Paraneoplastic Dermatomyositis in a Patient with Small Cell Bladder Cancer: A Case Report
by Anne Michel1*, Andreas Schmitt1, Cyrill Rentsch2, Thomas Daikeler3, Frank Stenner1
1Departement of Oncology, University Hospital Basel, Switzerland
2Departement of Urology, University Hospital Basel, Switzerland
3Departement of Rheumatology, University Hospital Basel, Switzerland
*Corresponding author: Anne Michel, Department of Oncology, University Hospital Basel, Petersgraben 4, CH-4031 Basel, Switzerland
Received Date: 25 July 2024
Accepted Date: 30 July 2024
Published Date: 01 August 2024
Citation: Michel A, Scmitt A, Rentsch C, Daikeler T, Stenner F (2024) Paraneoplastic Dermatomyositis in a Patient with Small Cell Bladder Cancer: A Case Report. Ann Case Report. 9: 1912. https://doi.org/10.29011/2574-7754.101912
Abstract
Introduction: Dermatomyositis (DM) is an idiopathic inflammatory myopathy characterized by muscle weakness and distinctive skin manifestations. It involves both muscular and extra muscular symptoms, with an unknown etiology likely rooted in autoimmune processes.
Case Presentation: We report on a 72-year-old male with hypertension and hypercholesterolemia, initially presenting with urinary symptoms and bladder tumours diagnosed as combined small cell carcinoma. After neoadjuvant chemotherapy and post-cystoprostatectomy, the patient developed progressive muscle weakness, cutaneous signs of DM, and dysphagia. Laboratory tests revealed elevated creatine kinase and anti-TIF1-gamma antibodies, with MRI and nail fold capillaroscopy confirming myositis.
Diagnosis and Treatment: After establishment of the diagnosis of paraneoplastic DM, the patient was treated with corticosteroids, intravenous immunoglobulins, and hydroxychloroquine, showing initial improvement. Persistent dermatitis required additional immunosuppressive therapies, including rituximab and upadacitinib.
Discussion: The link between DM and cancer is well-documented, with DM patients showing significantly higher cancer risks. Our patient's case underscores the complexity of managing DM in the context of cancer, necessitating adaptable and ongoing treatment strategies.
Conclusion: This case highlights the intricate interplay between DM and malignancy, demonstrating the importance of tailored therapeutic approaches to manage both muscular and cutaneous symptoms effectively.
Keywords: Paraneoplastic dermatomyositis, small cell bladder cancer, urothelial cancer
Introduction
Dermatomyositis (DM) is an idiopathic inflammatory myopathy characterized by muscle weakness and distinctive skin manifestations. It belongs to a group of conditions known as idiopathic inflammatory myopathies (IIMs) and exhibits both muscular and extra muscular involvement. The exact etiology of DM is unknown, but it is thought to result from an autoimmune process, potentially triggered by genetic predisposition, environmental factors, or infections [1]. The association between cancer and DM has been largely discussed. The incidence for cancer in a review article of literature was estimated 5 to 6 times higher in patients with DM [2]. Various studies have also shown a higher incidence of DM in the cancer population and an increased cancer risk in patients with DM. [3,4,5,6].
Clinically, DM presents with progressive symmetric proximal muscle weakness and cutaneous symptoms, such as heliotrope rash (a purplish discoloration around the eyes), Gottron's papules (raised, scaly eruptions on the knuckles), and a photosensitive rash on the face, neck, and chest. In some cases, patients may also experience systemic symptoms, including interstitial lung disease, esophageal involvement, and an increased risk of malignancies. Histopathologic ally, dermatomyositis is distinguished by perivascular and perimysial inflammation, with CD4+ T cells, B cells, and the presence of the membrane attack complex (MAC) on the endothelial cells of capillaries, leading to muscle fiber necrosis and per fascicular atrophy.
Diagnosis is based on a combination of clinical presentation, elevated muscle enzymes (such as creatine kinase), electromyography (EMG) findings, and muscle biopsy results. Magnetic resonance imaging (MRI) can also be used to identify muscle inflammation and edema. Autoantibodies, such as anti-Mi-2, anti-MDA5, and anti-NXP2, are helpful in diagnosing and prognosticating the disease.
