Pain and The UK Opioid Crisis: Time to Reconsider Pain Management Programmes and The Role for Specific Combination Analgesics
by Mark Burdon1, Nisa Aslam2, Pamela Mason3*
1Pharmacist, Northumberland, UK
2GP, Hertfordshire, UK
3Researcher, Brecon, UK
*Corresponding author: Pamela Mason, Researcher, Brecon, UK
Received Date: 29 March, 2025
Accepted Date: 08 April, 2025
Published Date: 16 June, 2025
Citation: Burdon M, Aslam N, Mason P (2025) Pain and The UK Opioid Crisis: Time to Reconsider Pain Management Programmes and The Role for Specific Combination Analgesics. J Community Med Public Health 9: 515. https://doi.org/10.29011/2577-2228.100515
Abstract
Pain is a complex experience that is both distressing for individuals and challenging for healthcare professionals (HCPs). It can range from mild to moderate or severe. Various pain relief options are available, including paracetamol, non-steroidal anti-inflammatory drugs (NSAIDs) like ibuprofen and naproxen, and opioids. In a survey of 1,000 consumers, 22 per cent reported taking painkillers two to three times a day. Opioids are linked to significant adverse effects, including dependency, and are frequently overused both in the community and in hospitals. The UK has been facing an opioid epidemic, with the highest global rate of opioid consumption recorded in 2019. Although opioid use in the UK has declined slightly since then, further efforts are needed to reduce it.
The various pain pathways for mild to moderate pain identify paracetamol, NSAIDs such as ibuprofen and naproxen, salicylate NSAIDs such as aspirin and weak opioids such as codeine, dihydrocodeine and tramadol as suitable for mild to moderate pain management.
Combination analgesics are identified in various pain pathways, but they do not include a combination of paracetamol/ ibuprofen in one dose. Paracetamol and ibuprofen reduce pain via different mechanisms of action, and in combination they act across the pain pathway and provide proven effective pain relief for mild to moderate pain at every recommended dose. Combogesic® is a patented double action formula containing paracetamol 500 mg and ibuprofen 150mg which work together in complementary ways to help relieve pain. A single dose of Combogesic is clinically proven to be 80 per cent more effective than paracetamol alone and 30 per cent more effective than ibuprofen alone. By contrast, the addition of codeine 60mg to paracetamol 1,000mg has been shown to provide only modest additional pain relief.
This paper includes a brief resumé of pain, an in-depth commentary of the high use of opioids with a focus on the UK, the adverse effects caused by the ‘opioid epidemic’ and the need to reduce their use in both the community and hospital settings. In addition, we review the management of mild to moderate pain and the clinical evidence around a specific combination of paracetamol and ibuprofen that could be put into place as a therapy option and included in recommendations for mild to moderate pain, with the potential to reduce the need for opioid use.
Keywords: Pain; UK opioid crisis; NSAIDs; Opioids; Ibuprofen; Paracetamol; Combination analgesic
Pain: An Introduction
Pain is defined as “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage.” [1] Causes of pain may include: an acute injury (e.g. wounds, cuts, sprains, strains, fractures, burns); an ongoing degenerative illness (e.g. musculoskeletal disorders such as arthritic conditions or ankylosing spondylitis, Chronic Obstructive Pulmonary Disease (COPD), infections, or a primary condition (e.g. endometriosis, cancer, headaches, trigeminal neuralgia). Medication overuse, medical interventions such as surgery, and conditions like anxiety, depression, and sleep disorders may also contribute to or lower the body’s tolerance to pain.
Pain Prevalence
Pain is very common and is considered a major clinical, social, and economic problem in communities globally [2]. A 2025 real- world survey of 2,003 people found that 16 per cent of respondents were in pain most of the time, 15 per cent were in pain two or three times a week, 12 per cent two or three times a month, 11 per cent experienced pain once a day and 10 per cent were in constant pain [3].
Pain prevalence identified in the literature varies depending on the criteria and definitions used but in general, data confirms the findings of the survey. Nearly 10 million Britons are estimated to suffer from pain almost daily resulting in a major impact on their quality of life and more days off work. The prevalence of significant persistent pain in the UK is around 23 per cent, and increases with age, affecting 30 per cent of older adults [4]. Women are more commonly affected by pain than are men. Socioeconomic deprivation and unemployment as well as manual jobs increase the risk of pain. Lifestyle factors such as smoking, alcohol and lack of physical activity, obesity and a history of trauma or pain are contributing factors.
Estimates in the literature of mild to moderate pain, which is the focus of this paper, also fluctuate based on study methodology and range from 20 to 50 per cent in different populations [5]. A 2023 study in 5,961 Germans aged 40-85 found 44 per cent suffered from at least mild pain [6]. Pains in the neck, shoulders, arms, back and legs were the most common in a study among a general population [5]. These findings were broadly confirmed in the consumer survey [3] with 38 per cent suffering from lower back pain, 30 per cent from leg pain, 23 per cent from neck pain, 18 per cent from pain in the arms and 15 per cent from shoulder pain. The real-world survey also identified more than half (51 per cent) with head pain, 18 per cent with knee pain, 17 per cent with upper back pain, 13 per cent had oral pain (teeth and gums) and 11 per cent uterine pain (period pain or endometriosis).
Types of Pain
Pain may be either acute or chronic:
- Acute pain is considered to be short term pain which resolves within 30 days or according to some definitions within three months. Acute pain is seen as a useful survival mechanism that serves a protective and healing function.
- Chronic pain or persistent pain is considered to be longterm, lasting for longer than three months [4]. Chronic pain is increasingly considered to be a disease in its own right [5] with syndromes like low back pain not caused by a serious pathology (e.g. cancer, or a broken bone) and fibromyalgia or arthritis considered to be chronic primary pain and a disease rather than a symptom [7]. Where pain may at least be initially conceived as a symptom only, there are now calls to refer to these instances as chronic secondary pain (e.g. chronic cancerrelated pain, chronic neuropathic pain, chronic secondary visceral pain, chronic post-traumatic and post-surgical pain, chronic secondary headache and orofacial pain and chronic secondary musculoskeletal pain) [7].
