One Sugar Too Many: A Rare Case of Fulminant Type 1 Diabetes Following Chemotherapy in Advanced Pancreatic Cancer and Comprehensive Literature Review.
by Gilles Boumaza, Ayoub Jaafari*, Rosine Chantal Tatou Mawamba, Andrea Gallerani, Sohaïb Mansour, Mircea T Talpos, Rachid Attou
Intensive Care Unit Department, C.H.U Brugmann,Belgium
*Corresponding author: Ayoub Jaafari, Intensive Care Unit Department, C.H.U Brugmann, Belgium
Received Date: 26 July 2024
Accepted Date: 30 July 2024
Published Date: 01 August 2024
Citation: Boumaza G, Jaafari A, Mawamba RCT, Gallerani A, Mansour S, et al (2024) One Sugar Too Many: A Rare Case of Fulminant Type 1 Diabetes Following Chemotherapy in Advanced Pancreatic Cancer and Comprehensive Literature Review. Ann Case Report. 9: 1915. https://doi.org/10.29011/2574-7754.101915
Abstract
Fulminant type 1 diabetes (FT1D) is a category of type 1 diabetes mellitus characterized by the rapid progression of hyperglycaemia, an absolute deficit in insulin secretion, and ketoacidosis due to pancreatic β-cell destruction. Owing to unspecific symptomatology and a hasty clinical course in most cases, patients with FT1D are sometimes untreated until they become unconscious and reach a life-threatening critical state. The exact aetiology and pathogenesis are relatively elusive and may be related to various conditions including genetic predisposition, autoimmunity, viral infection, and less commonly, medications. Combination chemotherapy is the current established first-line treatment approach for advanced pancreatic adenocarcinoma but these treatments may be fraught with infrequent complications as the onset of FT1D. According to the literature, several studies have reported the occurrence of FT1D by interferon-alpha or immune checkpoint inhibitors in various cancers. Still, no cases describing the development of FT1D in pancreatic carcinoma undergoing chemotherapy have been described. Here, we report a scarce case of FT1D in a patient with no prior history of diabetes mellitus during treatment of pancreatic adenocarcinoma with chemotherapy and a comprehensive literature review.
Keywords:Fulminant Type 1 Diabetes; FT1D; Pancreatic Cancer; Hyperosmolar Hyperglycaemia.
Introduction
For several decades now, pancreatic cancer remains one of the world’s most challenging diseases, both therapeutically and in terms of prognosis. According to the latest epidemiological data, it ranks as the 12th most common cancer and the 7th most common cause of cancer-related death worldwide [1,2]. Around 50-60% of patients show distant metastatic disease, 25-30% regional disease and only 10-15% local disease [1]. Therefore, improving the proportion of patients whose cancer is detected at an early stage, when treatment is likely more efficient, may enhance the outcome of pancreatic cancer. Combination chemotherapy is the current established first-line treatment approach for advanced pancreatic ductal adenocarcinoma (PDAC), specifically FOLFIRINOX (5-fluorouracil, leucovorin, irinotecan and oxaliplatin), NALIRIFOX (Fluorouracil, leucovorin, liposomal irinotecan and oxaliplatin), and gemcitabine with nab-paclitaxel (GEM-NABP) [3]. Unsurprisingly, these treatments are fraught with side effects such as nausea, vomiting, myelosuppression, and more rarely, severe hyperglycaemia, which can lead to the onset of fulminant type 1 diabetes.
First reported by Imagawa et al. in 2000, and commonly diagnosed in the Asian population, fulminant type 1 diabetes (FT1D) is a subtype of type 1 diabetes mellitus (T1DM) distinguished by the swift and almost destruction of the pancreatic B-cells, whose acute onset leads to serious metabolic disorders and may threaten the patient’s life [4]. According to the literature, several studies have reported the induction of FT1D by interferon-alpha or immune checkpoint inhibitors [5,6]. Only sporadic cases have been reported on the onset of this diabetes during or after chemotherapy in different cancers [7,8]. However, its development mechanism remains unclear. Up to now, there have been no cases describing the development of FT1D in pancreatic carcinoma undergoing chemotherapy. Herein, we report a rare case of fulminant type 1 diabetes in a patient with no prior history of diabetes mellitus during treatment of pancreatic adenocarcinoma with chemotherapy.