Case presentation
We present the case of a 72-year-old male patient. His co-morbidities and cvRF are hypertension and hypercholesterinaemia. The patient presented initially with pronounced nocturia and pollakisuria and recurrent episodes of macroscopic hematuria in October 2022. Cystoscopy revealed multiple solid tumours located on the posterior bladder wall, clinically staged at T2-T3 (Figure 1). The histological examination identified a combined small cell carcinoma with components of a poorly differentiated, partially spindle, and pleomorphic cell urothelial carcinoma (Figure 2).
The immunohistochemical analysis was performed: PD-L1 (SP263, Ventana): Tumour cells showed <1% positivity, while tumour-associated immune cells showed >10% positivity. The carcinoma was microsatellite stable. Furthermore, the Ki-67 was very high, surpassing 90%. Additionally, the tumour exhibited low to moderate expression of CD117.
The initial treatment was performed: In November 2022, a transurethral resection of the bladder tumour (TUR-B) was performed, diagnosing at least pT1 and carcinoma in situ (pTis) with clinical staging of cN0, cM0 From November 2022 to February 2023, the patient received neoadjuvant chemotherapy with dd MVAC (methotrexate, vinblastine, doxorubicin, cisplatin) for five cycles and gemcitabine plus carboplatin (GC) due to renal dysfunction (Figure 3).
On March 13, 2023, a robotic-assisted radical cystoprostatectomy with pelvic lymphadenectomy and intracorporeal orthotopic ileal neobladder was performed. Histology showed complete regression of the urothelial carcinoma following neoadjuvant therapy, with tumour-free and dysplasia-free surgical margins. The combined positive score (CPS) was less than 10. The pathological stage was ypT0 ypN0 (0/14) cM0, V0 L0 Pn0, R0, cM0.
Clinical presentation
On April 13th the patient subsequently described symmetric muscle weakness (proximal greater than distal), myalgias, severe dysphagia, Gottron's papules (Figures 4 and 5), facial erythema and dysphagia (Figure 6).
Imaging and Diagnostics
Laboratory findings showed a creatine kinase level of 2280 U/L. Anti-TIF1-gamma IgG antibodies were positive.
An MRI of the legs on April 12, 2023 (Figure 7), revealed edematous enlargement of the rectus abdominis and iliopsoas muscles bilaterally with associated contrast enhancement consistent with myositis. Additionally, patchy signal alterations were observed in several muscle groups of both thighs. A pulmonary function test on April 13, 2023, showed normal static and dynamic lung volumes.
Nail fold capillaroscopy on April 13, 2023, revealed severely disrupted capillary architecture in both hands, characterized by markedly varying capillary sizes and densities and numerous hemorrhages, consistent with dermatomyositis. A transthoracic echocardiogram on April 14, 2023, showed concentric remodelling of the left ventricle with normal systolic function (LVEF 65%) no regional wall motion abnormalities, normal diastolic function, and a normal-sized left atrium. No hemodynamically significant valvular defects were detected. A gastroscopy on April 9, 2023, revealed reflux esophagitis Grade A, a small axial hernia, haemorrhagic flat erosions in the stomach, and mucosal erythema in the antrum. No structural cause for dysphagia in the esophagus was identified, and a jejunal feeding tube was placed. A differentiated swallowing study on April 12, 2023, showed a normal oral phase but an abnormal pharyngeal phase with severely reduced hyolaryngeal anterior excursion, absent epiglottic tilt, and significantly reduced upper esophageal sphincter opening.
PET-CT on April 26, 2023, indicated status post-cystoprostatectomy with neobladder construction for bladder carcinoma without evidence of local recurrence. Compared to the CT from December 2022, the iliac lymph nodes on the right had regressed in size and were FDG-negative, indicating possible nonspecific findings or lymph node metastases that responded retrospectively to chemotherapy all these findings were indicative of paraneoplastic dermatomyositis.
Therapy
The patient received methylprednisolone 125 mg IV from April 12-14, followed by prednisone 60 mg/day from April 15-25, with subsequent tapering. Intravenous immunoglobulins (Octagam) 25 g IV were administered from April 13-17. Hydroxychloroquine was given at 400 mg from April 12-25, then at 200 mg/day from April 26-June 20, and back to 400 mg thereafter. The patient received enteral nutrition via a jejunal feeding tube until June 2023 and participated in speech therapy. Prednisone was reduced to 15 mg by June 20, 2023. Privigen 2 g/kg body weight was administered on May 25-26, then every four weeks. In September 2023, under 7.5 mg of prednisone, a marked increase in dermatitis was observed despite full muscle strength. Azathioprine 50 mg from September to December 2023, was ineffective in reducing steroids. There was no evidence of active HIV or hepatitis C infection. Rituximab (RTX) was initiated in February 2024 due to persistent dermatitis. Upadacitinib 15 mg was started in June 2024 (Figures 8 and 9), resulting in a rapid improvement of the skin condition.