Pathophysiology of Pain
Pain has sensory and emotional components. Acute pain is frequently associated with anxiety and hyperactivity of the sympathetic nervous system (e.g. tachycardia, increased respiratory rate and blood pressure, dilated pupils). Chronic pain does not involve sympathetic hyperactivity but may be associated with fatigue, loss of libido, loss of appetite and depressed mood. People vary considerably in their tolerance for pain.
Acute pain, which usually occurs in response to tissue injury, results from activation of peripheral pain receptors and their specific A delta and C sensory nerve fibres (nociceptors). Chronic pain related to ongoing tissue injury is caused by persistent activation of these fibres. Pain fibres enter the spinal cord at the dorsal root ganglia and synapse in the dorsal horn. From there, fibres cross to the other side and travel up the lateral columns to the thalamus and then to the cerebral cortex [8].
Repetitive stimulation (e.g. from a prolonged painful condition) can sensitise neurons in the dorsal horn of the spinal cord so that a lesser peripheral stimulus causes pain (wind-up phenomenon). Peripheral nerves and nerves at other levels of the Central Nervous System (CNS) may also be sensitised, producing long-term synaptic changes in cortical receptive fields (remodelling) that maintain exaggerated pain perception.
Substances released when tissue is injured, including those involved in the inflammatory cascade include vasoactive peptides (e.g. calcitonin gene-related protein, substance P, neurokinin A) and other mediators (e.g. prostaglandin E2, serotonin, bradykinin, epinephrine) [8]. The pain signal is modulated at multiple points by many neurochemical mediators, including endorphins (e.g. encephalin) and monoamines (e.g. serotonin, norepinephrine). These mediators interact in complex and not fully understood ways to amplify, sustain, diminish, or suppress pain perception and response. Psychological factors are also important mediators in the perception and experience of pain.
The Challenges of Pain Management
Managing mild to moderate or severe pain, is challenging and multifaceted. The experience of pain is complex and influenced by features such as the degree of tissue injury, current mood, previous experience of pain and understanding of the cause and significance of pain. Pain has historically been undertreated and 40 per cent of patients treated in primary care do not achieve adequate pain relief [9]. This highlights the difficulties many general practitioners face in pain management, likely due to limited training and education, insufficient knowledge of pain and its treatment, and a lack of confidence in prescribing analgesics and managing patient expectations regarding pain relief and outcomes.
A comprehensive patient pain assessment is essential. A thorough pain history should include a detailed description of the pain, such as its location, whether it radiates, its intensity, and its nature (sharp, dull, aching, stabbing, or burning). Additionally, the impact of pain on physical, mental, and social functioning should be considered. It’s important to identify co-morbidities, any current medications, the use and response to pain relief treatments, and complementary therapies. The patient’s current or past history of addiction, as well as their understanding of pain and its expected outcomes, should also be assessed. Pain can be assessed using self-reported measures. The most popular measures are numerical or visual analogue scales where patients will be asked to rate their pain on a scale of 0-10 or 0-100, including the 11-point Numerical Pain Rating Scale (NPRS-11) and the Wong-Baker FACES scale, which uses a series of facial expressions to help patients communicate their pain intensity [10].
Pain Medications
Medications for mild to moderate pain include paracetamol, nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen and naproxen and weak opioids such as codeine and dihydrocodeine. Several fixed dose preparations include an opioid (e.g. paracetamol with codeine, paracetamol with dihydrocodeine or aspirin with codeine). Many of these preparations are available Over The Counter (OTC) and on prescription.
There is a perception that the addition of an opioid to paracetamol improves mild to moderate pain control, though there is limited evidence for this. The addition of codeine 60mg to paracetamol has been shown to provide only modest incremental pain relief [11].
Opioids
Opioids are a class of natural, semi-synthetic, and synthetic drugs that include both prescription medications and illegal drugs like heroin. Strong opioids on prescription include morphine, diamorphine, buprenorphine, fentanyl, methadone, oxycodone, pentazocine and pethidine. Tramadol, codeine, dihydrocodeine and meptazinol are examples of weaker opioids. Some opioids are available in OTC formulations such as co-codamol. Opioid analgesics are commonly used to relieve moderate to severe pain particularly of visceral origin while weaker opioids in combination analgesics are used to treat mild to moderate pain.
The adverse effects associated with opioids are well established. Side effects include constipation, nausea and vomiting, headache, dizziness, depression, sexual dysfunction and sleepiness or sleep disturbances. Opioids can also impair the immune system, increasing the risk of infection. Repeated administration may cause dependence and tolerance. High doses, particularly when used for long periods of time, can make patients more sensitive to pain (a phenomenon known as opioid-induced hyperalgesia) and can lead to overdose and death. Even when used for a short time, opioids may result in adverse effects or lead to addiction and, too often, to misuse and overdose. Depending on the dose and how long people use them, opioids can cause cardiovascular changes, such as a slower heart rate, low blood pressure, heart failure, and cardiac arrest. Particular risks arise when opioids are combined with other medication used for pain such as benzodiazepines and gabapentinoids.
Opioid effectiveness has been evaluated in a 2022 meta-analysis [12]. Opioids are associated with small improvements versus placebo in pain and function, and increased risk of harms at short-term (1 to <6 months) follow-up. Research on long-term effectiveness is very limited, and there is evidence of increased risk of serious harms that appear to be dose dependent. At shortterm follow-up, evidence showed no differences between opioids versus non-opioid medications in improvement in pain, function, mental health status, sleep, or depression [12].
Opioid Misuse?
