s), nasojejunal feeding tubes and a percutaneous endoscopic gastrostomy (PEG) with a jejunal extension. Due to repeated catheter complications the creation of a forearm arteriovenous fistula became necessary. Multiple percutaneous transluminal angioplasty (PTA) and stent placements were performed due to recurrent stenosis of the arteriovenous fistula.
In 2018 and 2019, due to persistent symptoms with episodes of subileus and diarrhea with a frequency up to 30 times a day, two stool transplantations were performed with colonoscopic application of 300 ml stool suspension into the ileum. This measure transiently relieved the patient’s symptoms. Various pharmacological treatments have been attempted over the years, including octreotide, 5-HT4 agonists, metoclopramide, prucalopride, and several types of laxatives, without success.
Upon recurrent symptoms, after a multi-disciplinary discussion and considering the clinical presentation and the reduced motor neuron activity seen on the manometry, we performed a laparoscopic diagnostic small bowel segmental resection and implantation of a gastric pacemaker in the same session. Macroscopically, a piece of small intestine measuring 14 cm in length with a diameter of 3.5 cm was removed without significant macroscopic abnormalities. The histological analysis of the resected segment showed an intestinal myopathy of the polyglucosan body type. More specifically, the muscle-specific actin (MSA) and desmin stain showed an irregular wall structure of the lamina muscularis propria with marked thinning of the longitudinal outer muscle layer (Figure 2A,B). The periodic acid–Schiff (PAS) staining revealed polyglucosan inclusions in the inner muscle layer (Figure 2C).
Gastric pacemaker insertion did improve the general condition and the quality of life with a decrease in nausea and stool frequency as well as an increase in appetite. Parenteral nutrition could subsequently be reduced and the weight remained stable (BMI 21.2 kg/m²), whereas the need for parenteral liquid and micronutrients still remained high. Despite this, oral intake was adequate to allow for the subsequent removal of the PEG. However, the clinical condition could not completely be stabilized and an off-lable use of GLP-2 analog (teduglutide) was introduced aiming to improve small intestinal function. This measure led to further clinical improvement, including reduction of diarrhea episodes and use of parenteral nutrition and hydration. Nevertheless, some side effects, including nausea and vomiting, necessitated the discontinuation of the drug. The subsequent investigations were no longer performed at our center due to a change of patient’s place of residence.
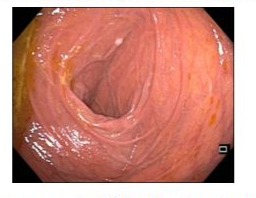
Figure 1: Gastroscopy in 2019: dilated and atonic proximal duodenum.
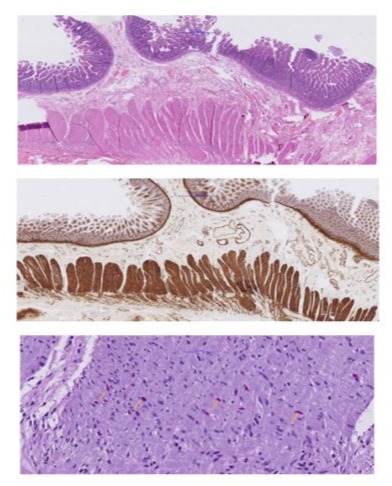
Figure 2: Histopathology of small intestinal full-thickness specimen: Overview on hematoxyllin&eosin-staining.
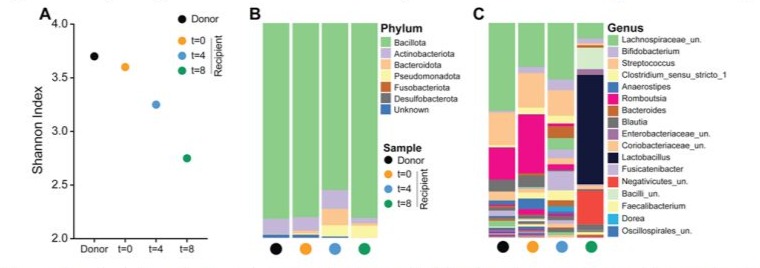
Figure 3: Shannon diversity index and taxonomic composition of gut microbiota from donor and FMT recipient samples. (A) Shannon index comparing alpha diversity across samples from the donor (black circle) and the recipient at baseline (t=0; orange circle), 4 weeks (t=4; blue circle), and 8 weeks (t=8; green circle) post-FMT. (B) Phylum-level taxonomic distribution showing the relative abundance of dominant bacterial phyla in each sample. (C) Genus-level taxonomic composition illustrating the shifts in microbial genera over time in the recipient compared to the donor sample.