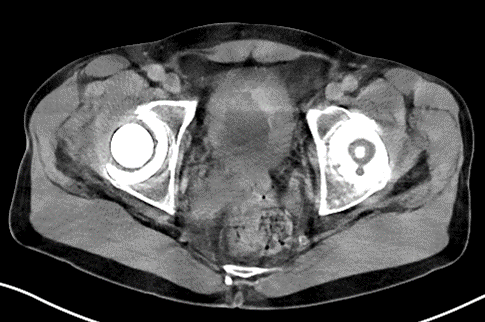
Figure 1: CT scan Multifocal Bladder Cancer.
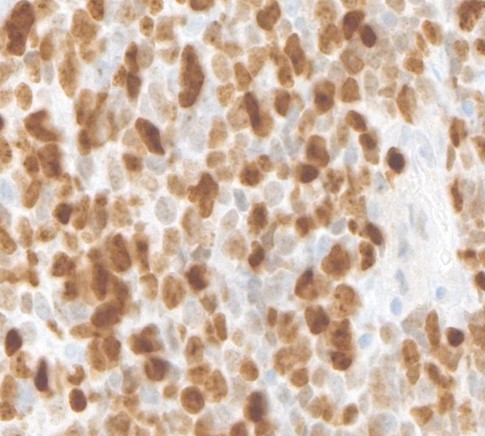
Figure 2: Small Cell bladder cancer.
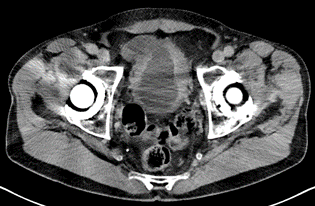
Figure 3: CT scan post neoadjuvant treatment.
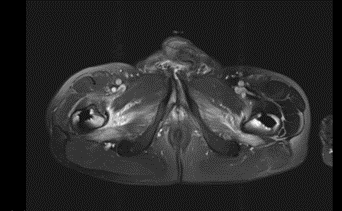
Figure 4: Gottrons papules.
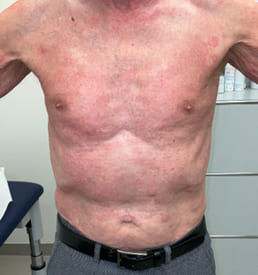
Figure 5: Gottrons papules.
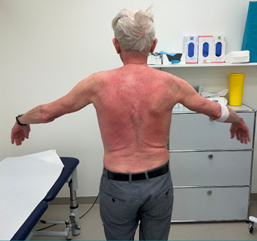
Figure 6: Nasogastric tube and facial erythema 03/23.
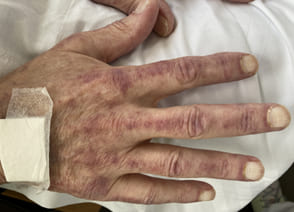
Figure 7: edema of sartorius and quadriceps femoris muscle (04/23).
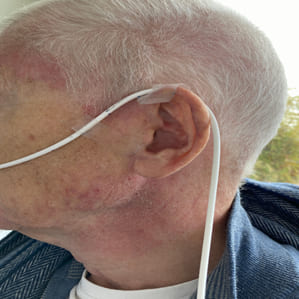
Figure 8: Skin rash before administering upadacitinib.
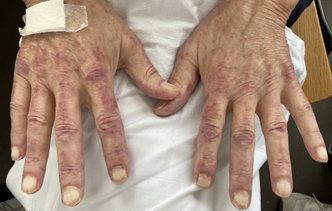
Figure 9: Skin rash before administering upadacitinib.
Discussion
The case presented illustrates the intricate relationship between dermatomyositis (DM) and malignancies, particularly the challenges in managing paraneoplastic DM in the context of small cell bladder cancer. DM is a rare autoimmune disease characterized by both muscular and cutaneous symptoms. The case under review presented significant diagnostic challenges due to the overlapping symptoms of DM and the patient's underlying malignancy. The patient's initial presentation with urinary symptoms and subsequent diagnosis of bladder cancer, followed by the development of DM, underscores the importance of considering paraneoplastic syndromes in patients with new-onset inflammatory myopathies. DM can appear before, during, or after the cancer diagnosis [6].
