Neural sensitivity in adults with autism spectrum disorder to an oxytocin trial: A proof of principle study
by Julie Sato1,2, Kristina Safar1,2, Celine Safati2,3, Diane Lamarle2,4, Alana Iaboni5, Veronica Yuk1,2,6, Evdokia Anagnostou2,6,7, Margot J. Taylor1,2,3,8*
1Department of Diagnostic Imaging, Hospital for Sick Children, Toronto, Ontario, Canada
2Program in Neurosciences & Mental Health, Hospital for Sick Children, Toronto, Canada
3Department of Psychology, University of Toronto, Toronto, Canada
4Biologie Intégrative et Physiologie, Sorbonne Université, Paris, France
5Autism Research Centre, Bloorview Research Institute, Holland Bloorview Kids Rehabilitation Hospital, Toronto, Canada.
6Laboratory for Autism and Neurodevelopmental Disorders, Istituto Italiano di Tecnologia, Rovereto, Italy.
7Department of Paediatrics, University of Toronto, Toronto, Canada
8Department of Medical Imaging, University of Toronto, Toronto, Canada
*Corresponding author: Margot J. Taylor, Department of Diagnostic Imaging, Hospital for Sick Children, Toronto, Canada.
Received Date: 27 November, 2024
Accepted Date: 12 December, 2024
Published Date: 16 December, 2024
Citation: Sato J, Safar K, Safati C, Lamarle D, Iaboni A, et al. (2024) Neural Sensitivity in Adults with Autism Spectrum Disorder to an Oxytocin trial: A Proof of Principle Study. J Psychiatry Cogn Behav 7: 190. https://doi.org/10.29011/2574-7762.000090
Abstract
The core characteristics of autism spectrum disorder (ASD) are restrictive and repetitive behaviours and interests and social and communication difficulties. A potential intervention for these difficulties is oxytocin, which has shown some success in ameliorating symptoms, but little is understood about the effects of oxytocin on neural processing. We recorded magnetoencephalography (MEG) during a social (emotional faces) and a cognitive (working memory) task in adults with ASD (n=14) undergoing a 12week randomized double blind intra- nasal oxytocin (IN-OXT) trial, pre- and post-trial. We compared the MEG metrics of brain connectivity in the two tasks both between groups (Placebo and IN-OXT) and between pre- and post-trial (Time 1 and Time 2).
Although there were no behavioural differences in task performance, there were significant MEG differences in functional brain networks. In the faces task, we found a significant increase in beta-band connectivity only in the IN-OXT group at Time 2 compared to Time 1, in a network strongly linked with face processing. This suggests that the IN-OXT contributed to greater efficiency within the face processing network post-treatment. In the working memory task, the IN-OXT group also showed increased connectivity at Time 2, but in the theta band and in a frontal-parietal network known to be involved in working memory.
Thus, network and frequency specific changes were seen to increase with IN-OXT administration post-trial, suggesting that oxytocin impacts neural functioning associated with these task-specific networks and may have positive effects that are worth pursuing in a larger clinical study.
Introduction
Autism spectrum disorder (ASD) is a heterogeneous neurodevelopmental lifetime condition. The main characteristics are restrictive and repetitive behaviours and interests and social and communication difficulties [1]. In particular, processing of facial expressions, a skill critical to successful peer interactions, is impaired in ASD [2,3] and may be a major contributor to the social deficits [4]. Individuals with ASD can also have cognitive difficulties in executive functioning, including working memory [5,6], which involves the ability to encode, store and/or manipulate information in the mind over a short delay (maintenance) and to retrieve or recognize information [7]. Both emotional face processing and working memory abilities play a central role in perceiving and adapting to social cues in one’s environment. However, despite widespread difficulties in these domains in those with ASD [8,9], relatively little is known about the underlying functional brain networks supporting emotional face processing and working memory in ASD and how they may be affected by pharmacological intervention.
Individuals with ASD display global alterations in the activation and connectivity of task-based neural networks [10-13]. While functional MRI has been traditionally used in this work, magnetoencephalography (MEG) is the optimal non-invasive neuroimaging approach to investigate functional connectivity, as it provides a combination of high spatial, temporal, and spectral (frequency) resolution of neural oscillatory activity and connectivity [14,15]. There have been some studies employing MEG that examined functional connectivity in children and adults with ASD, encompassing a range of task-based paradigms to better understand the neural underpinnings of social cognitive difficulties. Two oftenused protocols in the field include the n-back working memory and emotional faces tasks [13,16-18].
