Perioperative Management of a Complex Patient with Sheehan Syndrome and Myelodysplastic Syndrome for Double Valve Surgery: Case Report
by Despoina Sarridou1, Rafail Ioannidis2*, Sophia Anastasia Mouratoglou3, Giakoumis Mitos1, Fotini Ampatzidou4, Eleni Argiriadou1
1Department of Anesthesiology and Intensive Care, AHEPA University Hospital of Thessaloniki, Thessaloniki, Greece.
2Department of Anesthesiology and Pain Medicine, General Hospital of Drama, Drama, Greece.
33rd Department of Cardiology of Aristotle University of Thessaloniki, Hippocrates General Hospital of Thes-saloniki, Thessaloniki, Greece.
43rd Intensive Care Unit, General Hospital of Thessaloniki George Papanikolaou, Thessaloniki, Greece.
*Corresponding author: Rafail Ioannidis, Department of Anesthesiology and Pain Medicine, General Hospital of Drama, Drama, Greece.
Received Date: 23 January, 2026
Accepted Date: 29 January, 2026
Published Date: 02 February, 2026
Citation: Sarridou D, Ioannidis R, Mouratoglou SA, Mitos G, Ampatzidou F, et al. (2026) Perioperative Management of a Complex Patient with Sheehan Syndrome and Myelodysplastic Syndrome for Double Valve Surgery: Case Report. Cardiol Res Cardio vasc Med 11:293. https://doi.org/10.29011/2575-7083.100293
Abstract
Background and Clinical Significance: Sheehan syndrome is a postpartum irreversi-ble disorder characterized by panhypopituitarism and complete deficiency of pituitary hormone secretions, such as thyroid stimulating hormone, and adrenocorticotropic hormone. This deficiency impairs their response in stressful situations such the peri-operative period, increasing the risk of developing metabolic and haemodynamic in-stability. Case Presentation: We describe the case of a female patient with Sheehan syndrome and severe comorbidities, undergoing a complex cardiac surgery for double valve surgery and we highlight the importance of a coordinated, multidisciplinary ap-proach for a successful outcome. Conclusions: Meticulous endocrine optimization, vigilant hormonal monitoring, and coordinated multidisciplinary management are es-sential to ensure safe perioperative care and successful cardiac surgical outcomes in patients with Sheehan syndrome.
Keywords: Sheehan’s Syndrome; Perioperative Care; Hypopituitarism
Abbreviations
The following abbreviations are used in this manuscript:
TSH: Thyroid stimulating hormone; METs: Metabolic equivalent of tasks; ICU: Intensive care unit; CPB: Cardiopulmonary bypass
Introduction and Clinical Significance
Sheehan syndrome is a rare postpartum disorder, most often irreversible, caused by pituitary ischemic necrosis following severe obstetric hemorrhage [1]. It is charac-terized by deficiency of pituitary hormone secretions, including thyroid stimulating hormone (TSH), growth hormone, adrenocorticotropic hormone, luteinizing hormone, follicle-stimulating hormone, and prolactin [2], impairing their response to stressful situations. Although adults with Sheehan syndrome are more prone to develop cardiovascular disease and especially coronary artery disease [3], data on the perioperative management of cardiac surgical patients is lacking. Our aim is to highlight the im-portance of a coordinated, multidisciplinary approach for a successful outcome after complex cardiac surgery.