Case Presentation
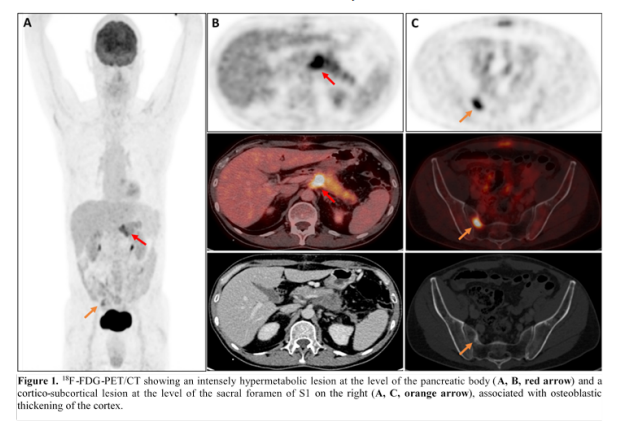
A 59-year-old patient with a history of hypertension treated with indapamide and perindopril was admitted to emergency with altered consciousness and hemodynamic instability. Three months before his admission, he had presented to the emergency with nausea and vomiting for several days. A lower bowel obstruction was diagnosed, caused by a 42 mm diameter sigmoidal lesion, which was treated by a Hartmann-type surgical procedure. Pathological analysis led to a diagnosis of high-grade invasive adenocarcinoma with local lymph node metastasis. In the extension work-up, a positron emission tomography scan (18F-FDG-PET/CT) revealed another lesion in the pancreas’s body and bone metastases in the sacrum (Figure 1). A final diagnosis of high-grade pancreatic ductal adenocarcinoma with lymph node, colonic, and bone metastases was made. Chemotherapy with FOLFIRINOX and dexamethasone was initiated, for which he has received a total of 3 courses to date.
Two days after completing his third cycle of FOLFIRINOX, the patient was found unconscious by his wife in the morning. According to her, it happened unexpectedly. At the arrival of the emergency physician, the Glasgow Coma Scale was at 3 on 15 (E1V1M1), associated with bradypnea at 8 cycles per minute. Oxygen saturation with an oximeter was not measurable. The patient had severe hypotension measured at 40/21 mmHg without compensatory tachycardia (regular sinus rhythm at 87 beats per minute on electrocardiogram). It is worth noting that the patient had expressed his refusal of any form of cardiopulmonary resuscitation and admission to intensive care, confirmed by his wife. Further assessment revealed hypothermia to 30 degrees Celsius, as well as severe hyperglycaemia (not measurable). Bearing in mind the patient’s condition, fluid resuscitation with crystalloid solutions (0.9% sodium chloride) was immediately initiated.
On admission to the hospital, the patient’s consciousness remained unchanged. The patient was placed on a 100% non-rebreathing mask and his respiratory rate was 12 cycles per minute. External rewarming with a heating blanket was undertaken on admission for his hypothermia. The patient appeared to respond slowly to fluid resuscitation but remained hypotensive after receiving the first 1000ml of 0.9% sodium chloride solution. Arterial blood gas analysis revealed mixed acidosis with a pH below 6.75, an unmeasurable bicarbonate level, hypercapnia with a pCO2 of 87 mmHg, and a lactic acid level of 4.40 mmol/L. Point-of-care blood glucose was elevated, above 847 mg/dL. There was no significant ketonuria and β-hydroxybutyrate levels were 0.9 mmol/L. After controlling potassium levels, which were moderately elevated, an intravenous infusion of rapid-acting fixed-rate insulin was started at 0.1 units/kg/hour, along with bicarbonate supplementation (100ml of 8.4% sodium bicarbonate solution, given in two doses). All laboratory results are presented in Tables 1 and 2. Additional laboratory investigations revealed stage 3 acute renal failure with hyperkalaemia of 5.6 mmol/L. Serum osmolality was significantly elevated at 445 mOsm/kg, with blood glucose at 1550 mg/dL. C-reactive protein levels were within the normal range and there were no clinical signs of infection either on urinalysis or on chest X-ray. Considering these findings, the retained diagnosis was a hyperglycaemic hyperosmolar syndrome due to new-onset fulminant diabetes mellitus after the third cycle of FOLFIRINOX, complicated by altered consciousness and respiratory acidosis. The respiratory acidosis was corrected, but there was no improvement in consciousness. The patient presented with multiple episodes of reentrant supraventricular tachycardia requiring the use of adenosine following European Resuscitation Council guidelines. Electrolyte levels were corrected. Regrettably, the patient progressed to ventricular fibrillation followed by asystole, resulting in his death without intensive resuscitation being carried out following the therapeutic limitations instituted by the patient and his wife.
