Palliative Care Integration in Neurosurgical Brain Tumor Patients: A One-Year Retrospective Analysis of Referral Patterns, Symptom Burden, and Missed Opportunities
by Dimitar Slavkov1, Svetoslava Troyanova-Slavkova2*
1Department of Neurosurgery, Helios Vogtland Hospital, Plauen, Germany
2Department of Dermatology, Helios Vogtland Hospital, Plauen, Germany
*Corresponding Author: Svetoslava Troyanova-Slavkova, Department of Dermatology, Helios Vogtland Hospital, Plauen, Germany
Received Date: 27 November 2025
Accepted Date: 04 December 2025
Published Date: 06 December 2025
Citation: Slavkov D, Troyanova-Slavkova S (2025) Palliative Care Integration in Neurosurgical Brain Tumor Patients: A One-Year Retrospective Analysis of Referral Patterns, Symptom Burden, and Missed Opportunities. J Surg 10: 11500 https://doi.org/10.29011/25759760.011500
Abstract
Neurosurgical brain tumor patients face complex, high-risk clinical courses but remain underrepresented in Palliative Care (PC) despite significant symptom burden. In this one-year retrospective study of 120 adults with primary or metastatic brain tumors, we assessed PC involvement, symptom profiles, and healthcare utilization. Glioblastoma (48%) and metastases (36%) predominated, with frequent motor deficits (60%), cognitive changes (45%), headaches (40%), and seizures (25%). Only 38% received PC consultation, and early referral occurred in just 15%. Delayed or missed opportunities were identified in 35% of cases. Patients without PC had higher 90-day readmission rates (30% vs. 15%) and more intensive end-of-life interventions, while those with PC had more documented goals-of-care discussions. These findings indicate that PC is underutilized and often introduced late. Earlier, structured referral pathways and routine needs assessment may improve quality of life, reduce unnecessary utilization, and support more goal-concordant care in neurosurgical oncology.
Keywords: Brain Tumors; Early Integration; Neurosurgery; Palliative Care; Symptom Burden
Introduction
Neurosurgical patients represent a unique and often underserved population within palliative care. Many neurosurgical oncological conditions produce severe symptoms, complex clinical trajectories, and high decision-making burdens that warrant early supportive involvement. Despite the significant morbidity and mortality associated with neurosurgical diseases, they remain underrepresented in palliative care structures. Individuals with malignant brain tumors such as glioblastoma or brain metastases commonly face severe neurologic deficits, cognitive deterioration, seizures, treatment-related toxicities, and progressive functional dependence [1]. These challenges profoundly affect both patients and caregivers and often persist throughout the entire disease course, regardless of surgical intervention. Palliative Care (PC) is a multidisciplinary approach aimed at improving quality of life for patients and families facing serious illness by preventing and relieving physical, psychological, and spiritual distress [2]. Although PC is firmly established in fields such as medical oncology, its integration into neurological and neurosurgical care has developed more slowly. Emerging evidence suggests that neurosurgical patients may significantly benefit from palliative approaches. Studies evaluating high-risk surgical populations have shown that perioperative palliative care is associated with better communication, improved symptom management, and higher family satisfaction at the end of life. Notably, neurosurgical patients demonstrate among the highest 90-day postoperative mortality rates and the highest proportion of palliative care consultations when compared with other surgical fields, underscoring their substantial unmet needs. However, many of these referrals occur late-often only when disease-directed treatment is no longer feasible-reflecting the persistent misconception that palliative care is synonymous with end-of-life care [3]. To address rising demand and limited specialist resources, current models emphasize distinguishing between basic “primary palliative care” skills that all clinicians should possess and “specialist palliative care,” reserved for more complex cases. For neurosurgical teams, this means that early discussions about symptoms, goals of care, functional expectations, and realistic outcomes should occur alongside surgical planning, while specialist consultation is sought for complex decision-making, refractory symptoms, or psychosocial distress [4]. Despite growing recognition of its importance, data regarding how and when palliative care is used in neurosurgery remain scarce. Understanding local practice patterns is an essential first step toward improving care delivery. Therefore, this study aims to retrospectively assess palliative care involvement in patients with brain tumors treated in our neurosurgical department, focusing on referral timing, symptom burden, and gaps in current care. By characterizing these patterns, we aim to identify opportunities for earlier, more effective integration of palliative principles into neurosurgical practice.