Atypical functional brain connectivity in adults with ASD has been found in theta and alpha frequency bands during an n-back working memory task using MEG [19,20]. For instance, Yuk et al. found decreased theta-band connectivity between inhibition and working memory brain areas in ASD during recognition of repeated visual stimuli (1-back) despite similar task performance to typically developed (TD) adults. As theta-band connectivity has been linked to the integration of brain regions during working memory [21], these results suggest difficulty inhibiting task-irrelevant stimuli during working memory in adults with ASD, which may be exacerbated at higher cognitive loads.
Similarly, atypical functional connectivity in several frequency bands has been observed in those with ASD to emotional face stimuli [13,16,22]. Leung et al. showed decreased beta-band connectivity to angry faces in adolescents with ASD, also shown in adults by Mennella et al., which could be related to deficits in top-down control, while Safar et al. showed that when a wide age range was studied, significant age-and group-related changes were seen particularly in the gamma band.
An intervention that is currently being explored for improving social skills in ASD is the administration of oxytocin (OXT), a nine-amino-acid peptide. Central release of OXT and its closely related peptide, arginine vasopressin (AVP), have been found to be involved in social functioning, including social recognition, social memory, and affiliative behaviours (e.g., [23]). A few studies have examined the effect of intranasal OXT (IN-OXT) in TD adults and showed that it improved trust and prosocial behaviour [24-26] and improved recognition memory for faces but not objects [27, 28].
Due to its impact on social behaviours, OXT has been trialled over the years as a potential treatment of social cognitive symptoms in autism. A single dose of oxytocin was reported to improve a number of social behaviours, such as affective speech in adults with ASD [29] and facial emotion recognition in youth [30]. Longerterm interventions have also shown improvements in core autism symptoms such as social reciprocity [31] or in secondary measures such as repetitive behaviour, social cognition and attachment [3234]. The literature, however, is not unanimous on these effects, with other studies showing few or no improvements with IN-OXT administration [35,36]. It is argued that although OXT has a role in modulating social-cognitive behaviour and has potential therapeutic value, more needs to be understood about its neurobiological mechanisms, including its influence on brain connectivity underlying emotion processing and working memory [37,38].
Using MEG, the current study is the first to examine whether INOXT, administered as part of a 12-week randomized clinical trial, enhanced social cognitive functioning related to brain-behaviour signatures in adults with ASD. We hypothesized that IN-OXT would effect changes in functional brain connectivity during emotional faces and working memory tasks that would be associated with improved social cognitive behaviour.
Methods
This study was part of a 12-week randomized, double-blind, parallel design clinical trial (NCT01788072), approved by the Research Ethics Boards of Holland Bloorview Kids Rehabilitation Hospital and the Hospital for Sick Children in Toronto, Canada, as a secondary, exploratory outcome. All participants had a primary diagnosis of ASD assigned by an experienced clinician and confirmed via the Autism Diagnostic Observation Schedule (ADOS-G or ADOS-2 [39,40]). Adults with ASD took 24 International Units of IN-OXT (Syntocinon® made by Novartis) or a placebo (manufactured by the Toronto Institute of Pharmaceutical Technology), twice daily (morning and afternoon), for a 12-week period. From the behavioural cohort of 42 adults with ASD (33 males), 18 participants agreed to participate in the neuroimaging component, to be scanned twice, pre- (Time 1) and post-treatment (Time 2).
Adults with ASD were eligible if they were between 18-45 years of age, had a primary diagnosis of ASD, a clinical global impressionseverity score ≥ 4, and a verbal scale IQ ≥ 70. Participants were ineligible if they were born before 28 weeks’ gestational age or had a medical history of neurological disease. All 18 participants underwent scanning; however, three did not return for the post-trial, Time 2 visit, one had poor quality MEG data due to too much movement for both tasks, and another had poor data for only the working memory task. Thus, there were 14 participants (seven in the oxytocin group, seven in the placebo group) in the emotional faces task, and 12 participants (six per group) in the working memory task (see Table 1 for demographics; there were no significant differences between the groups). Therefore, we had 52 MEG datasets over the two time points and tasks.
|
Emotional Faces task |
Placebo group (n=7) |
IN-OXT group (n=7) |
|
Sex (M:F) |
7:0 |
6:1 |
|
Age in years |
31.3 (6.6) |
29.8 (6.5) |
|
Full-Scale IQ |
95.6 (13.5) |
101.4 (17.3) |
|
ADOS Total |
12.4 (3.7) |
14.9 (3.5) |
|
N-Back task. |
Placebo group (n=6). |
IN-OXT group (n=6). |
|
Sex (M:F) |
6:0 |
5:1 |
|
Age in years |
32.6 (6.2) |
31.2 (6.3) |
|
Full-Scale IQ |
(10.9) |
103.8 (13.7) |
|
ADOS Total |
12.7 (4.0) |
13.3 (4.8) |
Table 1: Participant demographics; ADOS scores range from 3 to 20 with greater symptom severity reflected by higher scores; (S.D.).
