Outcomes of a Six-Month Real-World Pilot of a Thermal Neuromodulation Wearable for Chronic Pain in an Employer Setting
by Hapgood J1, Chabal C2*, Cipolli W3
1President, Soovu Labs Inc., USA
2Chief Medical Officer, Soovu Labs Inc, The Dementia Project Inc., USA
3Associate Professor of Mathematics, Colgate University, USA
*Corresponding author: Chabal C, Chief Medical Officer, Soovu Labs Inc, The Dementia Project Inc., USA
Received Date: 25 October 2025
Accepted Date: 04 November 2025
Published Date: 07 November 2025
Citation: Hapgood J, Chabal C, Cipolli W (2025) Outcomes of a Six-Month Real-World Pilot of a Thermal Neuromodulation Wearable for Chronic Pain in an Employer Setting. Chron Pain Manag 9: 175. https://doi.org/10.29011/2576-957X.100075
Abstract
Background: The Soovu™ Pain Relief System, a wearable thermal neuromodulation device delivering pulsed heat (42-45°C), was evaluated in a six-month real-world pilot among employees and dependents of a Pacific Northwest health plan for chronic musculoskeletal pain. Methods: This observational study enrolled 199 adults (pain ≥4/10 NPRS for ≥3 months; exclusions: neuropathy, adhesive allergy, pregnancy). Of 173 analyzed (76.7% women; mean age 43.5 years; primary sites: back 39.6%, neck 15.1%), outcomes included pain intensity, interference on quality of life (QoL), anxiety/tension/nervousness, productivity, and healthcare/medication utilization. Data was collected at baseline, after 6 and 26 weeks and was analyzed via mixed-effects and mediation models. Results: Pain intensity decreased significantly (baseline 6.01 to 26 weeks 4.70; p<0.001), with 39.3% achieving ≥30% reduction (42.1% in severe pain subgroup). Mediation showed pain reductions drove QoL improvements (e.g., enjoyment of life -0.61, sleep -0.51; all p<0.001), plus direct effects on anxiety (-11.01) and tension (-17.70). 32.4% of participants reduced/ eliminated services (e.g., PT, chiropractic, injections, operations); and 46.8% reduced or stopped medications. Engagement peaked early (1000 sessions/week 2), with 90.7% reporting pain management benefit. One minor adverse event. Conclusions: Soovu reduced pain, enhanced QoL/function, and lowered utilization, especially in severe pain cases (OR 1.31 per baseline point). Findings support targeted deployment in employer benefits, emphasizing patient-centered outcomes beyond intensity.
Keywords: Chronic pain; Pain modulation; Thermal; Long-term; Quality of life
Introduction
Soovu Labs has published a series of papers describing the Soovu™ Pain Relief System (“Soovu”), a novel device that uses high temperature pulses of heat to reduce pain in both chronic and acute conditions [1-3]. Soovu is a Bluetooth-enabled wearable device that delivers pulsing heat to the area of pain. The system includes a companion mobile app that initiates the 10-30-minute heat treatment and allows for customization of the treatment session.
Based on the clinical response to the device, fast onset and duration of treatment effects, it was hypothesized that the mechanism of action is likely activation followed by de-functionalization of the TRPV-1 receptor on peripheral nerves [1,4,5]. Similar effects are demonstrated with heat in laboratory studies and analogous to the clinical response associated with capsaicin treatment [4,6,7].
Having demonstrated the device’s ability to reduce pain in clinical settings [3], the company was contracted by a large Pacific NW health insurance company to provide the Soovu system to employees and dependents of the health plan in a six-month realworld pilot.
The primary outcome measures included pain interference on quality of life, impact of pain on users’ ability to function, the utilization of healthcare resources such as medications and interventions, and users’ pain ratings. In addition, the observational design allowed for statistical prediction of the characteristics of those most likely to benefit from this therapy. These predictive findings support better individualization of therapy and may help program sponsors target the therapy to those most likely to respond to it.
Materials and Methods
Study Design
The current study was done in a real-world home and work environment involving mostly office workers. The study used a long-term, six-month, observational design. The device was offered to employees and their dependents as an employee fringe benefit program for those who met the clinical criteria and were free from medical conditions that contraindicated its use. Per the health plan’s requirements, up to 220 eligible individuals were to be granted access to the device without randomization. This limitation is discussed in later sections. All employees using the device agreed to regular assessments as part of the employer’s evaluation of the effectiveness of the device.
Participants who passed the screening and opted into the pilot were treated remotely via the wearable medical device and asynchronous coaching program between July 8, 2024, and January 7, 2025.