Materials and Methods
This retrospective observational study was conducted over a one-year period at a neurosurgical center and included adult patients admitted with a diagnosis of a primary or metastatic brain tumor. All consecutive patients aged 18 years or older who were hospitalized under the neurosurgery service during the study period were identified through the electronic medical record system. Patients with incomplete inpatient documentation or those treated solely in outpatient settings were excluded. Data were collected using a standardized extraction form. Demographic information, presenting symptoms and tumor characteristics were recorded, along with treatment details such as type of neurosurgical intervention (resection, biopsy, or conservative management), urgency of surgery, and need for Intensive Care Unit (ICU) admission. Palliative care involvement was assessed by determining whether a consultation occurred, the timing relative to admission and diagnosis, and the primary indications for referral. Symptom burden-including pain, fatigue, and neurocognitive deficits-was extracted from neurosurgical, oncology, nursing, and palliative care notes. Cases with documented clinical deterioration prior to referral were classified as delayed or missed opportunities for palliative involvement. Healthcare utilization measures included total hospital length of stay, ICU stay, and 90-day readmissions. Discharge disposition, in-hospital mortality, and 30- and 90day mortality rates were recorded, along with documentation of goals-of-care discussions and advance care planning. Descriptive statistics were used to summarize all variables. Continuous data were reported as medians and ranges, while categorical data were expressed as frequencies and percentages.
Results
Patient Characteristics
A total of 120 neurosurgical patients with brain tumors were included in this retrospective analysis. The median age was 62 years (range 34-85), with 55% of patients being male. Glioblastoma was the most common diagnosis, affecting 48% of patients, followed by brain metastases in 36%, and other tumors such as meningioma and primary CNS lymphoma in 16%. Most patients presented with motor deficits (60%), cognitive changes (45%), and headaches (40%), while seizures were documented in 25% of patients. (Table 1) summarizes the demographic and clinical characteristics of the 120 neurosurgical brain tumor patients included in our study, highlighting age distribution, gender, tumor types, and presenting neurological symptoms.
|
Characteristic |
Number (%) / Median (Range) |
|
Age (years) |
62 (34-85) |
|
Gender |
|
|
- Male |
66 (55%) |
|
- Female |
54 (45%) |
|
Diagnosis |
|
|
- Glioblastoma |
58 (48%) |
|
- Brain metastases |
43 (36%) |
|
- Other tumors |
19 (16%) |
|
Presenting symptoms |
|
|
- Motor deficits |
72 (60%) |
|
- Cognitive changes |
54 (45%) |
|
- Headache |
48 (40%) |
|
- Seizures |
30 (25%) |
Table 1: Demographic and Clinical Characteristics of Neurosurgical Brain Tumor Patients (n = 120).
Treatment Characteristics Among the cohort, 65% underwent surgical resection, 20% received biopsy only, and 15% were managed conservatively without surgery. ICU admission occurred in 40% of patients, predominantly after major resections or during acute neurological deterioration. Emergency neurosurgical procedures accounted for 30% of operations, often due to sudden neurological decline or elevated intracranial pressure. (Table 2) presents the treatment characteristics and healthcare utilization of the cohort, including types of neurosurgical intervention, ICU admission rates, emergency surgeries, and median hospital length of stay.
|
Characteristic |
Number (%) / Median (Range) |
|
Type of neurosurgical intervention |
|
|
- Surgical resection |
78 (65%) |
|
- Biopsy only |
24 (20%) |
|
- Conservative management |
18 (15%) |
|
ICU admission |
48 (40%) |
|
Emergency surgery |
36 (30%) |
|
Median hospital length of stay (days) |
14 (5-45) |
Table 2: Treatment Characteristics and Healthcare Utilization of Neurosurgical Brain Tumor Patients (n = 120).