Social cognitive tasks
In the emotional faces task, participants were shown happy or angry faces beside a scrambled version of the face (target stimuli) in the MEG [13,16]. Happy and angry face stimuli (25 faces per emotion, 12 male faces) with a minimum validity rating of 0.8 were chosen from the NimStim Facial Expressions database [41]. Participants saw 50 trials per emotion condition, presented randomly, twice per hemifield, yielding a total of 200 face trials. Stimuli were presented for 80ms with an inter-stimulus interval that ranged from 1300-1500ms. They were instructed to fixate on the central cross and make a left or right button press as quickly as possible, corresponding to the side of the scrambled face.
To assess working memory, a 1-back task with complex multicoloured abstract images was used [19,42]. The protocol included 285 trials; 190 unique images were presented (‘New’), with 95 of these repeated on the subsequent trial (‘Repeat’). Stimuli were presented serially for 200ms each on a black background with an interstimulus interval with a white fixation cross randomly varying between 1050-1300ms. Participants were instructed to press a button as quickly as possible when they identified the repetition of an image presented one trial earlier. Behavioural accuracy and reaction time for Repeat trials were recorded. Participants were included in subsequent MEG analyses if they had a recognition accuracy of >55%; only correct trials were analyzed.
For both tasks, stimuli were presented using Presentation Software
(Neurobehavioral Systems Inc., https://www.neurobs.com/presentation) on a rear projection screen positioned 80cm from the participants’ eyes.
Neuroimaging acquisition and analyses
A 151-sensor CTF MEG system (Coquitlam, BC, Canada) recorded data with a 600 Hz sampling rate during the tasks, while participants lay supine. Head position was continuously tracked via three fiducial coils on the left and right pre-auricular points and nasion. An online anti-aliasing low-pass filter (0-150 Hz) and a 3rd order spatial gradient were applied to attenuate background environmental noise.
For co-registration, a structural T1-weighted MRI was obtained in all participants with a 12-channel head coil in a 3T MRI scanner (MAGNETOM, Siemens AG, Erlangen, Germany) using a sagittal 3D MPRAGE sequence (TR/TE =2300/2.96ms, FA =9º, FOV =192x240x256 mm, voxel size =1.0 mm isotropic). To allow for co-registration of MEG data, MRI radio-opaque markers were placed in the same positions as the MEG fiducial points.
MEG preprocessing and analyses
MEG data were preprocessed using the FieldTrip toolbox [43] in MATLAB. The continuous MEG data were band-pass filtered from 1 to 150 Hz using a 4th order Butterworth filter, with a notch filter at 60 and 120 Hz. For the working memory task, data were epoched from -500 to 600ms relative to the Repeat stimulus onset. For the emotional faces task, data were epoched from -600 to 600ms relative to stimulus onset (happy or angry faces).
Independent component analysis (ICA; ‘fastica’ function) was applied to identify ocular and cardiac artefacts, which were removed via manual inspection. Trials were further excluded if the signal exceeded +/-2000fT, and if initial median head position shifted greater than 5mm. one participant had to be removed from both analyses and another the memory task could not be used, as there were insufficient trials remaining (<25) after excluding trials with movement. For both the emotional faces and working memory tasks, there were no significant differences in head motion or number of trials analyzed between groups or sessions (Time 1 or Time 2), nor were there any significant group-by-session interactions (all p>0.05, see Supplementary Table 1 for descriptive summary).
A single shell head model was generated for each participant based on their anatomical MRI [44]. The centroids of the 90 cortical and subcortical coordinates of the Automated Anatomical Labelling (AAL; [45]) atlas were computed in standard template space (ICBM 152; [46] and non-linearly transformed onto analogous head space coordinates for each individual subject. A linearly constrained minimum variance beamformer [47] with 5% Tikonov regularization was used to estimate the broadband time series at each of the 90 AAL sources.