Case Presentation
This is the case of a 61-year-old female who presented for mitral valve replace-ment and tricuspid valve repair. Her medical history involved multi-valvular heart disease described as moderate mitral valve stenosis and regurgitation, severe tricuspid regurgitation and mild mixed aortic valve disease, chronic atrial fibrillation with slow ventricular rate, moderate to severe chronic kidney failure, arterial and postcapillary pulmonary hypertension. Her baseline functional status was good, with metabolic equivalent of tasks over four (METs>4). She was diagnosed with Sheehan syndrome complicating her third pregnancy, empty Sella syndrome (a condition where the Sella turcica, the bony structure that houses the pituitary gland, is filled with cerebrospinal fluid, causing the gland to appear flattened or shrunken on imaging), hypothyroidism and adrenal cortex deficiency. Additionally, she suffered myelodysplastic syndrome, iron deficiency anemia and ischemic stroke with no sequelae. The patient was receiv-ing anticoagulation therapy with rivaroxaban, which was discontinued four days prior to surgery. The platelet count was 180000/μL. Both TSH and cortisol levels were within normal range while free T4 levels were slightly decreased and an increase in daily levothyroxine dose was advised. The exact values are presented in (Table 1). The esti-mated EuroSCORE (a widely used clinical tool that predicts the risk of death or major complications for patients undergoing open heart surgery) was 11.3 and her complete medication is presented in (Figure 1). Transthoracic echocardiography revealed pro-gression of the mitral valve disease with severe tricuspid regurgitation, however pre-served biventricular systolic function, as well as left ventricular diastolic dysfunction and increased right ventricular systolic pressure. Hydrocortisone 100 mg was given as a bolus dose prior to induction of anaesthesia, as the typical dose of anesthesia man-agement. A pulmonary artery catheter was placed and connected to a Vigilance Con-tinuous Cardiac Output monitor. The choice of placement of a pulmonary artery cath-eter was based on the importance to enable continuous postoperative measurement of cardiac output in the intensive care unit (ICU) and to provide advanced hemodynamic monitoring in the setting of significant endocrine and cardiac complexity. Tempera-ture was measured continuously via the urinary bladder. Intraoperative transoesoph-ageal echocardiography was used and mitral valve was replaced with a tissue and tri-cuspid valve was repaired with a ring under cardiopulmonary bypass (CPB). Separa-tion from CPB was uneventful, initially following the local protocol of using adrenaline infusion 0,05 mg/kg/min and noradrenaline infusion 0,1-0,2 mg/kg/min. Inotropic vasopressor support was weaned off gradually and was completely discontinued after extubation on the first postoperative day. Duration of CPB was 144 minutes and aortic cross clamp (ischemia time) was 105 minutes. Post CPB biventricular systolic function was preserved with residual trivial mitral and tricuspid regurgitation central jets of no clinical significance. Cerebral oxygenation monitoring values were stable intraopera-tively. For the mitral valve replacement, a tissue mitral valve size 27 mm was used and a tricuspid valve ring size 26 mm was used for the tricuspid valve repair. According to thromboelastography, one gram of fibrinogen and one pool of platelets were given. The patient was transferred to the cardiac intensive care unit where she had an uneventful stay.
Hydrocortisone 50 mg four times daily was administered on first post-operative day tampered off per 50 mg daily thereafter, to complete discontinuation. Routine doses of levothyroxine were given orally. The patient was discharged on third post-operative day to the high dependency ward where she remained for two days un-til moved to the cardiac surgical ward. Consent was granted to report this case. To the best of our knowledge, this is the first reported case describing the perioperative course and management of a Sheehan syndrome patient undergoing the increased stress of a high risk double cardiac valve replacement surgery.
|
Hormone |
Preoperative value |
|
Cortisole |
8 μg/dL |
|
TSH |
2.1 mIU/L |
|
FT4 |
0.5 ng/dL |
|
ACTH (morning values) |
5.7 pg/mL |
Table 1: The exact values
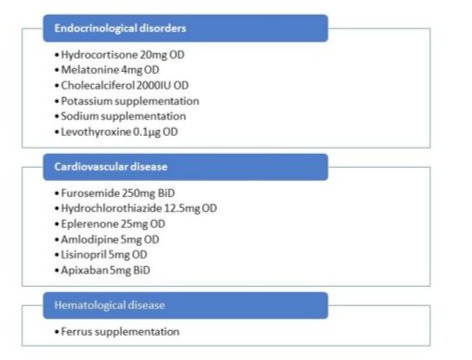
Figure 1: Patients complete medication.