Palliative Care Involvement Palliative care consultation was provided for 38% of patients. The median time from hospital admission to first palliative care contact was 12 days (range 1-45), while the median time from initial diagnosis to referral was 3 months. The most common reasons for palliative involvement included symptom management (70%), goals-of-care discussions (55%), and end-of-life planning (40%). Notably, only 15% of patients received early palliative care within the first week of admission. (Table 3) details the extent and timing of palliative care involvement in the cohort, including consultation rates, median time to first contact, primary reasons for referral, and the proportion of patients receiving early palliative care within the first week of admission.
|
Characteristic |
Number (%) / Median (Range) |
|
Palliative care consultation |
46 (38%) |
|
Median time from admission to PC contact (days) |
12 (1-45) |
|
Median time from diagnosis to PC referral (months) |
3 (0.5-12) |
|
Main reasons for palliative care |
|
|
- Symptom management |
32 (70%) |
|
- Goals-of-care discussions |
25 (55%) |
|
- End-of-life planning |
18 (40%) |
|
Early palliative care (within 1 week) |
7 (15%) |
Table 3: Palliative Care Involvement and Timing in Neurosurgical Brain Tumor Patients (n = 120)
Symptom Burden and Missed Opportunities High symptom burden was observed in most patients, including pain, fatigue, and neurocognitive deficits. In 35% of cases, documentation suggested that palliative care was initiated late despite clear indications, particularly in patients undergoing emergency neurosurgical interventions. Several patients experienced rapid neurological deterioration, leaving little time for structured goals-of-care discussions prior to critical surgical decisions. (Figure 1) illustrates the symptom burden among the cohort, highlighting the prevalence of motor deficits, pain, fatigue, cognitive decline, and seizures in neurosurgical brain tumor patients.
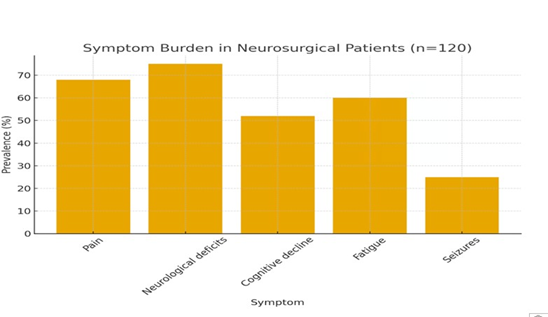
Figure 1: Symptom Burden in Neurosurgical Brain Tumor Patients (n = 120).
Patient Outcomes and Healthcare Utilization The median hospital length of stay was 14 days (range 5-45), with ICU stays ranging from 2 to 10 days among those admitted. Approximately 25% of patients experienced at least one hospital readmission within 90 days of discharge. Notably, readmissions were more frequent among patients who did not receive palliative care compared with those who did (30% vs. 15%). Patients without palliative care involvement also had higher ICU utilization and underwent more invasive interventions near the end of life, underscoring the potential impact of timely palliative care consultation on resource use and end-of-life management. (Table 4) presents patient outcomes, including discharge disposition, short- and mediumterm mortality, and 90-day readmission rates, highlighting the differences in readmissions between patients with and without palliative care involvement.