Following source reconstruction, the broadband MEG time series, at each AAL source location, were filtered into the following frequency bands: theta (4-7Hz), alpha (8-14Hz), beta (15-29Hz) and gamma (30-55Hz). To compute the instantaneous phase values for each source location and frequency band, the Hilbert transform was used. The cross-trial phase lag index (PLI; based on [48]) was computed to measure pair-wise functional connectivity between AAL source locations. For the emotional faces task, functional connectivity analyses were focused on the 0-200ms time window following stimulus onset using eight a priori regions of interest related to whole brain connectivity [13,49]: the bilateral fusiform gyri, amygdalae, insulae and anterior cingulate cortices (ACC). For the working memory task, we also took an a priori approach, focusing on the 100-400ms time window and the major hubs involved in working memory [19, 20]: the bilateral middle frontal gyri (MFG), the inferior frontal gyri (IFG), the inferior parietal lobes (IPL), the precuneus (PCUN) and the anterior cingulate cortices (ACC). Thus, for each subject (and frequency band) this yielded an 8-by-90 adjacency matrix and a 10- by-90 adjacency matrix for the emotional faces and working memory tasks, respectively. A baseline window of equal length to each of the active windows was selected (-200 to 0ms for the emotional faces and -300 to 0ms for the working memory task). The PLI values at each time point during the active window were z-scored relative to the baseline window.
The Network Based Statistic (NBS), a non-parametric technique for the statistical analysis of large networks, was used [50,51]. We ran between-group (Contrast: Placebo vs IN- OXT) and withingroup (Contrast: Time 1 [baseline] vs Time 2 [post-treatment]) ttests in NBS. A conservative primary component-forming threshold based on 5% of total possible connections (~40 connections) was used; 5000 permutations were run.
Results Behavioural results
In both the emotional faces and 1-back task, there was no significant group-by-time interaction, nor main effects of group or time for task accuracy or reaction time (all p>0.05). The descriptive statistics are summarized in Table 2.
|
Emotional Faces task |
||
|
Placebo (n=7) |
IN-OXT (n=7) |
|
|
Happy |
||
|
Accuracy (%), Time 1 |
99 [92, 100] |
97 [96, 98] |
|
Accuracy (%), Time 2 |
97 [86, 100] |
97 [93, 98] |
|
Reaction time (ms), Time 1 |
241.2 ± 43.5 |
216.8 ± 30.1 |
|
Reaction time (ms), Time 2 |
237.3 ± 44.0 |
234.1 ± 35.6 |
|
Angry |
||
|
Accuracy (%), Time 1 |
98 [92, 99] |
99 [95, 100] |
|
Accuracy (%), Time 2 |
99 [88, 100] |
99 [96, 99] |
|
Reaction time (ms), Time 1 |
237.7 ± 41.1 |
217.9 ± 31.1 |
|
Reaction time (ms), Time 2 |
238.7 ± 48.1 |
235.7 ± 40.9 |
|
1-Back task |
||
|
Placebo group (n=6) |
IN-OXT group (n=6) |
|
|
Accuracy (%), Time 1 |
94.1 [93.6, 98.9] |
93.6 [77.7. 97.9] |
|
Accuracy (%), Time 2 |
88.8 ± 9.1 |
80.0 ± 22.4 |
|
Reaction time (ms), Time 1 |
296.6 ± 63.2 |
290.8 ± 64.8 |
|
Reaction time (ms), Time 2 |
284.1 ± 48.7 |
285.8 ± 58.4 |
Table 2: Summary of behavioural performance; Values are described as mean ± SD when normally distributed and median [interquartile range] when skewed.
Emotional faces task
Findings revealed significant differences in connectivity at baseline between the Placebo and IN-OXT groups (IN-OXT > Placebo, pcorr = 0.02) in the gamma frequency band (30-55 Hz) to angry faces. This network involved connections predominantly among the bilateral limbic and frontal regions (Figure 1). The most highly connected areas (i.e., nodes with highest degree) included the right amygdala, right ACC and left insula. Within the IN-OXT group, we observed a significant difference in connectivity between baseline and posttreatment (Time 2 > Time 1; pcorr = 0.01) in the beta frequency-band (15-29 Hz) to angry faces. This network was anchored in the bilateral fusiform gyri and amygdalae, with connections predominantly among the limbic, occipital and frontal regions, largely in the left hemisphere (Figure 2). No significant changes over time were observed in the Placebo group within any frequency band, to angry or happy faces (all pcorr>0.05).