Population
Eligible participants were adult employees or spouses/partners/ other adult dependents of employees of the health plan who wished to use the device for musculoskeletal pain relief and selfreported having chronic pain for three or more months of a level 4 or greater on the numeric pain rating scale. Exclusion criteria included having any of the following conditions which are labeled contraindications for using the device: (1) neuropathy or nerve damage; (2) allergy to medical adhesives; (3) pregnant or possibly pregnant.
All participants reviewed and consented to Soovu Labs’ Terms & Conditions and the company’s Privacy Policy.
Of the 199 enrolled pilot participants, 173 participants had a baseline self-reported pain level of 4 or higher and used the device more than three times over the timeline of the study. These participants, who were included in the data analyses, were primarily women (76.7%), with 42.8% between the ages of 40 and 54 years old. Participants identified back (39.6%), neck (15.1%), shoulder/arm (13.2%), and other (32.1%; e.g., hip, legs, joints, etc.) as primary pain areas they would treat with the Soovu device. Of note, most participants (76.1%) reported suffering from pain in more than one area (Table 1).
|
Gender |
|||
|
Man |
20.8% |
||
|
Woman |
76.7% |
||
|
Non-binary |
2.5% |
||
|
Age |
|||
|
18-24 years |
0.6% |
||
|
25-39 years |
32.7% |
||
|
40-54 years |
42.8% |
||
|
55 and older |
22% |
||
|
Prefer not to say |
1.9% |
||
|
Pain Location |
|||
|
Back |
39.6% |
||
|
Neck |
15.1% |
||
|
Shoulder |
13.2% |
||
|
Other |
32.1% |
||
Table 1: Participant demographics for the sample of n=159 participants who screened into the data analyses and provided demographic information. Note demographic information was missing for 14 participants.
Program Design
The Soovu™ Pain Relief System is an FDA-registered Class II medical device indicated to provide topical heating for the purpose of elevating tissue temperature for the temporary relief of minor muscle and joint pain and stiffness; the temporary relief of joint pain associated with arthritis; the temporary relief of muscle spasms, minor sprains and strains, and minor muscular back pain; the temporary relief of menstrual discomfort; the relaxation of muscles; and the temporary increase of local circulation where applied.
The Soovu therapy consists of repetitive cycles of thermal energy applied to the area of pain where the heat cycles between 108°F (42°C) and up to 113°F (45°C) over a period of up to 30 minutes per therapy session. The wearer of the device determines the duration of the therapy (10, 20, or 30 minutes) and the maximum temperature for each session. The Soovu device is held on the body using a medical grade adhesive. The device can be worn on any flat surface of the body including back, neck, back of shoulders, abdomen, quadriceps, hamstrings, hips, glutes, calves, forearms, biceps, and triceps.
Throughout the six-month pilot, participants used the Soovu System at home and work as needed to manage pain. The device is controlled by the Soovu Mobile App which participants installed on their personal phones. Participants received guidance via the mobile app on how to place the device on the body and adjust the therapy settings. Participants received a printed Quick Start Guide and an electronic copy of the full owner’s manual.
The intervention also included asynchronous educational and Cognitive Behavioral Therapy (CBT) emails and text messages from customer care of approximately three messages per month. Participants were encouraged to ask questions and engage in messaging with the customer care team as needed.
Outcome Measures
Data was collected at baseline, 6 weeks (about 1 and a half months), 12 weeks (about 3 months), 18 weeks (about 4 months), and 26 weeks (about 6 months).
Pain intensity
Pain intensity was measured by the 11-point Numerical Pain Rating Scale (NPRS) for the participant’s primary reported pain area [8-10]. The following question was asked: “For your primary pain area (the location that typically bothers you the most), how do you rate your pain level over the last 7 days? (0=no pain at all; 5=moderate pain; 10=worst pain imaginable).
Responders to the Soovu treatment were those who decreased their pain rating by 2 points or 30%. The IMMPACT guidelines for clinical trials on pain treatment state that pain reduction of 2 points or 30% were associated with patient ratings of “much improved [11,12].”
Pain Interference on Quality of Life
Pain interference was measured with this question: “In the past 7 days, how much did pain interfere with each of the following?” The question was asked for: Enjoyment of life; Ability to concentrate: Day-to-day activities; Enjoyment of recreational activities; Outside of home tasks (e.g. getting groceries, running errands, etc.); Socializing with others; Sleep [13].
Anxiety
Anxiety was assessed with this question: “Over the past 7 days, how do you rate your level of anxiety? Select a number between 0 and 100 where 0 is no anxiety at all and 100 is anxiety at the highest level.”