Discussion
The importance of early diagnosis and therapeutic treatment of Sheehan syn-drome is well recognized as it can lead to catastrophic consequences. Although this syndrome is characterized by increased risk of coronary disease that may require sur-gical therapeutic interventions, only scarce data exists regarding the perioperative management of patients undergoing major cardiac surgery. Glucocorticoids and thy-roid hormones are well recognized hormones influencing the perioperative course. Cortisol, the main stress response hormone, apart from its inotropic effects, has well described metabolic, catabolic, vasoactive and anti-inflammatory properties on the cardiac muscle and the peripheral vasculature, while it also modulates free water dis-tribution within the vascular compartment. It is found to fluctuate between two to 10- times above normal limits in normal patients undergoing major stress or serious illness [4]. On the other hand, the hormonal stress after complex cardiac surgery is massive, therefore, a controlled patient with a hormonal profile within normal range preopera-tively and one resembling to normal response post-operatively, is crucial to avoid ad-renal crisis [2, 5, 6]. In addition, as thyroid hormones may lead to increased demands of enhanced metabolism and increase risk of adrenal insufficiency, glucocorticoid re-placement therapy must precede thyroid hormone replacement [2, 4]. Our patient re-ceived both hormonal replacement regiments and normal range of TSH and Free T4 levels were achieved. In patients with hypopituitarism, the indicated treatment for preventing adrenal insufficiency postoperatively is by supplementary administration of glucocorticoids and high postoperative doses of hydrocortisone (400 to 600 mg) on the first postoperative day are described in the literature [5-9]. In our case, we admin-istered 200 mg hydrocortisone on the first post-operative day, under close hormonal monitoring, as our patient suffered major comorbidities including hypertension and post-capillary pulmonary hypertension, in order to achieve the optimal balance be-tween cortisol levels and possible side effects by its administration. The strict transfu-sion and clotting screening protocols with the availability of perioperative thromboelastometry and platelet function tests aimed to eliminate the risk of bleeding due to the myelodysplastic syndrome. The insertion of a pulmonary artery catheter and the use of continuous cardiac output monitoring guaranteed accurate cardiovascular monitoring as these patients may present with higher requirements of vasoactive agents and in-otropes such as noradrenaline and adrenaline [4]. Subsequently the titration and weaning of the aforementioned agents may be more efficient.
Conclusions
In conclusion, this case highlights the crucial role of meticulous perioperative endocrine management in patients with Sheehan syndrome undergoing major cardiac surgery. Close hormonal monitoring, particularly of cortisol and thyroid levels, is es-sential to prevent adrenal crisis and hemodynamic instability. A tailored glucocorti-coid replacement strategy and multidisciplinary coordination among endocrinologists, anesthesiologists, and cardiac surgeons ensure optimal outcomes. Advanced hemodynamic monitoring further supports safe intraoperative and postoperative manage-ment. Ultimately, individualized and coordinated care is key to achieving a successful surgical recovery in such complex endocrine–cardiac cases.
Supplementary Materials: Table S1: Exact preoperative values of hormones, Figure S1: Detailed daily medication of the patient
Author Contributions: All authors equally contributed to this case and this manuscript and ap-proved the submitted version and version substantially edited by journal staff.
Funding: This research received no external funding.
Institutional Review Board Statement: There was written consent from the patient and ethics approval was not required.
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Data Availability Statement: Not applicable
Acknowledgments: No acknowledgments and No use of artificial intelligence (AI
Conflicts of Interest: The authors declare no conflicts of interest.
References
- Valassi E (2021) Pituitary disease and pregnancy. Endocrinol Diabetes Nutr (Engl Ed). 68:184-195.
- Fleseriu M, Hashim IA, Karavitaki N, Melmed S, Murad MH, et al. (2016) Hormonal replacement in hypopituita-rism in adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 101:3888-3921.
- Rosén T, Bengtsson BA (1990) Premature mortality due to cardiovascular disease in hypopituitarism. Lancet. 336:285-288.
- Munck A, Guyre PM, Holbrook NJ (1984) Physiological functions of glucocorticoids in stress and their relation to pharmacolog-ical actions. Endocr Rev. 5:25-44.
- Siminelakis S, Kotsanti A, Baikoussis NG, Papadopoulos G, Argiriou O, et al. (2010) Congenital hypopituitarism: monitoring after coronary artery bypass grafting. Ann Card Anaesth. 13:257-259.
- Takagi K, Tayama E (2020) Hormone replacement therapy for open heart surgery in a patient with panhypopituitarism and diabetes insipidus. Interact Cardiovasc Thorac Surg. 31:413-414.
- Raut MS, Kar S, Maheshwari A, Shivnani G, Dubey S (2019) Perioperative management in a patient with panhypopituitarism— evidence-based approach: a case report. Eur Heart J Case Rep. 3:ytz145.
- Sunoki K, Otsuka Y, Iwai H, Shimizu S, Nishikawa Y, et al. (2021) Perioperative management of Fontan operation for the child with panhypopituitarism: a case report. J Anesth. 35:303-306.
- Syed AU, Al Fagih MR, Fouda M (2001) Coronary bypass surgery in patients with Sheehan’s syndrome. Eur J Cardiothorac Surg. 20:12641266.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.