Substantial heterogeneity exists in global opioid consumption. An opioid crisis emerged across high-income countries in the 2000s. The United States led the opioid crisis with opioid analgesic poisoning death rates quadrupling between 1999 to 2011 due to abuse of prescription opioid analgesics. In the US, there were 50,000 opioid-related deaths in 2017, a four-fold increase from 2002. Death rates were 20.7 per 100,000 population in 2020, a situation which was described as a public health emergency [13]. There were three distinct hallmarks of the opioid crisis in the US:
- widespread increase in opioid prescribing
- increased use of street drugs such as heroin
- the introduction of highly potent synthetic opioids such as fentanyl.
From 2009-2019, other countries like Canada, Sweden, Norway, Ireland, and the UK saw a surge in opioid-analgesic poisoning deaths but death rates in these countries have since fallen [13]. Fentanyl deaths are not as common in Europe as the US but are increasing. In the case of France, opioid prescriptions more than doubled from 2004 to 2017. Opioid related hospitalisations increased from 15 to 40 per 1,000,000 population and opioidrelated deaths increased from 1.5 to 3.2 per 1,000,000 population (2000-2015) [13].
The UK has also seen increases in opioid use and may be considered to be suffering an opioid epidemic. In a 2025 real-world survey among 111 HCPs, 79 per cent agreed there is an opioid crisis [3]. Research from the peer reviewed literature found that between 1998 and 2016 opioid prescriptions increased by 34 per cent in England (equivalent to a 127 per cent increase in terms of total oral morphine) [14,15] In 2019, the UK had the world’s highest rate of opioid consumption [14].
The consequences of this epidemic are extremely serious. Opioidrelated hospitalisations rose by 49 per cent from 2008-2018, with an estimated healthcare cost of £137 million [15]. Opioid-related drug poisoning deaths have also increased significantly, rising by 388 per cent in England and Wales over the past decades [15]. In 2020, the death rate stood at 4.0 per 100,000.
Nearly half of all drug-poisoning deaths registered in England and
Wales in 2023 were confirmed to involve an opiate (46.8 per cent; 2,551 deaths) [16]. According to the latest Adult Substance Misuse Treatment Statistics 2023–2024, nearly half of adults in treatment programs are addressing opiate-related issues, with 137,965 individuals receiving treatment for opiate misuse in England alone [17].
Despite these concerning figures, death rates in the UK remain lower than in the US, likely due to differences in health care systems. The UK’s centralised NHS and GP registration contrast with the decentralised healthcare system of the US which may contribute to the disparity in opioid-related mortality rates.
A 2020 UK retrospective cohort study [18] among nearly two million primary care patients without cancer found that codeine was the most commonly prescribed opioid, with its use increasing fivefold from 2006 to 2017. Morphine, buprenorphine and oxycodone prescribing rates also rose throughout the study period. A total of 14.6 per cent became long-term opioid users in the first year. Factors associated with long-term (>two years) use included age, deprivation, comorbidities such as fibromyalgia, rheumatological conditions, as well as recent major surgery, history of substance and alcohol misuse and self-harm/suicide. Regional and practicelevel variation was also observed.
Notably, in the UK, the number of people receiving treatment for prescription and OTC opioid-related misuse increased by 38 per cent from 2009 to 2016 [15].
But why is England suffering an opioid epidemic at a time when death rates from opioid use in the US, Germany and Sweden have fallen? There are a couple of reasons. GPs may have faced additional pressure to prescribe due to patient expectations for pain relief. Access to pain services is also inadequate [19].
According to the IWOTCH study from Warwick University, there are currently over one million people in the UK on prescription opioids. More than 50,000 have been taking them for six months or longer, at an estimated cost of £500 million to the NHS annually [19]. However, according to the IWOTCH study, prescriptions for opioids have fallen over the past four years by only eight per cent. [20] This fall in prescriptions is thought to be due to increased GP and pharmacist awareness. Furthermore, an NHS Action Plan was developed in 2023 to support further reduction in prescribing. Also, the NICE guidelines for chronic pain [21], which do not include analgesics at all, may have contributed to reduced opioid prescribing. However, a 2022 study evaluating pain management in a community-dwelling sample in England found that since the introduction of the NICE guideline for chronic pain, 47 per cent of people treated for chronic pain continue to use opioids [22]. Additionally, the data in Table 1 shows that opioid prescribing remains high and despite prescriptions seeming to decrease, related deaths have continued to rise (Table 1).
|
Year |
Number of opioid prescriptions |
|
2019 |
24,464,291 |
|
2020 |
23,285,877 |
|
2021 |
23,001,489 |
|
2022 |
22,881,196 |
|
2023 |
22,270,950 |
Table 1: Prescriptions for opioids in England since 2019 [23].
Reconsideration of the Management of Mild to Moderate Pain Pathways
Various pain pathways identify the following analgesics as suitable for the use in the management of mild to moderate pain.
- Paracetamol
- NSAIDs – classified as non-selective NSAIDs (e.g. naproxen, diclofenac, ibuprofen, indomethacin and mefenamic acid) and selective NSAIDS or coxibs (e.g. celecoxib and eterocoxib)
- Aspirin (a salicylate NSAID)
- Weak opioids (codeine, dihydrocodeine and tramadol).
Fixed dose combination preparations are also listed in the various pathways:
- Paracetamol with codeine
- Paracetamol with dihydrocodeine
- Paracetamol with tramadol
- Aspirin with codeine.
However, a fixed-dose combination of paracetamol with ibuprofen is not included for mild to moderate pain in various pain pathways. As studies have shown synergies between paracetamol and ibuprofen when used in combination [25] the lack of inclusion is surprising..
Of particular note, these studies demonstrate the importance of the paracetamol-to-ibuprofen ratio in fixed-dose combinations. When the ratio is high (3.3:1), there is a distinct synergistic effect in both acute and chronic pain models compared to ibuprofen alone, while still maintaining dosing of both active ingredients within OTC limits [26].
Table 2: Effect of paracetamol/ibuprofen ratios on additive efficacy vs comparable doses of the single components. |
||||||||||||||||||||||||||||||||||||
However, as the ratio decreases, i.e. ibuprofen increases relative to paracetamol, the synergistic effect of paracetamol becomes less apparent. A 2.5:1 is effective in acute pain, but not chronic pain [27-29]. At even lower ratios, such as 2:1, 1.67:1 or 1.25:1, no synergistic effect in acute pain is observed [31-35] (Table 2).