Nervousness
Nervousness was assessed with this question: “Over the past 7 days, how do you rate how nervous you’ve been? Select a number between 0 and 100 where 0 is not nervous at all and 100 is nervous
at the highest level.”
Tension
Tension was assessed with this question: “Over the past 7 days, how do you rate how tense you’ve been? Select a number between 0 and 100 where 0 is not tense at all and 100 is tense at the highest level.”
Productivity
Productivity was assessed with this question: “During the past 7 days, how much did your pain affect your productivity while you were working? Think about days you were limited in the amount or kind of work you could do, days you accomplished less than you would like, or days you could not do your work as carefully as usual. If pain affected your work only a little, choose a low number. Choose a high number if pain affected your work a great deal.”
Healthcare utilization
Changes in healthcare utilization for pain management was assessed with this question: “Have you eliminated or reduced your use of therapeutic services such as chiropractor, physical therapist or massage since using Soovu?”
Medication utilization
Changes in medication utilization for pain management was assessed with this question: “Have you eliminated or reduced your use of pain medications (either prescription or over the counter) since using Soovu?
Engagement
Engagement with the Soovu System was assessed with the following measures: 1) number of weeks wherein participant completed 1 or more Soovu therapy sessions; 2) total number of Soovu therapy sessions completed.
Safety and Adverse Events
Patients were instructed to report any adverse events when they occurred to Soovu Customer Care and to their manager at the sponsoring health plan.
There was one adverse event reported to Soovu Customer Care during the six-month period in which an individual failed to operate the Soovu device according to the use instructions resulting in minor skin damage. The individual acknowledged ignoring several layers of safety warnings when using the device.
Statistical Analysis
For simplicity, we report statistical models fitted using mixedeffects models with the lmer function from the lmerTest package for R. This approach enables us to assess the treatment effect while accounting for the repeated measures on participants. We used the emmeans package for R to compare outcomes across baseline, 12 weeks, and 26 weeks. We note here that several outcomes are on the ordinal scale: Self-Reported Pain Intensity (0-10), Pain Interference on Quality of Life (0-10), Anxiety (0-100), Nervous Feelings (0-100), Tension (0-100). Parallel analyses using cumulative link mixed models, fitted with the clmm function from the ordinal package in R, yielded consistent significance results and interpretation with those reported below.
To formally evaluate whether treatment reduced outcome measures through reduction in Self-Reported Pain Intensity, we fit mediation models using the mediate function from the mediation package in R. These models enable us to untangle a total treatment effect into an average causal indirect effect, which quantifies the effect of the treatment on each outcome measure through reductions in Self-Reported Pain intensity, and an average causal direct effect, representing the effect of the treatment on each outcome measure.
Results
Completion Rates
We obtained responses from 199 participants at baseline. Six participants who reported a pain level of 4 or greater at prescreening but then reported a pain level of 3 or lower at the baseline survey were excluded from analysis. Further, twenty participants who used the device fewer than 3 times over the timeline of the study were also excluded from analysis. Of the 173 participants included, we obtained 159 (91.9%) at the six-week follow-up, 147 (85%) at the 12-week follow-up, 151 (87.3%) at the 18-week follow-up, and 150 (86.7%) at the 26-week follow-up.
Pain Intensity
Self-Reported Pain Intensity was significantly lower at 12 Weeks, and lowest by 26 Weeks compared to baseline (Tables 2-3, Figure 1). Patients experienced an initial statistically significant decrease in self-reported pain intensity at 12 weeks (t=5.99, p<0.001) and a smaller subsequent statistically significant decrease at 26 weeks (t=2.77, p=0.0163). Of note, 39.3% of the 150 participants providing self-reported pain intensity at baseline and 26 weeks showed a clinically meaningful reduction in pain intensity, defined as ≥30% reduction in pain intensity. By baseline pain intensity, 37.6% of 93 patients with moderate pain intensity (4-6), and 42.1% of 57 participants with severe pain intensity (7-10) experienced a clinically meaningful reduction in pain intensity (Figure 2).
|
Fixed Effect |
Estimate |
SE |
t (df) |
p-value |
|
Intercept (Baseline) |
6.012 |
0.122 |
49.11 (387.3) |
< 0.001 |
|
12 Weeks |
-0.889 |
0.148 |
-5.99 (317.1) |
< 0.001 |
|
26 Weeks |
-1.314 |
0.147 |
-8.93 (315.5) |
< 0.001 |
Table 2: Linear mixed effects model for self-reported pain.
|
Contrast |
Estimate |
SE |
t (df) |
p-value |
Lower |
Upper |
|
Baseline – 12 Weeks |
0.889 |
0.148 |
5.99 (311) |
<0.001 |
0.540 |
1.238 |
|
Baseline – 26 Weeks |
1.314 |
0.147 |
8.93 (310) |
<0.001 |
0.967 |
1.660 |
|
12 Weeks – 26 Weeks |
0.425 |
0.153 |
2.77 (307) |
0.0163 |
0.637 |
0.785 |
Table 3: Contrasts of estimated marginal means comparing Self-Reported Pain Intensity at baseline, 12 weeks and 26 weeks.