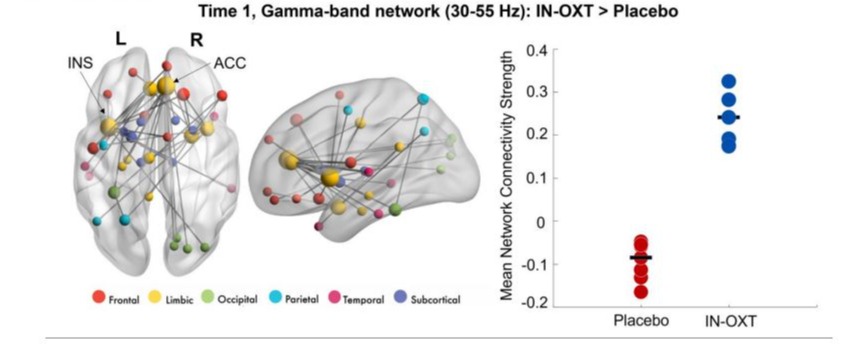
Figure 1: Significant between-group gamma network at baseline (Time 1);significant differences in connectivity were observed at baseline between the Placebo and IN-OXT groups in the gamma band to angry faces. The network involved connections predominantly among the bilateral limbic and frontal regions. Most highly connected areas included right amygdala, right ACC, and left insula. The node sizes in the gamma network (on the left) are scaled by degree (i.e., the number of connections). On the right, the mean network connectivity strengthw as extracted and plotted for both groups.
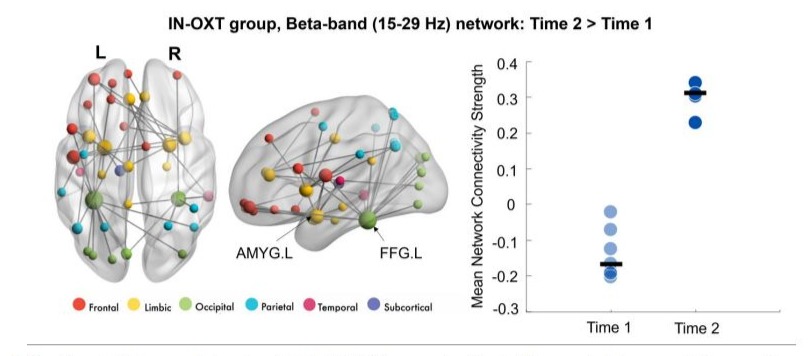
Figure 2: Significant within-group beta network in the IN-OXT group;significant differences in beta connectivity were observed in the IN-OXT group between the baseline and post-treatment to angry faces. The network involved connections predominantly among the limbic, occipital, and frontal regions, largely in the left hemisphere. This network was anchored in the bilateral fusiform gyri and amygdalae. The node sizes in the brain network (on the left) are scaled by degree and mean connectivity strength for this network at both time points are plotted on the right-side graph.
Working memory task
Similar to the emotional faces task, we ran between-group and within-group t-tests in NBS for the 1-back task. Significant differences in connectivity were observed at baseline between groups (Figure 3), with the Placebo group showing higher theta connectivity compared to the IN-OXT group (pcorr=0.039). Within the IN-OXT group, higher theta connectivity was found at Time 2 compared to Time 1 (pcorr=0.029; Figure 4). The two theta-band networks had a similar distribution, involving fronto-parietal connections with major hubs in the left MFG, the left IFG and in the right ACC. There were no significant differences in connectivity between baseline and posttreatment in the Placebo group (all pcorr>0.05).
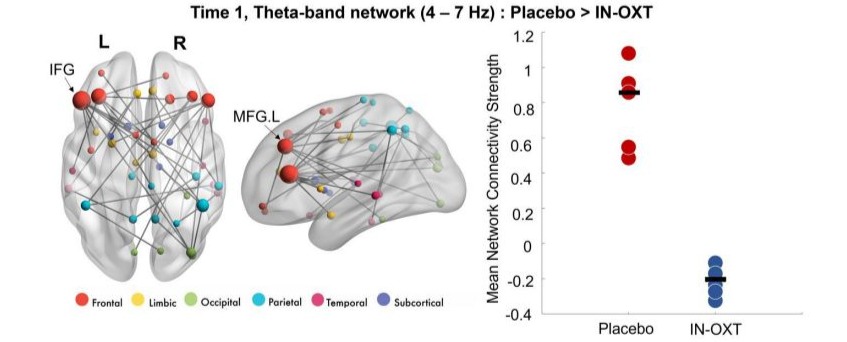
Figure 3: Significant between-group gamma network at baseline (Time 1); The Placebo group showed higher theta connectivity compared to the IN-OXT group, anchored in bilateral frontal regions. Node sizes are scaled by degree. The mean connectivity strength for this network for both groups are plotted on the right-side graph.