In a study of adults undergoing dental surgery, two tablets of paracetamol 500 mg/ibuprofen 150 mg or two tablets of paracetamol 500 mg or two tablets of ibuprofen 150mg were taken before wisdom tooth removal and every six hours for 48 hours post-surgery. The Area Under the Curve (AUC) for the 100 mm Visual Analogues Scale for Pain demonstrated the superior pain relief of the paracetamol 500mg/ibuprofen 150 mg combination [27].
A prospective, multicentre, randomised, double-blind, placebocontrolled, Phase III trial included 408 adult volunteers aged 18 to 60 years experiencing moderate to severe pain after surgical removal of at least two impacted third molars. Patients were randomised to paracetamol 975 mg/ibuprofen 292.5 mg (3.3:1), paracetamol 975mg, ibuprofen 292.5 mg or placebo. Pain intensity was measured using a 100mm Visual Analogue Scale and time to perceptible and meaningful pain relief was assessed by using the two-stop-watch method.
The paracetamol 975 mg/ibuprofen 292.5 mg 3.3:1 combination provided better pain control than either component alone or placebo [28].
In a multicentre, two-stage, randomised, double-blind, parallelgroup, placebo-controlled, factorial study, 735 adults with moderate to severe dental pain were assigned to various 2.5:1 combination groups of paracetamol/ibuprofen (250 mg/100 mg, 500 mg/200 mg, 1000 mg/400 mg) or paracetamol alone (500 or 1000mg) or ibuprofen alone (200 or 400 mg). The two higher-dose combinations provided improved pain relief compared with either paracetamol or ibuprofen alone, or placebo [29]. Similar findings were observed in a study in 234 adolescents and adults aged 1640 with post-operative dental pain following the extraction of impacted molars [30].
A randomised, double-blind, four-arm, parallel-group, active controlled trial in 892 participants with knee arthritis investigated short-term (day 10) and long-term (week 13) benefits and sideeffects of four regimens, each taken three times a day: ibuprofen (400 mg); paracetamol (1000 mg); one fixed-dose combination tablet (ibuprofen 200mg/paracetamol 500mg); two fixed-dose combination tablets (ibuprofen 400mg/paracetamol 1000mg). The fixed dose combination (paracetamol/ibuprofen 2.5:1) was more effective than paracetamol alone [31].
A study among 792 patients with soft tissue injuries attending an emergency department found that patients experienced no greater pain relief with a combination of paracetamol/ibuprofen in the specific ratio of 2.5:1 in alternating doses compared with either component alone [32]. A 2:1 paracetamol/ibuprofen combination was not superior to either constituent alone in a study in children post tonsillectomy [33]. Combinations of paracetamol/ibuprofen in ratios of 1.66:1 [33,34] and 1.25:1 [34,35] did not impact acute pain compared with paracetamol or ibuprofen alone.
Paracetamol/Ibuprofen 3.3:1 (Combogesic)
Combogesic is the only combination analgesic that provides paracetamol and ibuprofen at a synergistic 3.3:1 ratio. Each Combogesic tablet contains 500 mg paracetamol and 150 mg ibuprofen (3.3:1). In a single dose, this combination is indicated for mild to moderate pain. The recommended dose is one to two tablets every six hours (up to a maximum of six tablets daily) [38]. It is available OTC as a General Sales List (GSL) and as a Pharmacy (P) medicine.
Mechanisms of Action of Paracetamol and Ibuprofen
Paracetamol has analgesic and anti-pyretic activity but has little anti-inflammatory activity. It has been used clinically for over a hundred years and is the most widely used analgesic in the world. When combined with ibuprofen, paracetamol allows for lower doses of ibuprofen to be used because of the analgesic effectiveness of the combination, thereby reducing the risk of adverse effects that may be observed at higher doses [39].
Paracetamol undergoes a two-stage metabolic process to produce the active metabolite N-(4-Hydroxyphenyl) arachidonylphenolamine (AM404). AM404 acts as a potent agonist of the transient receptor potential vanilloid type 1 (TRPV1), a low-affinity ligand of the cannabinoid receptor type 1 (CB1) and a Cyclo-Oxygenase (COX) inhibitor. Additionally, AM404 inhibits sodium channels in a similar manner to anaesthetics. These actions individually contribute to pain reduction and are considered potential mechanisms of action for paracetamol [38]. Paracetamol primarily acts in the brain and Central Nervous System (CNS) and interacts through several other pathways including serotonergic, opioid and Nitric Oxide (NO) pathways [40].
Ibuprofen works by non-selectively and reversibly inhibiting the cyclooxygenase enzymes COX-1 and COX-2, leading to the reduction in inflammatory prostanoids. The activity of the COX enzyme depends on it being in the oxidised form, which paracetamol can reduce in the central nervous system but not in the peripheral tissues. This suggests a possible synergistic effect in action between paracetamol and ibuprofen in the CNS [41].
The combination of paracetamol and ibuprofen at a 3.3:1 ratio offers an effective alternative to either component alone, without the adverse effects of opioid-based therapy.
Efficacy
Combogesic oral tablets offer stronger, faster pain relief than paracetamol or ibuprofen tablets alone [27] (Figure 1). Its 3.3:1 ratio is clinically proven to be 80 per cent more effective than
paracetamol alone and 30 per cent more effective than ibuprofen alone. It also provides a faster onset of meaningful pain relief than paracetamol or ibuprofen alone [27]. It is indicated for the temporary pain relief of headaches and migraines, back pain and period pain.