|
Variable |
Reference Range |
Value |
|
Haemoglobin (g/dL) |
13.0 – 18.0 |
9.9 |
|
Erythrocytes (<106/μL) |
4.40 – 5.90 |
3.20 |
|
Haematocrit (%) |
40.0 – 53.0 |
33.5 |
|
MeanCorpuscular Volume (fL) |
80 – 100 |
104 |
|
MeanCorpuscularHaemoglobin Concentration (g/dL) |
31 – 36 |
29.6 |
|
Platelets (<103/μL) |
150 – 440 |
178 |
|
Leucocytes (<103/μL) |
3.50 – 11.00 |
63.27 |
|
Neutrophils (<103/μL) |
1.50 – 6.70 |
47.30 (76.1%) |
|
Lymphocytes (<103/μL) |
1.20 – 3.50 |
1.07 (1.7%) |
|
Eosinophils (<103/μL) |
0.10 – 0.50 |
0.00 (0.0%) |
|
Basophils (<103/μL) |
< 0.10 |
0.08 (0.1%) |
|
C-ReactiveProtein (mg/L) |
< 5.0 |
2.8 |
|
Sodium (mmol/L) |
136 – 145 |
137 |
|
Potassium (mmol/L) |
3.5 – 4.5 |
5.6 |
|
Chloride (mmol/L) |
98 – 107 |
89 |
|
Bicarbonate (mmol/L) |
23 – 29 |
15 |
|
Anion gap (mmol/L) |
9 – 21 |
39 |
|
Calcium (mmol/L) |
2.20 – 2.55 |
2.30 |
|
Magnesium (mmol/L) |
0.63 – 1.05 |
1.88 |
|
Phosphate (mmol/L) |
0.75 – 1.39 |
2.87 |
|
Osmolality (mOsm/kg) |
275 – 295 |
445 |
|
Urea (mg/dL) |
16.6 – 48.5 |
239 |
|
Creatinine (mg/dL) |
0.70 – 1.20 |
3.97 |
|
CKD-EPI (mL/min/1.73m2) |
> 60 |
16 |
|
Uremicacid (mg/dL) |
3.5 – 7.2 |
17.7 |
|
Glucose (mg/dL) |
70 – 100 |
1550 |
|
C-peptide (nmol/L) |
0.370 – 1.470 |
0.799 |
|
Aspartate aminotransferase (UI/L) |
10 – 50 |
22 |
|
Alanine aminotransferase (UI/L) |
10 – 50 |
47 |
|
Gamma-glutamyltransferase (UI/L) |
10 – 71 |
35.5 |
|
Alkaline phosphatases (UI/L) |
40 – 129 |
203 |
|
Total bilirubin (mg/dL) |
< 1.2 |
< 0.2 |
|
Troponin (ng/L) |
< 14.0 |
38.0 |
|
Urine glucose (g/L) |
< 0.15 |
53.64 |
|
Dipstickacetone |
Traces / + |
|
|
Point-of-care ketones (mmol/L) |
< 0.6 |
0.9 |
|
HbA1c (%) |
< 6.5% |
5.8% |
Table 1: All laboratory results.
|
Variable |
Reference Range |
Admission |
First Hour |
Third hour |
|
pH |
7.35 – 7.45 |
< 6.75 |
6.81 |
7.15 |
|
pCO2 (mmHg) |
32 – 45 |
87 |
52 |
44 |
|
pO2 (mmHg) |
75 – 104 |
323 (NRM) |
55 (FiO2 21%) |
200 |
|
Bicarbonate (mmol/L) |
23 – 30 |
8 |
15 |
|
|
Base Excess (mEq/L) |
-24.9 |
-13.0 |
||
|
Sodium (mmol/L) |
135 – 145 |
144 |
148 |
152 |
|
Potassium (mmol/L) |
3.4 – 4.5 |
4.8 |
5.7 |
3.4 |
|
Chloride (mmol/L) |
95 – 107 |
103 |
108 |
|
|
Calcium (mmol/L) |
1.12 – 1.32 |
1.30 |
1.26 |
1.05 |
|
Lactate (mmol/L) |
0.70 – 2.00 |
4.40 |
8.00 |
2.60 |
|
Hemoglobin (g/dL) |
12 – 18 |
11.9 |
9.0 |
10.2 |
|
Glucose (mg/dL) |
70 – 100 |
>847 |
809 |
>847 |
Table 2: All laboratory results.