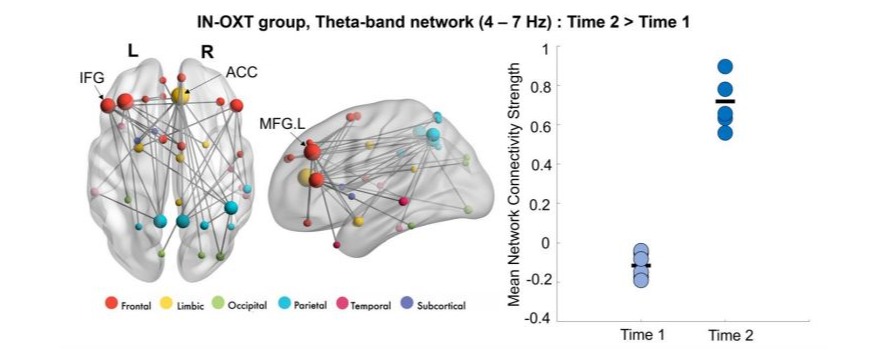
Figure 4: Significant within-group theta network in the IN-OXT group;Higher theta connectivity was found at Time 2 compared to Time 1 only in the IN-OXT group, involving fronto-parietal connections with major hubs in the left MFG, the left IFG and in the right ACC. Node sizes are scaled by degree. The mean connectivity strength for this network at both time points are plotted on the right-side graph.
Discussion
This is the first study to investigate the effect of oxytocin on two social-cognitive tasks in adults with ASD, using MEG to determine underlying brain functional connectivity changes.
Although our sample was small, we had longitudinal data on two MEG tasks in the participants pre- and post-treatment, yielding a considerable amount of neuroimaging data, and found significant effects of IN-OXT on underlying neural processing.
Emotional Face Processing
Between-group contrasts revealed increased gamma connectivity to angry faces in the IN-OXT compared to the Placebo group at baseline (Time 1). This fronto-temporal network in the gammaband may reflect discrepancies in attention to angry faces at Time 1 between IN- OXT and Placebo groups, as gamma oscillations in fronto-temporal regions have been implicated in attentional control mechanisms [52]. This finding suggests that the Placebo group may have shown decreased engagement with angry faces at Time 1. Given the randomized design of the study, we did not expect to find significant group differences in connectivity at Time 1; however, due to the relatively small sample size, we would not anticipate this between- group differences to be replicated in a larger sample
More interestingly, we also found a network of increased beta connectivity to angry faces (Time 2 > Time 1) only in the IN-OXT group, involving the bilateral amygdalae and fusiform gyri, regions known to be important in emotional face processing [13]. This increase in beta connectivity post-treatment suggests that IN-OXT may improve recruitment of task-relevant networks during angry face processing in adults with ASD. Beta is linked with top-down processes [59] and may reflect increased top-down monitoring of the negative stimuli. Beta- band hypo-connectivity during angry face processing has previously been associated with emotional face processing impairments in ASD [16,53], potentially signalling a reduced ability to extract emotional information from these salient stimuli. Behavioural studies have also shown that individuals with ASD have more difficulty processing/interpreting angry faces compared to TD peers [54]. Thus, our findings suggest that this increase in beta-band connectivity seen in the IN-OXT group post-treatment may reflect the improved efficiency of the functional networks underlying processing of angry faces, consistent with previous findings in TD adults [27].
Working Memory task
Increased theta connectivity in the Placebo compared to the INOXT group was observed at Time 1, but not at Time 2. This was again an unexpected result as the groups were well matched and thus likely due to the small sample. Interestingly, the IN-OXT group showed greater theta band connectivity post-treatment (Time 2 > Time 1). Larrain-Valenzuela et al. also found that adults with ASD showed alterations in the modulation of theta oscillations with working memory, while Yuk et al. found that adults with ASD showed a significant decrease in theta-band connectivity during recognition (in a 1-back task) compared to controls, involving major frontal and parietal hub regions. Prior work has established the critical role of frontal and parietal regions for working memory processing [55,56]. Thus, the increase of theta-band connectivity at Time 2 within this fronto-parietal network in the IN-OXT group may reflect improved functional connectivity during working memory recognition post- treatment in adults with ASD. We did not, however, find significant differences between working memory scores at the behavioural level between Time 1 and 2, likely due to the high level of performance on this 1-back task. Future studies are needed to confirm our results with a larger sample size, and with more challenging working memory protocols, to support the effective role of IN-OXT administration in improving socialcognitive functioning.
General discussion
We found distinct and task appropriate brain areas activated for each of our two tasks, as well as differing frequencies band effects, indicating task-specific effects, and not attributed to global effects of time. There have been many studies on the use of IN-OXT, either single dose or in trials over longer periods, as used the current study, driven by the initial reports of positive effects on social behaviour in TD adults [25,26] and subsequently shown in those with ASD [29, 31]. There is considerable variability in the reports, however, suggesting that more focused studies on the effects of OXT are needed [38,57]. Although the primary outcome measures of social symptoms are often not improved significantly in the INOXT compared to the placebo group, secondary outcomes (e.g., repetitive behaviour, attachment), show more subtle, positive effects, suggesting that there may be a therapeutic potential [32-34].