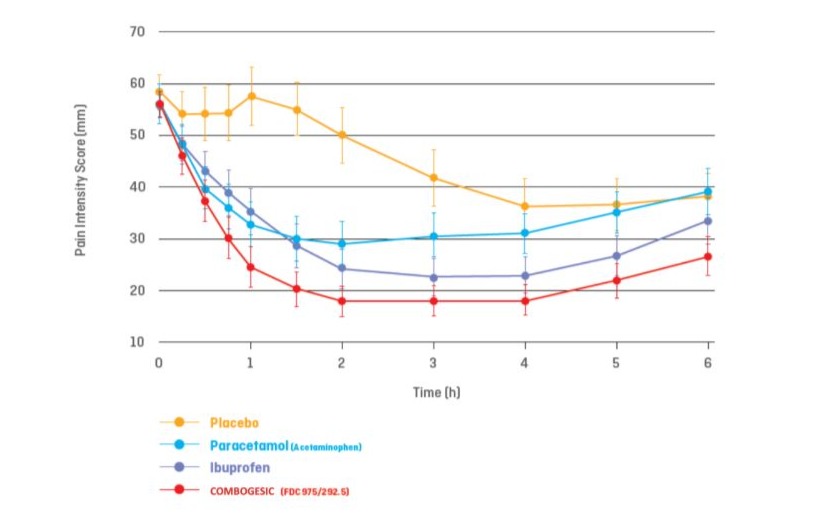
Figure 1: Pain intensity scores over six hours: Combogesic compared with paracetamol alone, ibuprofen alone and placebo. (Data from [28]).
Safety
Combogesic has a strong safety profile. Pooled safety data from 922 patients [39] who received full doses of either paracetamol 500 mg/ibuprofen 150 mg fixed-dose combination across four studies showed the incidence of adverse events for paracetamol 500 mg/ ibuprofen 150 mg fixed dose combination were similar to or below either paracetamol monotherapy or ibuprofen monotherapy or placebo. Combogesic did not alter the incidence and percentage of gastrointestinal events and post-operative bleeding compared with paracetamol or ibuprofen alone (Figure 2).
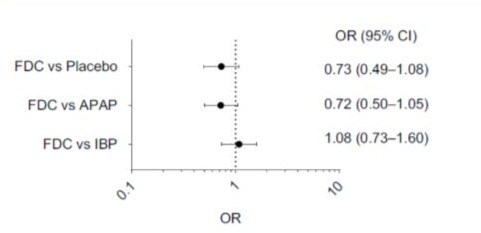
Figure 2: Odds ratio of experiencing at least one adverse event during the double-blind treatment period. A Comparison of the Fixed Dose Combination (FDC) (Combogesic) of Acetaminophen (Paracetamol) and Ibuprofen Compared with Paracetamol or Ibuprofen Monotherapy (data from [39]). OR: odds ratio; FDC: Fixed Dose Combination (Combogesic) of Acetaminophen (Paracetamol) and Ibuprofen; APAP: Acetaminophen
(Paracetamol); IBU: Ibuprofen.
In this analysis [39], the percentage of patients requiring rescue medication was pooled and assessed. The percentage of subjects that required at least one dose of rescue medication (oxycodone) was highest in the placebo group (70 per cent) and lowest in the fixed dose combination group (34 per cent). In the active comparator groups, over 39 per cent taking ibuprofen and 50 per cent taking paracetamol required at least one dose of rescue medication. These findings suggest that use of Combogesic can reduce the use of an opioid rescue medication to a greater extent than paracetamol or ibuprofen monotherapy. This could potentially contribute to reducing the use of opioid medications in the management of pain.
NSAIDs are particularly associated with risk of gastrointestinal (GI) complications. Dosage is an important consideration. When increasing the dose above 1200 mg per day, the risk profile of ibuprofen, particularly the relative risk of GI complications, appears to increase and ibuprofen begins to demonstrate relative risks similar to that of naproxen and indomethacin. From a meta-analysis of 28 studies [41] the pooled relative risk of GI complications with ibuprofen was almost doubled (pooled relative risk 1.8; Figure 3) with higher doses (>1200mg daily).
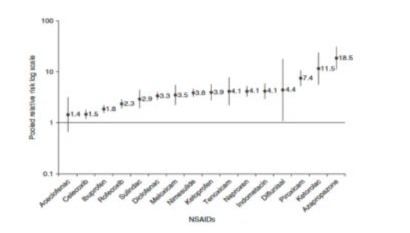
Figure 3: Pooled Relative Risks and 95% CIs of upper GI symptoms associated with individual NSAIDs (data from [41]).
A systematic review found that low-dose ibuprofen (<1500 mg/ day) is associated with a lower risk of serious gastrointestinal complications compared to other NSAIDs. However, this advantage diminishes at higher doses (>1800mg/day), where ibuprofen’s gastrointestinal risks become comparable to those of other NSAIDs like naproxen and indomethacin [42].
Combogesic was developed with this dose-dependent risk profile in mind. It contains 300 mg of ibuprofen per dose (maximum 900 mg/day), a dosage range specifically chosen to minimise NSAIDrelated adverse events while maintaining analgesic efficacy.
As Combogesic contains no opioid the risk of addiction is low. A new drug driving law was introduced in the UK in 2015 makes it illegal to drive if someone is unfit to do so due to legal or illegal drugs. This includes the OTC medicine codeine, which can potentially affect an individual’s ability to drive. Paracetamol and ibuprofen do not fall under the drug driving law, making Combogesic a useful alternative to recommend for pain relief without concerns about driving impairment.
Is It Time To Revisit The Mild To Moderate Pain Pathways?
Various international pain management guidelines recommend adopting a multimodal analgesic approach that combines differing mechanisms of action. This strategy enhances pain relief through synergistic effects while minimising safety risks associated with high-dose monotherapy.
Fixed-dose combination analgesics like Combogesic offer a number of advantages in terms of providing multimodal analgesia to patients. The benefits include reduced tablet burden and better adherence, a broader analgesic spectrum well-suited to multimechanistic pain conditions and more predictable pharmacokinetic and pharmacodynamic properties. These advantages may outweigh disadvantages such as reduced flexibility in dose adjustment. Combinations which use differing mechanisms of action (synergy) allow prescribers to minimise the dosing of the NSAID component thereby reducing the potential for adverse events.