Discussion
Polyglucosans are amylopectin-like polysaccharides associated with borderline glycogen metabolism characterized by a deficiency of the glycogen-branching enzyme. The assembly of polyglucosans can form what are called polyglucosans bodies. These bodies can accumulate in various tissues (renal, neurological, cardiac, etc).Histopathologically, polyglucosan bodies are highlighted by periodic acid Schiff staining (PAS), which can be combined with diastase treatment [4]. In case of the digestive tract, they can be observed in the smooth muscle of the muscularis externa of colon, jejunum and stomach [6,11,12]. The first time polyglucosan bodies were described was in the 1980s in the smooth muscles of the digestive tract of dogs that had no symptoms of neuromuscular dysfunction [13]. Regarding human investigations, Knowler et al. analysed 104 colectomy cases demonstrating presence of polyglucosan bodies in 4 individuals all of which were presenting with intestinal dysmotility symptoms [11].
Polyglucosan inclusion myopathy is a disease that includes a large spectrum of gastrointestinal symptoms. A case of proctalgia fugax with biopsy at the level of the external anal sphincter showing with polyglucosan inclusion bodies, was treated with lateral internal anal sphincterotomy after 18 years of clinical history [14]. It was also observed in a potential case of scleroderma with symptoms of chronic intestinal pseudo-obstruction [15]. The presence of inclusion bodies in the intestine has also been reported in cases of diabetic autonomic neuropathy and slow transit constipation [16]. Finally, polyglucosan inclusion bodies are a common feature of idiopathic and secondary megarectum being present in up to 47% of patients suffering medically intractable megarectum having undergone surgical resection [17].
Intestinal motility disorders such as polyglucosan inclusion myopathy are one cause of chronic intestinal failure (CIF). CIF is a rare condition, in Europe the prevalence of home parenteral nutrition for CIF due to benign diseases is estimated to be about 5 to 20 persons per 1 million population. Of these, motility problems account for 18% [18]. Intestinal failure is defined as the inability of the intestine to absorb the minimum amount of fluids, macro- and micronutrients necessary for the body’s growth and function. This status requires a parenteral nutrition supplementation. When this condition persists and prolonged parenteral nutrition over periods of weeks or months is needed, it develops into chronic intestinal failure [5,19]. The diagnostic process and treatment of CIF is complex and must be carried out in an interdisciplinary and multiprofessional way. As in the case presented here, symptoms and diagnostic-therapeutic history often last for years with examinations and treatment received at different centers.
An endoscopic macroscopic and histologic evaluation (important to take deep biopsies with submucosa present) is fundamental [20].
A study of bowel motility by manometry of the upper and lower intestinal tract is important to identify a neuropathic (disrupted contractility pattern, but contractions with normal amplitude) or myopathic (contractions with decreased amplitude) problem, and to highlight problems related to bowel atonicity or anorectal dysfunction [21]. A radiologic evaluation (RX abdomen, CT abdomen) is essential to distinguish the presence of an obstruction or pseudo-obstruction [22]. Moreover, amyloidosis should always be considered and excluded histopathologically [14] e.g. by fullthickness rectal biopsies which can be performed by so-called endoscopic FTRD (Full Thickness Resection Device). If all the diagnostic tests followed do not lead to a conclusion, we recommend a surgical step-up approach to deliver sufficient representative intestinal tissue as full-thickness specimen. However, there is no recommendation for the exact location of the sampling-site.
From the therapeutic point of view, after failure of standard prokinetic, anti-emetic and supportive measures an experimental approach by endoscopic delivery of FMT was initiated since this has been suggested as a therapeutic option for treating intestinal failure [23]. The patient presented temporary improvement after FMT.