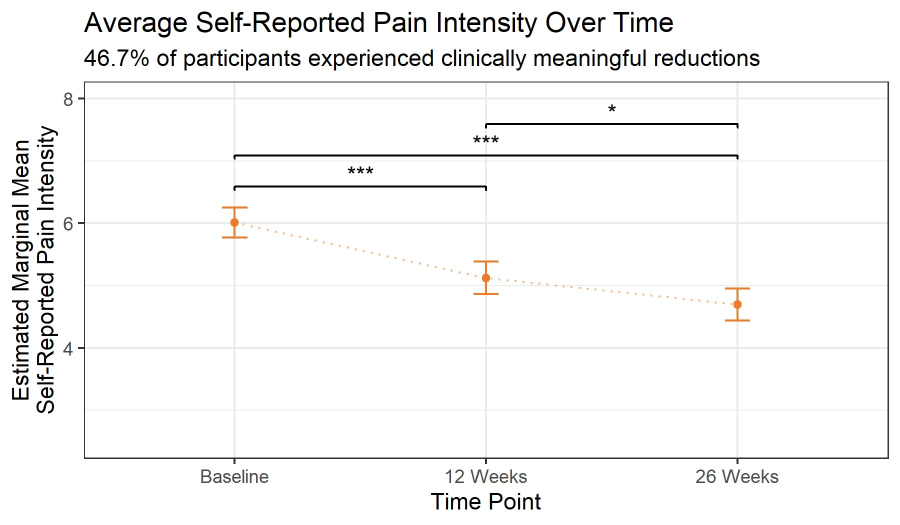
Figure 1: Estimated marginal mean self-reported pain intensity at baseline, 12 weeks, and 26 weeks.
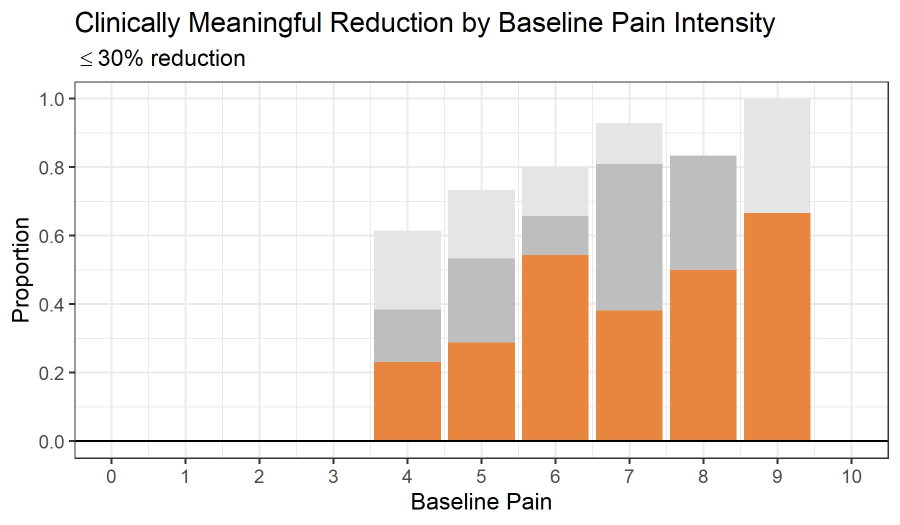
Figure 2: The proportion of participants who experienced a clinically meaningful reduction in pain intensity (a ≥30% reduction; orange), reduced (grey), same or reduced (light grey). Estimated marginal mean self-reported pain intensity at baseline, 12 weeks, and 26 weeks.