Discussion
Several biological criteria are necessary to establish the diagnosis of FT1D: abrupt onset of ketosis or ketoacidosis, absence or reduction in C-peptide secretion, and elevation of plasma glucose with normal glycosylated haemoglobin, as in our patient (Table 1) [4]. However, our clinical presentation was slightly unusual. First, he presented with a state of severe hyperosmolar hyperglycaemia (HHS) with loss of consciousness (known as “hyperosmolar hyperglycaemic coma”), which manifested 48 hours after his third chemotherapy cycle, without any history of diabetes mellitus. Secondly, HHS is a clinical condition that arises more frequently in type 2 diabetes, while diabetic ketoacidosis (DKA) is more commonly encountered in type 1 diabetes [9].
Although DKA and HHS have been described for type 1 and type 2 diabetes mellitus respectively, there is a shared pathophysiological pathway: insulin starvation followed by the discharge of counter regulatory hormones promoting gluconeogenesis and glycogenolysis [10]. Hyperglycaemia, generally over 600 mg/dL, leads to an osmotic gradient increase with displacement of free water. The resulting osmotic gradient and transfer of free water induce dehydration and may even cause cardiovascular collapse. The remaining insulin secretion will inhibit ketogenesis and thus limit ketonemia, as in our patient, but also limit acidaemia, which may be aggravated by other mechanisms (mixed respiratory and metabolic acidosis) [9,10].
The exact aetiology and pathogenesis of FT1D are relatively elusive and may be related to genetic predisposition and environmental factors [11]. Anticancer agents may also act as an environmental factor leading to FT1D. For some time now, the new immunotherapies have revolutionized advanced neoplasia management by blocking certain immune checkpoints and thus un-inhibiting the immune system, with a beneficial anti-neoplastic impact [12]. Nevertheless, many of these therapies frequently induce immune-related adverse events (IRAEs) that impact numerous organ systems, most commonly endocrine (hypophysitis, thyroid dysfunctions), including FT1D [12].
According to the literature, reports of FT1D acquired with chemotherapy agents are extremely uncommon and rarely reported [7,8]. Our case is very scarce and noteworthy as there are no reported cases of FT1D with chemotherapy in advanced pancreatic cancer and presented with HHS.
The underlying mechanisms of the aggressive and uncontrolled destruction of β-cells remain among the most challenging questions concerning this subtype of type 1 diabetes. Our patient was receiving chemotherapy combining 5-fluorouracil (5FU), leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX). Interestingly, some articles report a potential propensity for diabetes induction with oxaliplatin, and cisplatin, but particularly with 5-FU [13]. Basically, 5-FU exerts its antineoplastic effects through the inhibition of RNA synthesis by its active metabolites and through the action of thymidylate synthase [13-14]. In our view, two mechanisms have been suggested. The first involves immune suppression or an immunological reaction. At lower concentrations, 5-FU has been demonstrated to enhance the release of interferon-gamma in activating lymphocytes [14]. Interferongamma stimulates the chemokine ligand 10 CXC (CXCL10) and interleukin-18 (IL-18) in pancreatic islet beta cells. The CXCL10 also activates T cells and macrophages and IL-18 additionally enhances the expression of interferon-gamma and CXCL10. This mutual positive feedback loop encourages an adaptive immune response that destroys beta cells by infiltrating T-lymphocytes via a cell-mediated mechanism. In this case, the rest period after the patient’s 3rd cycle of chemotherapy might have resulted in a lower concentration of 5-fluorouracil, leading to an increase in interferon-gamma in activated lymphocytes.
The second mechanism is related to increased levels of thymidine phosphorylase. Thymidine phosphorylase promotes endothelial cell migration in vitro and angiogenesis in vivo, and 5-FU has previously been shown to have antiangiogenic effects by inhibiting thymidine phosphorylase [15]. Furthermore, the combination of different chemotherapies, as in our case, and stress conditions increase the expression of thymidine phosphorylase which may contribute to TH1 activity [16]. Therefore, the post-3rd cycle chemotherapeutic interruption in our patient may induce increases in thymidine phosphorylase expression, leading to an increase in CXCL10 expression.
Up to now, there is no consensus or standardized protocol for blood glucose monitoring in pancreatic cancer undergoing chemotherapy. Given the current use of FOLFIRINOX in various cancers, identification, and prevention of hyperglycaemic emergencies, which influence mortality and morbidity, is crucial. Further research is required to establish evidence-based guidelines, but implementing blood glucose monitoring into current pancreatic cancer chemotherapy protocols may improve patient safety and treatment outcomes.