|
Outcome |
Number (%) |
|
Discharge destination |
|
|
Home |
48 (40%) |
|
Rehabilitation facility |
30 (25%) |
|
Hospice / palliative care unit |
42 (35%) |
|
In-hospital mortality |
22 (18%) |
|
30-day mortality |
24 (20%) |
|
90-day mortality |
34 (28%) |
|
Readmissions within 90 days |
30 (25%) |
|
Readmissions without PC |
23/78 (30%) |
|
Readmissions with PC |
7/42 (15%) |
Table 4: Patient Outcomes, Discharge Disposition, and Readmissions (n = 120)
Discussion
In our one-year retrospective cohort of 120 neurosurgical brain tumor patients, we documented a notably high burden of symptoms, delayed palliative care integration, and considerable healthcare utilization. The predominance of glioblastoma (48%) and brain metastases (36%) in our sample aligns with the demographic and diagnostic trends described in other neurooncology settings. These tumor types are well-known for their aggressive clinical course and rapid neurological decline, which often necessitates intensive neurosurgical interventions. Despite this, only 38% of our patients received palliative care consultation, and a mere 15% were referred within the first week of admission. This underutilization mirrors recent findings: Rhee et al. note in their review that palliative care in neuro-oncology is still underdeveloped, with many patients referred late despite high symptom burden and unmet needs [5]. Moreover, benchmarking data from German neuro-oncology centers show that only about 30% of surveyed centers consistently integrate palliative care at the time of diagnosis [6]. The limited timing of palliative care referral in our study likely reflects entrenched misconceptions that restrict palliative care to the very end of life, rather than appreciating it as a complementary form of support throughout disease progression. Indeed, late palliative decisions have been associated with more aggressive end-of-life interventions and higher reliance on acute hospital services. Supporting this, a study in neuro-oncology demonstrated that early palliative care integration can improve quality of life [7]. Our observation that a substantial proportion of patients had indications for palliative care well before referral underscores the urgent need for earlier, structured referral pathways. The symptom burden in our cohort was similarly high: pain, fatigue, and neurocognitive decline were common. These findings reflect the complex palliative needs of neuro-oncological patients. Prior work underscores that neurosurgical tumor patients face not only physical symptoms but also profound psychosocial and existential distress, driven by cognitive impairment, seizures, personality changes, and difficulties in communication [5]. In this context, early palliative involvement is critical: it can facilitate goal-setting, Advance Care Planning (ACP), and shared decisionmaking-especially given that neurologic decline may rapidly compromise a patient’s capacity to express preferences. Our data further reveal important differences in healthcare utilization. Patients without palliative care involvement had higher 90-day readmission rates and underwent more intensive interventions near the end of life, whereas those who received palliative care had more documented goals-of-care discussions. This mirrors prior research showing that palliative care engagement is associated with more goal-concordant care and less aggressive end-of-life treatment. For instance, in a neuro-ICU setting, palliative consultation has been linked to a shift toward comfort-oriented code status.
The clinical and ethical challenges in neurosurgery are especially pronounced in brain tumor patients. These patients often confront urgent, high-stakes decisions in the face of cognitive decline, fluctuating capacity, and complex prognoses. A neuro-oncology survey in Germany revealed wide variability in when palliative care is involved-many centers wait until neurological or general deterioration, while only a minority introduce palliative care at initial diagnosis [6]. Our findings reinforce that delayed involvement represents missed opportunities to support patients and families during vulnerable moments. The benchmarking survey of German neurooncology centers identified substantial heterogeneity in practice: while 76.1% of centers have a dedicated palliative care unit, only 34.8% of centers include palliative specialists in tumor board meetings, and only 30.4% routinely initiate palliative care at diagnosis [6]. These data reveal structural gaps in care that limit early integration. Another important gap relates to in-patient malignant glioma care. A recent German nationwide analysis found that only about 10% of hospitalized glioma patients received complex or specialized palliative care, and among those who died in the hospital, only ~40% were documented to have received it [8]. This substantial under-provision clearly indicates that even when patients are hospitalized and face severe disease, palliative services are not consistently applied. Finally, caregiver needs remain inadequately addressed. Research among family caregivers of patients with primary brain tumors shows that many report substantial unmet needs - emotional, informational, practical and psychosocial - despite providing long-term, intensive care [9]. Several studies highlight that caregivers often feel unprepared and unsupported as the patient’s condition evolves, coping with neurocognitive and behavioural changes, financial and family-role shifts, and enduring psychological distress (including high rates of depressive symptoms) [10].