A recent MEG study in adolescents with a single dose of IN-OXT, found reduced connectivity in gamma that correlated with performance on a task that involved inferring emotional and cognitive states from partial facial expressions (Reading the Mind in the Eyes Task; [58]). IN-OXT also increased connectivity in the adolescents in alpha and beta bands, which play key roles in visual attention [59], regardless of stimulus type. The authors argued that although it was a preliminary study, MEG allowed investigation of neural mechanisms underlying these social processes. A study with 3-8-year-old children, however, showed both primary and secondary effects with a six-week course of oxytocin [60], including with emotional faces. We would also suggest that with MEG, we are able to determine the effects of IN-OXT that impact relevant neural processes underpinning social cognitive functions, even though behavioural measures may not show overt effects. This finding is consistent with other studies that have found improvements in secondary effects that were reported as being valued by the participants with ASD, although classic primary symptoms showed no improvements [34].
Our groups were randomized and well-matched, therefore differences at baseline were not anticipated and likely attributable to the small sample size. Our post-treatment findings of increased beta-band connectivity in a network involving the bilateral amygdalae and fusiform gyri for the emotional faces task and greater theta-band connectivity in a fronto-parietal network for the working memory task, suggest that IN-OXT improves recruitment of specific task-relevant networks in adults with ASD, impacting process-specific networks and frequencies. These promising results support the literature that suggests IN-OXT may have a therapeutic potential for social cognitive deficits in adults with ASD [33], and they emphasize the need for further clinical trials with larger sample sizes. Importantly, these results start to assess specific neural underpinnings of the oxytocin effects on brain function; this is critical for understanding the therapeutic mechanisms in the future.
Conflict of Interest
E. Anagnostou has served as a consultant to Roche and Quadrant Therapeutics, has received consultation fees from Roche, holds a patent for the device, “Anxiety Meter”, and has received in kind support from AMO pharma, royalties from APPI and Springer, and editorial honoraria from Wiley. The remaining authors declare that there are no competing financial interests in relation to the work described.
Ethical statement
This research was conducted in accordance with the Declaration of Helsinki and was reviewed and approved by the Holland Bloorview Kids Rehabilitation Hospital and the Hospital for Sick Children. None of the data reported in this manuscript have been published or are under consideration elsewhere. All the authors have seen and reviewed the manuscript and have contributed to it in a meaningful way.
Acknowledgements
This work was funded by Canadian Institutes of Health Research grants MOP-119541 and MOP- 142379 to MJT and the SickKids Research Institute LiUNA! Fellowship to JS. The authors thank Marc Lalancette, Tammy Rayner and Ruth Weiss for their help in data collection and Kaela Amorim for her contributions to reviewing the manuscript. We are also very grateful to all individuals who participated in this study.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5.
- Harms MB, Martin A, Wallace GL (2010) Facial emotion recognition in autism spectrum disorders: a review of behavioral and neuroimaging studies. Neuropsychol Rev 20: 290-322.
- Lozier LM, Vanmeter JW, Marsh AA (2014) Impairments in facial affect recognition associated with autism spectrum disorders: a metaanalysis. Dev Psychopathol 26: 933-945.
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, et al. (2008) Abnormal functional connectivity in autism spectrum disorders during face processing. Brain 131: 1000-1012.
- Frith U (2012) Why we need cognitive explanations of autism. Q J Exp Psychol (Hove) 65: 2073-2092.
- St John T, Woods S, Bode T, Ritter C, Estes A (2022) A review of executive functioning challenges and strengths in autistic adults. Clin Neuropsychol 36: 1116-1147.
- Baddeley A (2012) Working memory: theories, models, and controversies. Annu Rev Psychol 63: 1-29.
- Leung RC, Vogan VM, Powell TL, Anagnostou E, Taylor MJ (2016) The role of executive functions in social impairment in autism spectrum disorder. Child Neuropsychol 22: 336-339.
- Barendse EM, Hendriks MPH, Jansen JFA, Backes WH, Hofman PAM, et al. (2013) Working memory deficits in high-functioning adolescents with autism spectrum disorders: neuropsychological and neuroimaging correlates. J Neurodev Disord 5: 1-11.
- Just MA, Keller TA, Malave VL, Kana RK, Varma S (2012) Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neurosci Biobehav Rev 36: 1292-1313.
- Uddin LQ (2015) Idiosyncratic connectivity in autism: developmental and anatomical considerations. Trends Neurosci 38: 261-263.