Diagnostic tools such as elevated muscle enzymes, electromyography (EMG), muscle biopsy, and autoantibodies (e.g., anti-TIF1-gamma IgG) were crucial in confirming the diagnosis of DM [7,8]. The use of MRI to detect muscle inflammation and nail fold capillaroscopy to identify capillary changes provided additional diagnostic confirmation. These diagnostic modalities are essential for accurately diagnosing DM and differentiating it from other inflammatory and non-inflammatory myopathies. The management of paraneoplastic DM involves addressing both the underlying malignancy and the autoimmune manifestations. The initial treatment of the patient’s bladder cancer with neoadjuvant chemotherapy, followed by radical cystoprostatectomy was effective in achieving complete remission of the malignancy. However, the development of DM post-treatment posed additional therapeutic challenges. Corticosteroids and intravenous immunoglobulins (IVIG) are the mainstays of DM treatment, aimed at reducing inflammation and improving muscle strength [9].
In this case, the patient responded well initially, with significant improvement in muscle strength and skin lesions. However, persistent dermatitis required the introduction of additional immunosuppressive agents, such as rituximab and upadacitinib. This highlights the need for a tailored and dynamic treatment approach, considering the variable response of DM to different therapies. This case emphasizes the association between DM and malignancies, reinforcing the need for vigilance in cancer screening among DM patients. The literature suggests a significantly higher risk of malignancies in DM patients, with the highest risk observed within the first year of DM diagnosis [2-5,9]. This case adds to the body of evidence, suggesting that comprehensive cancer screening should be an integral part of the diagnostic workup in DM patients.
Moreover, the role of specific autoantibodies, such as anti-TIF1-gamma, in predicting cancer-associated DM is noteworthy. The presence of these autoantibodies can guide clinicians in identifying patients at higher risk of underlying malignancies, facilitating early diagnosis and treatment. The therapeutic journey of the patient also underscores the complexity of managing DM in the context of cancer. The need for ongoing evaluation and adjustment of immunosuppressive therapy is critical in managing persistent or recurrent symptoms. Future research should focus on identifying biomarkers that can predict treatment response and on developing targeted therapies that address both the autoimmune and oncologic aspects of paraneoplastic DM.
Conclusion
This case of paraneoplastic DM associated with small cell bladder cancer highlights the complex interplay between autoimmune diseases and malignancies. It underscores the importance of comprehensive diagnostic strategies and the need for personalized therapeutic approaches. Ongoing research and clinical vigilance are essential to improving outcomes for patients with paraneoplastic syndromes and advancing our understanding of their underlying pathophysiology.
Disclosure
Author Contributions: Conceptualization, writing review and editing A.M and F.S: supervision A.S. T.D and F.S; Images A.M., A.S.
Acknowledgments: We kindly thank the patient who has generously agreed to the presentation of his case.
Conflicts of Interest: None.
References
- Marinos C D, Reinhard H (2003) Polymyositis and dermatomyositis, The Lancet. 362: 971-982.
- Barnes BE, Mawr B (1976) Dermatomyositis and malignancy. A review of the literature. Ann Intern Med. 84:68.
- Chow WH, GG (1995) Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer Causes Control. 6:9.
- Buchbinder R, Forbes A, Hall S, Denett X, Giles G (2001) Incidence of malignant disease in biopsy-proven inflammatory myopathy. A population-based cohort study. .Ann Intern Med. 134:1087.
- Chow WH, Gridley G, Mellemkjaer L, McLaughlin JK, Olsen JH, et al (1995) Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer Causes Control. 6:9
- Didona D, Fania L, Didona B, Eming R, Hertl M, et al (2020) Paraneoplastic dermatoses: A brief general review and an extensive analysis of paraneoplastic pemphigus and paraneoplastic dermatomyositis. Int J Mol Sci. 21:2178.
- Best M, Molinari N, Chasset F, Vincent T, Cordel N, et al (2019) Use of anti-transcriptional intermediary factor-1 gamma autoantibody in identifying adult dermatomyositis patients with cancer: a systematic review and meta-analysis. Acta Derm Venereol. 99:256–62.
- De Vooght J, Vulsteke J-B, De Haes P, Bossuyt X, Lories R, et al. (2020) Anti-TIF1-γ autoantibodies: warning lights of a tumour autoantigen. Rheumatology (Oxford). 59:469–77.
- Chen YJ, Wu CY, Huang YL, Wang CB, Shen JL et al. (2010) Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther. 12: R70.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.