Alternate dosing of paracetamol 2x500 mg and ibuprofen 2x200 mg is sometimes recommended but this can result in the patient taking as many as 14 tablets in a day. Moreover, paracetamol may be taken four times daily and ibuprofen three times daily leading to possible confusion and the patient has to take a dose of paracetamol or ibuprofen every two hours. With its combination of paracetamol and ibuprofen in one tablet, Combogesic requires fewer doses and therefore reduces the risk of over and underdosing.
Combogesic shows superior efficacy compared with other combination paracetamol/ibuprofen preparations, such as those providing paracetamol to ibuprofen ratios of 2.5:1 and less. Combogesic also shows superior efficacy compared with paracetamol or ibuprofen monotherapy with favourable safety data.
All of the combination analgesics in various pathways for mild to moderate pain contain an opioid (codeine, dihydrocodeine or tramadol). A systematic review of 24 clinical trials showed that in single dose studies, adding codeine to paracetamol produced a five per cent increase in analgesic effect as measured by the sum pain intensity difference [42]. Multiple dose studies showed an increased risk of side effects with paracetamol and codeine combined [42].
In summary, Combogesic is a patented double action formula containing paracetamol 500 mg and ibuprofen 150mg which work together in complementary ways to help relieve pain. A single dose of Combogesic is clinically proven to be 80 per cent more effective than paracetamol alone and 30 per cent more effective than ibuprofen alone [27,38]. By contrast, the addition of codeine 60mg to paracetamol has been shown to provide only modest incremental pain relief [11]. All of these factors should be taken into account to allow the inclusion of paracetamol/ibuprofen 3.3:1 (Combogesic) with various pain pathways.
Combogesic could be considered for mild to moderate pain relief in a patient where paracetamol or ibuprofen monotherapy is not adequate. Combogesic is more effective than monotherapies and allows for lower dosing of ibuprofen so reducing the risk of side effects. Combogesic should also be considered before the use of an opioid either as monotherapy (e.g. codeine) or as a combination preparation (e.g. codeine with paracetamol or aspirin). This is because of the limited efficacy of added codeine and the reduced risk of adverse effects including elimination of opioid addiction.
Use of Combogesic in the Community and Hospital
Combogesic, both the tablet and the Intravenous Infusion (IV), is also used in hospitals. Combogesic IV is being integrated into hospital pain management systems where it can be given for many types of pain - mild to moderate pain, moderate to severe pain and severe pain. The goal is to reduce opioid requirements and improve pain control at each step of the analgesic ladder, allowing for use of lower doses of opioids or avoiding them completely. Both formulations of Combogesic contain the same 3.3:1 paracetamol/ ibuprofen ratio making it easy to transfer from one formulation to another, for example when a patient is discharged from hospital and may transfer from the intravenous infusion to the tablet.
Reducing Opioid Usage
The use of Combogesic can potentially address opioid usage, so tackling the opioid epidemic. England had the highest use of opioids in the world in 2019 [14]. A retrospective cohort study using UK primary care electronic health records evaluating opioid prescribing for non-cancer pain demonstrated a rise in opioid prescribing up to 2017 [18]. In this study opioid prescribing varied between practices with older age and social deprivation being risk factors for incremental increases in long term opioid use [18]. Following opioid initiation 14.6 per cent became long-term opioid users during the first year. Of those who started on high or very high opioid doses, 10.3 and 18.7 per cent respectively remained in the same dose category at two years. Opioid use from 2020 to 2023 reduced by approximately eight per cent, [20,23]. However, the NHS Prescribing Analysis Data [23] (see Table 1) does not include OTC use of opioids, so estimates of opioid use are likely underestimates. NICE guidelines for chronic pain recommend no analgesic, including opioids, yet research has shown that almost half of patients in the community suffering from pain continue to use them [22].
Consideration should also be given to opioid prescribing post hospital discharge. A systematic search of MEDLINE, EMBASE, and Cochrane databases at the cut-off date of 1 December 2018 was conducted for studies reporting on various harmful effects of discharge opioids after inpatient care. Sixteen studies reported the overall rate of persistent opioid use after inpatient care. The proportion of all patients still consuming opioids three months postdischarge ranged from two to 82 per cent, with an adjusted mean of 22.6 per cent. At six months, the proportion ranged from four to 69 per cent, with an adjusted mean of 19.2 per cent. Discharge opioids contribute to prolonged opioid use; the proportion of opioid-naïve patients still consuming opioids three months after hospital discharge is 10.4 per cent [44].
The adverse effects associated with opioid use and the potential for addiction make it imperative to reduce their use further. Treatment with Combogesic potentially contributes to reducing the opioid usage in both the community (prescription and OTC) and in hospital.
In a randomised, double blind, parallel group, placebo-controlled trial of post-operative pain relief following the removal of at least two molars, less than a quarter (23.9 per cent) taking Combogesic required rescue medication (oxycodone). However, over half (53.2 per cent) of those taking paracetamol required rescue medication as did 43.2 per cent taking ibuprofen and 81.3 per cent taking a placebo [27]. Mean consumption of opioid rescue medication was 3.7 mg in those taking Combogesic, but 11 mg in those taking paracetamol, 7.1 mg in those taking ibuprofen and 17.9 mg among those taking a placebo.
In mild to moderate pain where a combination analgesic is the preparation of choice, Combogesic should be used preferentially to combinations containing opioids such as codeine or dihydrocodeine.
A pharmacoeconomic analysis is required to estimate how much opioid use could be saved and hence the extent to which the adverse effects of opioids could be reduced across the population. Hospital stays could potentially be shortened by using Combogesic instead of opioids wherever possible. Opioid related hospitalisations rose by 49 per cent from 2008-2018 with an estimated healthcare cost of £137 million [15). Opioid-related drug poisoning deaths increased by 388 per cent in England and Wales during the same period [15) Just under half of all drug-poisoning deaths registered in 2023 in England and Wales were confirmed to involve an opiate (46 per cent; 2,551 deaths) [16]. This compares with figures in 2023 when 46.8 per cent of drug-related deaths were due to an opioid [17]. In 2021/22 the NHS spend on opioid drugs was £307 million [45] and a 10 per cent reduction in opioid consumption could save approximately £30 million in prescription costs in addition to possibly £10-20 million on opioid related hospitalisations and costs related to adverse effects.