Pain Interference with Activities and Quality of Life
The causal mediation model results in Table 4 demonstrate that treatment had statistically significant direct, mediated, and total effects decreasing pain interference with Enjoyment of Life, Day to Day Activities, Recreational Activities, Outside of Home Tasks, Sleep, as well as Anxiety and Tension by 26 weeks. Additionally, treatment had a statistically significant total and mediated effects decreasing pain interference with the Ability to Concentrate, Socializing with Others, and Productivity. Finally, we observed statistically significant total and direct effects decreasing Nervous Feelings.
|
Outcome |
Mediated Effect |
Direct Effect |
Total Effect |
|
Enjoyment of Life |
-0.350 (p < 0.001) |
-0.263 (p = 0.002) |
-0.613 (p < 0.001) |
|
Ability to Concentrate |
-0.333 (p < 0.001) |
-0.140 (p = 0.162) |
-0.473 (p < 0.001) |
|
Day to Day Activities |
-0.369 (p < 0.001) |
-0.334 (p < 0.001) |
-0.703 (p < 0.001) |
|
Recreational Activities |
-0.366 (p < 0.001) |
-0.432 (p < 0.001) |
-0.799 (p < 0.001) |
|
Outside of Home Tasks |
-0.366 (p < 0.001) |
-0.321 (p = 0.002) |
-0.687 (p < 0.001) |
|
Socializing with Others |
-0.297 (p < 0.001) |
-0.115 (p = 0.256) |
-0.413 (p < 0.001) |
|
Sleep |
-0.301 (p < 0.001) |
-0.208 (p = 0.038) |
-0.510 (p < 0.001) |
|
Anxiety* |
-3.428 (p = 0.004) |
-7.579 (p = 0.004) |
-11.01 (p < 0.001) |
|
Nervous Feelings* |
-0.905 (p = 0.448) |
-8.781 (p = 0.004) |
-9.686 (p < 0.001) |
|
Tension* |
-5.101 (p < 0.001) |
-12.59 (p < 0.001) |
-17.70 (p < 0.001) |
|
Productivity |
-0.683 (p < 0.001) |
-0.374 (p = 0.202) |
-0.956 (p < 0.001) |
Table 4: Causal mediation model results assessing the total treatment effect, direct treatment effect, and mediated effect through reduction in Self-Reported Pain Intensity on Pain Interference on Quality of Life, Anxiety, Nervousness, Tension, and Productivity from baseline to 26 weeks. Note an asterisk indicates the scale is 0-100 instead of 0-10.
Medication and Healthcare Usage
At the end of the pilot, 32.4% of participants reported reducing or eliminating services that help manage pain. Services that were reduced or eliminated included: massage; physical therapy; chiropractic work; acupuncture; and injections. Three participants reported cancelling scheduled medical procedures because of the relief they received from the Soovu device. The procedures canceled were: 1 hysterectomy; 1 back surgery; and 1 round of injections.
Additionally, 46.8% of subjects who had been using medication to manage pain either reduced or stopped using medication to manage pain. Among the medication users, 80% of the subjects were using over-the-counter medication while 5% used prescription medication and 15% were using a combination of over-the-counter medication and prescription medication to manage pain.
Engagement with Soovu System
Study participants were instructed to use the Soovu System on an as-needed basis whenever pain arose or was anticipated. In general, the Soovu System was used more in the first months of the pilot (nearly 1000 times in week 2) and declined over time (just under 100 times at week 26) (Figure 3). Approximately 12.7% of participants remained consistently engaged (at least one session per week) at 6 months (Figure 3). All participants were asked if they felt Soovu helped manage their pain; 90.7% responded in the affirmative. While some fall off in device usage is expected with all medical wearables, it can be hypothesized that Soovu helps users reduce pain, and in some cases, resolve pain to a low enough level that ongoing use of the device is not necessary.
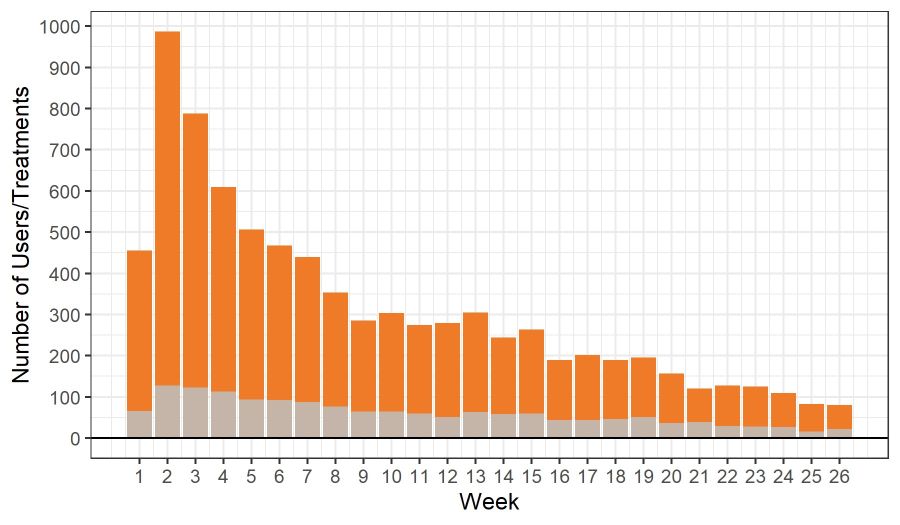
Figure 3: The Number of Soovu users (grey) and treatments (orange) conducted per week through week 26.