We analysed one donor sample and three fecal samples from a recipient of fecal microbiota transplantation (FMT) collected at baseline (t=0), 4 weeks (t=4), and 8 weeks (t=8) post-transplant. Over time after FMT, there was a notable reduction in species richness (Figure 3A) which coincided with an increase in the relative abundance of the phyla Bacteroidota and Pseudomonadota (Figure 3B). At the genus level, key changes included an increase in Lactobacillus, Bacteroides, and unassigned Negativicutes, alongside a decrease in Romboutsia and unassigned Lachnospiraceae (Figure 3C). Romboutsia, a genus within the Bacilliota phylum, is known for its roles in carbohydrate metabolism and fermentation. Its decrease following FMT suggests a shift away from a gut environment that previously supported its proliferation, possibly indicating a transition towards a more balanced metabolic state. This shift could reflect a broader reconfiguration of the gut microbiota, particularly as it coincides with an increase in Lactobacillus. Lactobacillus, known for its protective and healthpromoting properties, may contribute to stabilizing gut health and potentially modulating disease progression or symptom severity in the context of neuromuscular disease. The overall microbial changes suggest a movement towards a microbiome composition that supports a healthier and more resilient gut environment, which could be crucial in the therapeutic effects of FMT.However, the clinical benefit was limited in duration and not sustained.
Finally, gastric pacemaker, as device utilized in cases of gastroparesis without responding to conservative therapies [24], was implemented aiming to achieve improved gastric motility and triggering downstream intestinal effects as well. However, benefits were limited and only transient as well. Finally, teduglutide, a glucagon-like peptide 2 (GLP-2)-analogue was used ultimately as attempt at healing. GLP2 is a pleiotropic intestinotrophic hormone and stimulates intestinal mucosal crypt-cell proliferation leading to increased villus height and intestinal length [25].Teduglutide, a GLP-2 analogue, has been approved for clinical use in intestinal failure associated with short bowel syndrome [26]. This is based on the peptide’s ability to increase intestinal surface area, reducing intestinal motility and thus increasing absorption capacity [27]. Schoeler et. all demonstrated a reduction in thirst, stool frequency of parenteral nutrition intake by 20 percent as early as 24 weeks after starting therapy [28]. Very little is known about its effects in SBS patients associated with GINMDs. GLP-2 administration inhibits gastric acid secretion and motility [29]. It’s use in these patients thus carries risks of further ileus episodes. Our patient reported increased nausea and vomiting shortly after starting the therapy, which is why the therapy had to be discontinued. It can therefore be assumed that this patient group does not benefit from GLP-2 treatment.
In summary, polyglucosan inclusion myopathy is a rare but for the individual patient critical differential diagnosis within the group of gastrointestinal neuromuscular diseases. Increased awareness should enable earlier diagnosis being based on histomorphological PAS-staining of intestinal smooth muscle and thus, ultimately help to guide treatment. The latter is mainly supportive in nature with particular care needed for nutritional optimal care. However, electrical stimulation devices and pharmacological GLP2agonists may help to counteract the underlying neuro-myopathic pathophysiology which is so far unfortunately, still not completely understood in terms of intracellular mechanism.
References
- Bernardini N, Ippolito C, Segnani C, Mattii L, Bassotti G, et al. (2013) Histopathology in gastrointestinal neuromuscular diseases: methodological and ontological issues. Adv Anat Pathol. 20:17-31.
- Paine P, McLaughlin J, Lal S. (2013) Review article: the assessment and management of chronic severe gastrointestinal dysmotility in adults. Aliment Pharmacol Ther. 38:1209-29.
- Knowles CH, De Giorgio R, Kapur RP, Bruder E, Farrugia G, et al. (2010) The London Classification of gastrointestinal neuromuscular pathology: report on behalf of the Gastro 2009 International Working Group. Gut. 59:882-7.
- Knowles CH, Martin JE. (2009) New techniques in the tissue diagnosis of gastrointestinal neuromuscular diseases. World J Gastroenterol. 15:192-7.
- Pironi L, Corcos O, Forbes A, Holst M, Joly F, Jonkers C, et al. (2018) Intestinal failure in adults: Recommendations from the ESPEN expert groups. Clin Nutr. 37:1798-809.
- McCulloch A, Malhi H, Parkinson S, Martin JE, Cooper SC. (2019) Spontaneous Restoration of Nutrition Autonomy in a Case of Intestinal Failure Secondary to a Gastrointestinal Neuromuscular Disease. Nutr Clin Pract. 34:935-9.
- Martin JE, Chowdhury AH, McElwaine S, Fikree A, Broad J, et al. (2011) A Novel Phenotype of Glycogen Storage Disease Presenting as Gastrointestinal Neuromuscular Disease. Gastroenterology. 140.