Recommendations
A proportion of these serious consequences could be saved through appropriate opioid stewardship, HCP training and patient education on the use of opioids. The following recommendations could contribute to appropriate use of opioids.
- Include combination paracetamol/ibuprofen in a ratio of 3.3:1 in the pathways for mild to moderate pain after paracetamol and ibuprofen used singly.
- Develop evidence-based guidelines for effective stewardship of opioids. These could be developed by one of the healthcare professional bodies such as the Royal Pharmaceutical Society (RPS) or the British Medical Association (BMA).
- Train all HCPs, including prescribers in hospitals and community, and community pharmacists, on the place of nonopioid medications in mild to moderate pain and appropriate use of opioids, including OTC fixed dose combinations of paracetamol and ibuprofen.
- Educate prescribers to be vigilant about patients who may become a long-term opioid user (e.g. patients of older age, deprivation, substance misuse, alcohol excess, CNS medications and musculoskeletal conditions).
Provide information (already under development by NHS BSA) to help GP practices:
- Understand the scale of their local opioid issues
- Understand which areas of opioid prescribing are most problematic locally
- Identify patients who are deemed to be at the greatest risk from harm to be prioritised for a structured medication review
- Measure the impact of any interventions aimed at reducing harm from opioids
- Understand the local scale of use of dependency and withdrawal forming medicines in the short and long term
- Educate patients on the proper storage and disposal of opioids and the relatively limited effectiveness of opioids in mild to moderate pain
- Ensure patients understand the risks of opioids, including misuse and long-term dependence.
Conclusion
The UK is suffering from an opioid crisis. In 2019, England had the highest use of opioids in the world. Though use has fallen by an estimated eight per cent since then, the adverse effects, addiction, drug-related deaths and hospitalisations from opioids misuse make it mandatory to reduce opioid use further. Evidence based guidelines on opioid stewardship, plus HCP training, prescriber education on patients at risk and patient education are essential to help stem the tide on this serious situation.
Inclusion of Combogesic (paracetamol/ibuprofen 3.3:1) in the preparation of choice for adults aged 18 years and over for mild to moderate pain after paracetamol and ibuprofen used singly could help to reduce the use of opioids in the community and hospitals. The benefits of a combination preparation include reduced pill burden and better adherence, a broader analgesic spectrum well-suited to multi-mechanistic pain conditions and more predictable pharmacokinetic and pharmacodynamic properties. These advantages may outweigh disadvantages such as reduced flexibility in dose adjustment with single ingredient analgesics. Clinical studies have shown Combogesic to be more effective than either paracetamol or ibuprofen alone and the dose of ibuprofen is low enough to reduce the risk of side effects.
References
- Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, et al. (2020) The revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain 161: 1976-1982.
- Henschke N, Kamper SJ, Maher CG (2015). The epidemiology and economic consequences of pain. Mayo Clin Proc 90: 139-147.
- Perspectus Global. 9/02/2025 to 11/02/2025; 2003 Respondents. Data on File.
- British Medical Association (BMA) (2017) Chronic Pain. Supporting Safer Prescribing of Analgesics.
- Brattberg G, Thorslund M, Wikman A (1989). The prevalence of pain in a general population. The results of a postal survey in a county of Sweden. Pain 37: 215-222.
- Wettstein M, Tesarz J (2023) Increasing pain prevalence and intensity among middle-aged and older adults: Evidence from the German Ageing Survey. J Psychosom Res 168: 111233.
- Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, et al. (2019) Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 160: 19-27.
- Johnson M, Collett B, Castro-Lopes JM (2013). The challenges of pain management in primary care: a Pan-European Survey. J Pain Res 6: 393-401.
- Watson J (2022) Overview of Pain. MSD Manual, Professional Version.
- British Pain Society & Faculty of Pain Medicine (2019) Outcome Measures.
- Toms L, Derry S, Moore R, McQay HJ (2009) Single Dose Oral Paracetamol (Acetaminophen) plus Codeine for Post-Operative Pain Relief in Adults.
- Chou R, Hartung D, Turner J, Blazina I, Chan B et al (2022) Opioid Treatments for Chronic Pain. Comparative Effectiveness Review No. 229. (Prepared by the Pacific Northwest Evidence-based Practice Center under Contract No. 290-2015-00009-I.) AHRQ Publication No. 20-EHC011. Rockville, MD: Agency for Healthcare Research and Quality.
- Jayawardana S, Forman R, Johnston-Webber C, Campbell A, Berterame S, et al. (2021) Global consumption of prescription opioid analgesics between 2009-2019: a country-level observational study. EClinicalMedicine 42: 101198.
- The Lancet Public Health (2022) Opioid Overdose Crisis: Time for a Radical Rethink. Lancet Public Health 7: e195.
- Roberts AO, Richards GC (2023) Is England facing an opioid epidemic? Br J Pain 17: 320-324.
- Office for National Statistics (2023) Deaths Related to Drug Poisoning in England and Wales: 2023 Registrations.
- Office for Health Improvement and Disparities (2024) Adult Substance Misuse Treatment Statistics 2023-2024 report.
- Office for National Statistics (2021) Deaths Related to Drug Poisoning in England and Wales: 2021 Registrations.
- Jani M, Birlie Yimer B, Sheppard T, Lunt M, et al. (2020) Time trends and prescribing patterns of opioid drugs in UK primary care patients with non-cancer pain: A retrospective cohort study. PLoS Med 17: e1003270.
- Deslauriers S, Roy JS, Bernatsky S, Blanchard N, Feldman DE, et al. (2021) The burden of waiting to access pain clinic services: perceptions and experiences of patients with rheumatic conditions. BMC Health Serv Res 21: 160.