Limitations
The study was done as an observational assessment of a medical device in an office / home environment over a six-month period. As the study was designed to mimic the roll-out of a health benefit to an employee population, employees self-screened via the study website rather than being screened and referred by a medical professional. Though participants were required to have a baseline pain level of 4 or greater on the NRS, three participants who qualified on the screening later reported baseline pain levels of less than four. They were subsequently removed from the analysis. Even so, it is hypothesized that a portion of participants in the study enrolled just to gain access to the medical device being offered by their employer at no cost and did not necessarily require the use of the device to treat their chronic pain and impacts of pain on their quality of life.
Further, all outcomes were collected via self-report without objective verification of pain levels. Pain, at its core, is highly subjective and variable between individuals and over time. Nonetheless, the numeric pain rating scale is a widely used standard to assess pain relief interventions, technologies, and medications.
This was an observational study; as such, there was no control group, no randomization and no blinding. The authors have previously published shorter term studies that were designed as randomized controlled trials.
Discussion
This study aimed to evaluate patient-centered outcomes such as quality of life (QoL), physical function, stress, medical resource utilization, and self-reported pain levels among individuals using a novel medical wearable device for pain relief. The overarching goal was to enhance well-being and reduce pain-related productivity loss and usage of pain-related services and medication.
Clinically meaningful improvement was observed in 39.3% of participants [14,15]. For those with severe chronic pain at baseline, clinically meaningful improvement was observed in 42.1% of participants at 26 weeks. Importantly, significant reductions were also reported in pain interference across domains critical to QoL, including daily activities outside the home, social and recreational engagement, sleep, and cognitive function.
Additionally, participants demonstrated improvements in emotional well-being, including reductions in anxiety, tension, and enhanced cognitive clarity. These benefits were sustained over the six-month period, suggesting durable relief and a lower risk of functional regression.
This study reinforces a pivotal principle in pain management: while pain relief is important, it does not always correlate directly with improved function or QoL. This paradox became particularly evident during the opioid crisis, in which pain scores sometimes improved while cognitive and functional outcomes declined [16]. For instance, opioids may enhance function briefly in the perioperative period, but long-term use often impairs sleep, mood, and cognitive function. This disconnect between pain intensity and overall outcomes has been recognized by many pain researchers [11-18]. Notably, multidisciplinary pain programs have been shown to consistently improve function even in the absence of significant reductions in pain intensity, highlighting the dissociation between symptom relief and meaningful life outcomes [19-21].
Mixed modeling in this study facilitated analysis of repeated measures over time within a heterogeneous population. Statistically significant improvements were noted in enjoyment of life, concentration, day to day activities, recreational activities, outside of home tasks, socializing with others, sleep, anxiety, nervous feelings, tension and productivity (all p<0.001). There was also decreased reliance on healthcare resources including chiropractic care, physical therapy, massage, acupuncture, and cancellation of planned interventions. However, the lack of a control group and the self-reported nature of findings necessitates cautious interpretation of these results.
Overall, these findings underscore that even modest reductions in pain can produce meaningful functional and QoL improvements. This is consistent with the evolving framework advocated by expert panels and federal initiatives such as IMMPACT and NIH HEAL, which emphasize patient-centered outcomes over simple numerical pain scores [22,23].
Numeric rating tools such as the Visual Analog Scale (VAS) and Numeric Rating Scale (NRS) remain prevalent due to their ease of use and historical acceptance by regulatory agencies. These metrics offer clear advantages in acute pain trials where pharmacokinetics play a pivotal role. However, their application to chronic pain— characterized by long-term, multifactorial symptoms—is limited. Chronic pain management benefits from outcome measures that reflect function, satisfaction, and quality of life rather than isolated pain intensity.
Measuring function and QoL are more complex, requiring consideration of psychosocial variables, comorbidities, and timedependent changes [24,25]. These assessments demand larger sample sizes, longer follow-up, and increased study costs. As a result, although patient-centered outcomes may better reflect realworld efficacy, they are not yet universally adopted as clinical trial endpoints.
In this study, patients with pain levels >7 showed the most pronounced improvements in both pain and broader outcomes such as healthcare utilization. While a placebo effect cannot be ruled out, sustained benefits over six months reduce that likelihood. Emerging research supports additional mechanisms. For instance, a recent study involving the same device showed sustained anxiety reduction during use [26]. Moreover, anecdotal reports suggest users find the device beneficial for stress relief even after pain subsides.