Recommendations for Improving Integration
Based on both our findings and the recent literature, several strategies may help to close these gaps. First, institutions should implement routine screening of physical, cognitive, emotional, and spiritual needs for all neuro-oncology patients at defined time points—at diagnosis, post-surgery, and at discharge-to identify palliative needs early. Second, referral pathways should be formalized: risk-based triggers (e.g., high-grade glioma, ICU admission, signs of neurological decline) can prompt automatic palliative care consultation, rather than waiting until late-stage disease [11]. Embedding palliative specialists within neuro-oncology tumor boards can help normalize early referral and ensure multidisciplinary collaboration. Third, education and training are essential: neurosurgeons, neuro-oncologists, ICU staff, and palliative care teams should develop shared competencies in symptom management, communication, and ACP. Fourth, to overcome capacity constraints, palliative care services should be expanded through telehealth, home-based care, and additional staffing, ensuring a broader reach. Fifth, caregiver support must be strengthened by using formal assessment tools to identify unmet needs and offering psychosocial, educational, and spiritual interventions tailored to the unique burden of brain tumor care. Sixth, advance care planning should be initiated early and revisited over time, using a patient-centered, conversational approach that respects autonomy and de-stigmatizes palliative care, emphasizing its role beyond just end-of-life care [12].
Conclusion
In summary, our study reaffirms that many neurosurgical brain tumor patients suffer significant symptoms and follow high-risk clinical trajectories, yet palliative care is underutilized and often initiated too late. When integrated earlier, palliative care has the potential to improve quality of life, facilitate shared decision-making, and better align care with patients’ values. The literature underscores persistent gaps-structural, educational, and perceptual-that hinder early integration. To address these, neuro-oncology practices must evolve: palliative care should be embedded proactively, with routine screening, structured referral pathways, interprofessional collaboration, and strong caregiver support. By doing so, we can provide more holistic, patient-centered care that truly meets the needs of both patients and families.
References
- Calisaya-Madariaga IG, Acurio K, Callapiña-Sumaran R (2024) Beyond the scalpel: the role of palliative care in Neurosurgery. Neurosurg Rev 47: 472.
- Virdun C, Singh GK, Yates P (2025) Understanding the acute care context to inform palliative care improvements: a qualitative study of hospital-based multidisciplinary clinicians. BMC Palliat Care 24: 185.
- Pottash M, McCamey D, Groninger H (2020) Palliative Care Consultation and Effect on Length of Stay in a Tertiary-Level Neurological Intensive Care Unit. Palliat Med Rep 1: 161-165.
- Harrison DJ, Wu E, Singh R (2023) Primary and Specialist Palliative Care in Neurosurgery: A Narrative Review and Bibliometric Analysis of Glioblastoma and Stroke. World Neurosurg 180: e250-e257.
- Rhee JY, Strander S, Podgurski A (2023) Palliative Care in Neurooncology: an Update. Curr Neurol Neurosci Rep 23: 645-656.
- Lawson McLean AC, Lawson McLean A, Ernst T (2024) German Consortium for Excellence in Neurooncology, Palliative Care (GCENPC). Benchmarking palliative care practices in neurooncology: a german perspective. J Neurooncol 168: 333-343.
- Golla H, Nettekoven C, Hellmich M (2024) EPCOG study group. Effect of early integration of palliativecare ont the quality of life in glioblastoma patients. Neuro Oncol 26: v20.
- Fink L, van Oorschot B, von Saß C (2024) Palliative care for in-patient malignant glioma patients in Germany. J Neurooncol 167: 323-338.
- Pointon L, Grant R, Peoples S (2023) Unmet needs and wish for support of family caregivers of primary brain tumor patients. Neurooncol Pract 10: 271-280.
- Schubart JR, Kinzie MB, Farace E (2008) Caring for the brain tumor patient: family caregiver burden and unmet needs. Neuro Oncol 10: 61-72.
- Bar B, Creutzfeldt CJ, Rubin MA (2020) Palliative Care in the Neuro-ICU: Perceptions, Practice Patterns, and Preferences of Neurointensivists. Neurocrit Care 32: 302-305.
- Yefimova M, Aslakson RA, Yang L (2020) Palliative Care and End-ofLife Outcomes Following High-risk Surgery. JAMA Surg 155: 138-146.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.