- IWOTCH (2022) The University of Warwick Clinical Trials Unit.
- National Institute for Health and Care Excellence (NICE) Chronic Pain (primary and secondary) in over 16s: Assessment of all Chronic Pain and Management of Chronic Primary Pain. NICE Guideline [NG193] Published: 07 April 2021.
- Zambelli Z, Halstead EJ, Iles R, Fidalgo AR, Dimitriou D (2022). The 2021 NICE Guidelines for Assessment and Management of Chronic Pain: A Cross-Sectional Study Mapping Against a Sample of 1,000* in the Community. Br J Pain 16: 439-449.
- NHSBSA (2023) Prescription Cost Analysis (PCA) Data.
- Anekar A, Hendrix J, Cascello M (2023) WHO Analgesic Ladder.
- Miranda HF, Puig MM, Prieto JC, Pinardi G (2006). Synergism Between Paracetamol and Non-steroidal Anti-inflammatory Drugs in Experimental Acute Pain. Pain 121: 22-28.
- Merry AF, Gibbs RD, Edwards J, Ting GS, Frampton C, et al. (2010) Combined Acetaminophen and Ibuprofen for Pain Relief after Oral Surgery in Adults: a Randomized Controlled Trial. Br J Anaesth 104: 80-88.
- Daniels SE, Atkinson HC, Stanescu I, Frampton C (2018). Analgesic Efficacy of an Acetaminophen/Ibuprofen Fixed-dose Combination in Moderate to Severe Postoperative Dental Pain: A Randomized, Double-blind, Parallel-group, Placebo-controlled Trial. Clin Ther 40: 1765-1776.
- Mehlisch DR, Aspley S, Daniels SE, Bandy DP (2010a) Comparison of the analgesic efficacy of concurrent ibuprofen and paracetamol with ibuprofen or paracetamol alone in the management of moderate to severe acute postoperative dental pain in adolescents and adults: a randomized, double-blind, placebo-controlled, parallel-group, singledose, two-center, modified factorial study. Clin Ther 32: 882-895.
- Mehlisch DR, Aspley S, Daniels SE, Southerden KA, Christensen KS (2010b) A single-tablet fixed-dose combination of racemic ibuprofen/ paracetamol in the management of moderate to severe postoperative dental pain in adult and adolescent patients: a multicenter, two-stage, randomized, double-blind, parallel-group, placebo-controlled, factorial study. Clin Ther 32: 1033-1049.
- Doherty M, Hawkey C, Goulder M, Gibb I, Hill N, et al. (2011) A randomised controlled trial of ibuprofen, paracetamol or a combination tablet of ibuprofen/paracetamol in community-derived people with knee pain. Ann Rheum Dis 70: 1534-1541.
- Hung KKC, Graham CA, Lo RSL, Leung YK, Leung LY, et al. (2018) Oral paracetamol and/or ibuprofen for treating pain after soft tissue injuries: Single centre double-blind, randomised controlled clinical trial. PLoS One 13: e0192043.
- Merry AF, Edwards KE, Ahmad Z, Barber C, Mahadevan M, et al. (2013) Randomized comparison between the combination of acetaminophen and ibuprofen and each constituent alone for analgesia following tonsillectomy in children. Can J Anaesth 60: 1180-1189.
- Menhinick KA, Gutmann JL, Regan JD, Taylor SE, Buschang PH (2004) The efficacy of pain control following nonsurgical root canal treatment using ibuprofen or a combination of ibuprofen and acetaminophen in a randomized, double-blind, placebo-controlled study. Int Endod J 37: 531-541.
- Wells LK, Drum M, Nusstein J, Reader A, Beck M (2011). Efficacy of Ibuprofen and ibuprofen/acetaminophen on postoperative pain in symptomatic patients with a pulpal diagnosis of necrosis. J Endod 37: 1608-1612.
- Dahl V, Dybvik T, Steen T, Aune AK, Rosenlund EK (2004) Ibuprofen vs. acetaminophen vs. ibuprofen and acetaminophen after arthroscopically assisted anterior cruciate ligament reconstruction. Eur J Anaesthesiol 21: 471-475.
- Bondarsky EE, Domingo AT, Matuza NM, Taylor MB, Thode HC Jr, et al. (2013). Ibuprofen vs acetaminophen vs their combination in the relief of musculoskeletal pain in the ED: a randomized, controlled trial. Am J Emerg Med 31: 1357-1360.
- Electronics Medicine Compendium (EMC) Combogesic 500 mg/150 mg film-coated tablets SmPC.
- Aitken P, Stanescu I, Playne R, Zhang J, Frampton C, et al. (2019) An Integrated Safety Analysis of Combined Acetaminophen and ibuprofen (MAXIGESIC®/Combogesic®) in Adults. J Pain Res 12: 621-634.
- Anderson BJ (2008) Paracetamol (Acetaminophen): mechanisms of action. Paediatr Anaesth 18: 915-921.
- Castellsague J, Riera-Guardia N, Calingaert B, Varas-Lorenzo C, Fourrier-Reglat A, et al. (2012) Individual NSAIDs and upper gastrointestinal complications: a systematic review and meta-analysis of observational studies (the SOS project). Drug Saf 35: 1127-1146.
- Henry D, McGettigan P (2003) Epidemiology overview of gastrointestinal and renal toxicity of NSAIDs. Int J Clin Pract Suppl 135: 43-49.
- de Craen AJ, Di Giulio G, Lampe-Schoenmaeckers JE, Kessels AG, Kleijnen J (1996) Analgesic efficacy and safety of paracetamolcodeine combinations versus paracetamol alone: a systematic review. BMJ 313: 321-325.
- Arwi GA, Schug SA (2022) Potential for Harm Associated with Discharge Opioids After Hospital Stay: A Systematic Review. Drugs 80: 573-585.
- NHSBSA (2022) Dependency Forming Medicines – England 2021/22.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.