Patient distress is often amplified by a lack of control over pain, including unpredictability and ineffective treatments [27]. This concept was illustrated in the development of PatientControlled Analgesia (PCA), where rapid, on-demand relief provided psychological reassurance and improved outcomes, even if absolute pain scores were not minimized [28]. Similarly, the device’s quick onset and user-directed application may have enhanced perceived control, contributing to observed QoL and functional benefits. This mechanism could explain why some participants experienced marked QoL improvement despite only modest changes in reported pain.
Predictors of Treatment Success
A secondary analysis was conducted to identify predictors of treatment success—defined as a clinically meaningful reduction in pain (>30% reduction). Specifically, for every 1-point increase in baseline pain, the odds of achieving treatment success empirically increased by approximately 1.31 (OR = 1.31; 95% CI: 0.99–1.7; p = 0.062), which did not reach traditional levels of statistical significance.
These results contrast with the conventional expectation that higher pain severity, often associated with complex comorbidities, would predict poorer outcomes. However, as reported by others in an analysis of physical therapy for chronic pelvic pain, our data similarly show a positive association between higher baseline pain and better outcomes [29].
A review publication reported that mean baseline pain explains two thirds of the variation in absolute Minimum Significant Clinical Important Difference across chronic pain trials, with a 10 mm increment in baseline pain (on a 100 mm VAS) correlating with roughly a 10 mm increase in clinically meaningful change [14].This aligns well with our observations where individuals with baseline pain of 8–10/10 experienced mean pain reductions of 2.87 points vs. 1.16 points in those with baseline pain ≤ 7/10 (mean difference = 1.71, 95% CI 0.04 – 3.38; p = 0.045).
As anticipated, the predictive ability of baseline pain intensity is expected to improve with larger sample sizes.
This long-term observational study demonstrates that the device induces significant pain reduction, particularly in participants with severe chronic pain. Not only was each point higher at baseline linked to ~1.31× increased odds of success, but even modest reductions in pain correlated with clinically meaningful improvements in quality of life and function. These results are supported by evidence from other chronic pain treatments [29,30].
Conclusions
This six-month real-world pilot demonstrates the Soovu™ Pain Relief System’s efficacy as a non-pharmacological tool for chronic musculoskeletal pain in employed adults. Participants showed significant pain reductions (39.3% achieving ≥30% improvement, rising to 42.1% for severe baseline pain), alongside enhanced quality of life—reduced interference in daily activities, sleep, social/recreational engagement, and productivity, plus lower anxiety and tension. Notably, 46.8% cut pain medications and 32.4% reduced complementary therapies, easing healthcare burdens.
Modest pain relief yielded substantial functional gains, aligning with patient-centered pain management paradigms. Discreet, ondemand use empowered autonomy in work/home settings. Despite observational limitations, sustained six-month benefits and 90.7% user endorsement support its role as an employee benefit.
The device could be used discreetly in the workplace and required a low time burden for users. Our findings advocate for prioritizing patients with severe baseline pain for device-based therapy. Incorporating thermal neuromodulation such as delivered by the study device into tailored treatment protocols could enhance efficacy, especially among populations traditionally resistant to standard interventions.
References
- Chabal C (2021) Fundaments of Thermal Analgesia in Humans: Exploring New Methods of Pain Relief. Anesth Pain Res 5: 1-8.
- Chabal C, Dunbar PJ, Painter I, Young D, Chabal DC (2020) Properties of Thermal Analgesia in a Human Chronic Low Back Pain Model. J Pain Res 13: 2083-2092.
- Hapgood JE, Chabal C, Dunbar PJ (2021) The Effectiveness of Thermal Neuromodulation Using Precise Heat in the Treatment of Chronic Low Back Pain Over 60 Days: An In-Home User Trial. J Pain Res 14: 2793-2806.
- Sánchez-Moreno A, Guevara-Hernández E, Contreras-Cervera R, Rangel-Yescas G, Ladrón-de-Guevara E, et al. (2018) Irreversible temperature gating in trpv1 sheds light on channel activation. Elife 7: e36372.
- Lima PMA, Reis TO, Wanner SP, Chianca-Jr DA, de Menezes RC (2022) The role of peripheral transient receptor potential vanilloid 1 channels in stress-induced hyperthermia in rats subjected to an anxiogenic environment. J Therm Biol 106: 103191.
- Liu B, Qin F (2016) Use Dependence of Heat Sensitivity of Vanilloid Receptor TRPV2. Biophys J 110: 1523-1537.
- Anand P, Bley K (2011) Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new highconcentration capsaicin 8% patch. Br J Anaesth 107: 490-502.