- Yilmaz B, Fuhrer T, Morgenthaler D, Krupka N, Wang D, et al. (2022) Plasticity of the adult human small intestinal stoma microbiota. Cell host & microbe. 30:1773-87 e6.
- Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, et al. (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 37:852-7.
- McMurdie PJ, Holmes S. (2013) phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PloS one. 8.
- Knowles CH, Nickols CD, Feakins R, Martin JE. (2003) A systematic analysis of polyglucosan bodies in the human gastrointestinal tract in health and disease. Acta Neuropathol. 105:410-3.
- Hedberg-Oldfors C, Oldfors A. (2015) Polyglucosan storage myopathies. Mol Aspects Med. 46:85-100.
- Kamiya S, Suzuki Y, Sugimura M. (1983) Polyglucosan bodies in the digestive tract of the aged dog. Acta Neuropathol. 60:297-300.
- Panagiotopoulou IG, Miller R, Powar MP, Chan JYH, Davies RJ. (2018) Proctalgia and constipation secondary to hypertrophic polyglucosan inclusion body myopathy of the internal anal sphincter: a case report. J Med Case Rep. 12:315.
- Venizelos ID, Shousha S, Bull TB, Parkins RA. (1988) Chronic intestinal pseudo-obstruction in two patients. Overlap of features of systemic sclerosis and visceral myopathy. Histopathology. 12:533-40.
- Knowles CH, Nickols CD, Scott SM, Bennett NI, de Oliveira RB, et al. (2001) Smooth muscle inclusion bodies in slow transit constipation. J Pathol. 193:390-7.
- Martin JE, English W, Kendall JV, Sheshappanavar V, Peroos S, et al. (2021) Megarectum: systematic histopathological evaluation of 35 patients and new common pathways in chronic rectal dilatation. J Clin Pathol. 75:651-7.
- Cuerda C, Pironi L, Arends J, Bozzetti F, Gillanders L, et al. (2021) ESPEN practical guideline: Clinical nutrition in chronic intestinal failure. Clin Nutr. 40:5196-220.
- Pironi L, Konrad D, Brandt C, Joly F, Wanten G, Agostini F, et al. (2018) Clinical classification of adult patients with chronic intestinal failure due to benign disease: An international multicenter cross-sectional survey. Clin Nutr. 37:728-38.
- den Braber-Ymker M, Heijker S, Lammens M, Croockewit S, Nagtegaal ID. (2018) Intestinal involvement in amyloidosis is a sequential process. Neurogastroenterol Motil. 30:e13469.
- De Giorgio R, Camilleri M. (2004) Human enteric neuropathies: morphology and molecular pathology. Neurogastroenterol Motil. 16:515-31.
- Rohrmann CA, Jr., Ricci MT, Krishnamurthy S, Schuffler MD. (1984) Radiologic and histologic differentiation of neuromuscular disorders of the gastrointestinal tract: visceral myopathies, visceral neuropathies, and progressive systemic sclerosis. AJR Am J Roentgenol. 143:93341.
- Pironi L, Joly F, Forbes A, Colomb V, Lyszkowska M, Baxter J, et al. (2011) Long-term follow-up of patients on home parenteral nutrition in Europe: implications for intestinal transplantation. Gut. 60:17-25.
- Rajamanuri M, Mannava SM, Chhabra J, Karwarker GV, Chahal M, et al. (2021) A Systematic Review of the Therapeutic Role of Gastric Pacemakers in Adults with Gastroparesis. Cureus. 13:e18152.
- Drucker DJ, Yusta B. (2014) Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu Rev Physiol. 76:561-83.
- Schwartz LK, O’Keefe SJ, Fujioka K, Gabe SM, Lamprecht G, et al. (2016) Long-Term Teduglutide for the Treatment of Patients With Intestinal Failure Associated With Short Bowel Syndrome. Clin Transl Gastroenterol. 7:e142.
- Jeppesen PB, Hartmann B, Thulesen J, Graff J, Lohmann J, et al. (2001) Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology. 120:806-15.
- Schoeler M, Klag T, Wendler J, Bernhard S, Adolph M, et al. (2018) GLP-2 analog teduglutide significantly reduces need for parenteral nutrition and stool frequency in a real-life setting. Therap Adv Gastroenterol. 11:1756284818793343.
- Wøjdemann M, Wettergren A, Hartmann B, Holst JJ. (1998) Glucagonlike peptide-2 inhibits centrally induced antral motility in pigs. Scand J Gastroenterol. 33:828-32.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.