Conclusion
In conclusion, fulminant type 1 diabetes is an entity characterized by a rapid progression of hyperglycaemia that might be potentially life-threatening for the patient if not diagnosed in time. The clinical presentation can be variable but sometimes severe, leading to hyperosmolar coma. Clinicians should be vigilant in cancer patients undergoing hyperglycaemia-inducing chemotherapy and must consider this diagnosis in patients as promptly as possible.
Acknowledgment:No Acknowledgments
Disclosure and Conflict of Interest: The authors declare no conflict of interest.
Informed Consent Statement:Written informed consent was obtained from the individual(s) to publish any potentially identifiable images or data in this article.
Data Availability Statement: The data used and analysed in this study are available from the corresponding author on reasonable request.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, et al(2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 71:209-249.
- Chandana SR, Woods LM, Maxwell F, Gandolfo R, et al. (2024) Risk factors for early-onset pancreatic ductal adenocarcinoma: A systematic literature review. Eur J Cancer. 198:113471.
- Nichetti F, Rota S, Ambrosini P, Pircher C, Gusmaroli E, et al (2024) NALIRIFOX, FOLFIRINOX, and Gemcitabine With Nab-Paclitaxel as First-Line Chemotherapy for Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis. JAMA Netw Open. 7:e2350756.
- Imagawa A, Hanafusa T, Miyagawa I, Matsuzawa Y (2000) A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. Osaka IDDM Study Group. New Engl J Med. 342:301–7.
- Fabris P, Betterle C, Floreani A, Greggio NA, de Lazzari F, et al (1992) Development of type 1 diabetes mellitus during interferon alfa therapy for chronic HCV hepatitis. Lancet. 340: 1992.
- Uto H, Matsuoka H, Murata M, Okamoto T, Miyata Y, et al (2000) A case of chronic hepatitis C developing insulin-dependent diabetes mellitus associated with various autoantibodies during interferon therapy. Diabetes Res ClinPract, 49: 101-106.
- Iwata Y, Matsuhashi N, Takahashi T, Suetsugu T, Fukada M, et al (2019) Diabetic ketoacidosis caused by fulminant type 1 diabetes during adjuvant chemotherapy for colon cancer: A case report. MolClinOncol 11: 189-191.
- Stalcup S, Dahlin A, Pau, D. (2020) A Case of Fulminant Type 1 Diabetes Mellitus and Diabetic Ketoacidosis Caused by Adjuvant Chemotherapy for Colon Cancer. American Thoracic 2020 International Conference, May 15-20, 2020. Critical Care Case Reports: Metabolic, Renal, and Endocrine.
- Adeyinka A, Kondamudi NP. (2024) Hyperosmolar Hyperglycemic Syndrome. 2023 Aug 12. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
- Pasquel FJ, Umpierrez GE. (2014) Hyperosmolar hyperglycemic state: a historic review of the clinical presentation, diagnosis, and treatment. Diabetes Care. 37:3124-31.
- Fu J, Shi BY. (2013) Fulminant type 1 diabetes. Int J EndocrinolMetab. 30:185–8.
- Ying Li, Junfeng Zhao, Yue Wang, Yali Xu, Ruyue Li, et al (2024) Common endocrine system adverse events associated with immune checkpoint inhibitors, Cancer Pathogenesis and Therapy. 2: 164-172.
- Adachi J, Mimura M, Gotyo N, Watanabe T. (2015) The development of fulminant type 1 diabetes during chemotherapy for rectal cancer. Intern Med. 54:819-22.
- Tanaka S, Nishida Y, Aida K, Maruyama T, Shimada A, et al. (2019) Enterovirus infection, CXC chemokine ligand 10 (CXCL10), and CXCR3 circuit: a mechanism of accelerated-cell failure in fulminant type 1 diabetes. Diabetes. 58: 2285-2291.
- Toyoda Y, Tabata S, Kishi J, Kuramoto T, Mitshuhashi A, et al. (2014) Thymidine phosphorylase regulates the expression of CXCL10 in rheumatoid arthritis fibroblastlikesynoviocytes. Arthritis Rheumatol. 66: 560-568.
- Toi M, Rahman MA, Bando H, Chow Louis WC. (2005) Thymidine phosphorylase (platelet-derived endothelial-cell growth factor) in cancer biology and treatment. Lancet Oncol. 6: 158-166.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.