- Chiarotto A, Maxwell LJ, Ostelo RW, Boers M, Tugwell P, et al. (2019) Measurement Properties of Visual Analogue Scale, Numeric Rating Scale, and Pain Severity Subscale of the Brief Pain Inventory in Patients With Low Back Pain: A Systematic Review. J Pain 20: 245263.
- Hartrick CT, Kovan JP, Shapiro S (2003) The numeric rating scale for clinical pain measurement: a ratio measure? Pain Pract 3: 310-316.
- Childs JD, Piva SR, Fritz JM (2005) Responsiveness of the numeric pain rating scale in patients with low back pain. Spine 30: 1331-1334.
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, et al. (2005) Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 113: 9-19.
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, et al. (2008) Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 9: 105121.
- Cleeland CS, Ryan KM (1994) Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap 23: 129-138.
- Olsen MF, Bjerre E, Hansen MD, Tendal B, Hilden J, et al. (2018) Minimum clinically important differences in chronic pain vary considerably by baseline pain and methodological factors: systematic review of empirical studies. J Clin Epidemiol 101: 87-106.e2.
- Ostelo RWJG, de Vet HCW (2005) Clinically important outcomes in low back pain. Best Pract Res Clin Rheumatol 19: 593-607.
- Chou R, Deyo R, Devine B, Hansen R, Sullivan S, et al. (2014) The Effectiveness and Risks of Long-Term Opioid Treatment of Chronic Pain. Evid ReportTechnology Assess 1-219.
- Deyo RA, Dworkin SF, Amtmann D, Andersson G, Borenstein D, et al. (2015) Report of the NIH Task Force on research standards for chronic low back pain. Phys Ther 95: e1-e18.
- Taylor AM, Phillips K, Patel KV, Turk DC, Dworkin RH, et al. (2016) Assessment of physical function and participation in chronic pain clinical trials: IMMPACT/OMERACT recommendations. Pain 157: 1836-1850.
- Turk DC, Wilson HD, Cahana A (2011) Treatment of chronic noncancer pain. Lancet 377: 2226-2235.
- Kamper SJ, Apeldoorn AT, Chiarotto A, Smeets RJEM, Ostelo RWJG, et al. (2014) Multidisciplinary biopsychosocial rehabilitation for chronic low back pain. Cochrane Database Syst Rev 2014: CD000963.
- Guzmán J, Esmail R, Karjalainen K, Malmivaara A, Irvin E, et al. (2001) Multidisciplinary rehabilitation for chronic low back pain: systematic review. BMJ 322: 1511-1516.
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services (2022) Accelerating the Use of Findings from Patient-Centered Outcomes Research in Clinical Practice to Improve Health and Health Care: Proceedings of a Workshop Series. (Alper J, Applegate A, Carrera L, Bell C, eds.). National Academies Press (US).
- Patient-Centered Outcomes (2025) Rethinking Clinical Trials.
- Lin XJ, Lin IM, Fan SY (2013) Methodological issues in measuring health-related quality of life. Tzu Chi Med J 25: 8-12.
- Thompson GN, Chochinov HM (2006) Methodological Challenges in Measuring Quality Care at the End of Life in the Long-Term Care Environment. J Pain Symptom Manage 32: 378-391.
- Natarelli N, Subramanyam C, Gahoonia N, Burney W, Sivamani RK, et al. (2023) Effect of Pulsing Digital Heating Devices on Skin Parameters, Subjective Pain, Mood, and Anxiety. J Clin Med 12: 7206.
- Ghoussoub K, Côté CI, Fortier M, Nauche B, Rainville P, et al. (2024) Investigating the Impact of Stress on Pain: A Scoping Review on Sense of Control, Social-Evaluative Threat, Unpredictability, and Novelty (STUN Model). J Pain Res 17: 737-751.
- Patak L, Tait AR, Mirafzali L, Morris M, Dasgupta S, et al. (2013) Patient Perspectives of Patient-Controlled Analgesia (PCA) and Methods for Improving Pain Control and Patient Satisfaction. Regional Anesthesia and Pain Medicine 38: 326-333.
- Nygaard AS, Haugstad GK, Wilsgaard T, Øian P, Stedenfeldt M (2020) Baseline pain characteristics predict pain reduction after physical therapy in women with chronic pelvic pain. Secondary analysis of data from a randomized controlled trial. Scand J Pain 20: 793-800.
- Schein JR, Kosinski MR, Janagap-Benson C, Gajria K, Lin P, et al. (2008) Functionality and health-status benefits associated with reduction of osteoarthritis pain. Curr Med Res Opin 24: 1255-1265.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.