- Maximo JO, Cadena EJ, Kana RK (2014) The implications of brain connectivity in the neuropsychology of autism. Neuropsychol Rev 24: 16-31.
- Safar K, Vandewouw MM, Taylor MJ (2021) Atypical development of emotional face processing networks in autism spectrum disorder from childhood through to adulthood. Develop Cogn Neurosci 51: 101003.
- Hämäläinen M, Hari R, Ilmoniemi RJ, Knuutila J, Lounasmaa OV (1993) Magnetoencephalography theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev Mod Phys 65: 413.
- Hari R, Salmelin R (2012) Magnetoencephalography: From SQUIDs to neuroscience. Neuroimage 20th anniversary special edition. NeuroImage 61: 386-396.
- Leung RC, Ye AX, Wong SM, Taylor MJ, Doesburg SM (2014) Reduced beta connectivity during emotional face processing in adolescents with autism. Mol Autism 5: 51.
- Barendse EM, Schreuder LJ, Thoonen G, Hendriks MPH, Kessels RPC, et al. (2018) Working memory network alterations in high-functioning adolescents with an autism spectrum disorder. J. Psychiatry Neurosci 72: 73-83.
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, et al. (2005) Functional connectivity in an fMRI working memory task in high-functioning autism. NeuroImage (3): 810-821.
- Audrain SP, Urbain CM, Yuk V, Leung RC, Wong SM, et al. (2020) Frequency-specific neural synchrony in autism during memory encoding, maintenance and recognition. Brain Commun 2: fcaa094.
- Yuk V, Urbain C, Anagnostou E, Taylor MJ (2020) Frontoparietal network connectivity during an N-back task in adults with autism spectrum disorder. Front. Psychiatry 11: 551808.
- Sauseng P, Klimesch W, Doppelmayr M, Hanslmayr S, Schabus M,et al. (2004) Theta coupling in the human electroencephalogram during a working memory task. Neurosci Lett 354: 123-126.
- Khan S, Gramfort A, Shetty NR, Kitzbichler MG, Ganesan S, et al. (2013) Local and long-range functional connectivity is reduced in concert in autism spectrum disorders. Proc Natl Acad Sci U S A 110: 31073112.
- Bakermans-Kranenburg MJ, van IJzendoorn MH (2013) Sniffing around oxytocin: review and meta- analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl Psychiatry 3: e258.
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E (2005) Oxytocin increases trust in humans. Nature 435: 673-676.
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, et al. (2005) Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci 25: 11489-11493.
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC (2007) Oxytocin improves “mind-reading” in humans. Biol Psychiatry 61: 731-733.
- Savaskan E, Ehrhardt R, Schulz A, Walter M, Schachinger H (2008) Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology 33: 368-374.
- Rimmele U, Hediger K, Heinrichs M, Klaver P (2009) Oxytocin makes a face memory familiar. J Neurosci 29: 38-42.
- Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, et al. (2007) Oxytocin increases retention of social cognition in autism. Biol Psychiatry 61: 498-503.
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, et al. (2010) Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry 67: 692-694.
- Watanabe T, Kuroda M, Kuwabara H, Aoki Y, Iwashiro N, et al. (2015) Clinical and neural effects of six-week administration of oxytocin on core symptoms of autism. Brain 138: 3400-3412.
- Yamasue H, Okada T, Munesue T, Kuroda M, Fujioka T, et al. (2020) Effect of intranasal oxytocin on the core social symptoms of autism spectrum disorder: a randomized clinical trial. Mol Psychiatry 25: 1849-1858.
- Anagnostou E, Soorya L, Chaplin W, Bartz J, Halpern D, et al. (2012) Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: A randomized controlled trial. Mol Autism 3: 16.
- Bernaerts S, Boets B, Bosmans G, Steyaert J, Alaerts K (2020) Behavioral effects of multiple-dose oxytocin treatment in autism: a randomized, placebo-controlled trial with long-term follow-up. Mol Autism 11: 6.
- Guastella AJ, Gray KM, Rinehart NJ, Alvares GA, Tonge BJ, et al. (2015) The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: A randomized controlled trial. J Child Psychol Psychiatry 4: 444-452.
- Yamasue H, Kojima M, Kuwabara H, Kuroda M, Matsumoto K, et al. (2022) Effect of a novel nasal oxytocin spray with enhanced bioavailability on autism: a randomized trial. Brain 142: 490-499.
- Carter CS, Kenkel WM, MacLean EL, Wilson SR, Perkeybile AM, et al. (2020) Is oxytocin “Nature’s Medicine”? Pharmacol Rev 72: 829-861.
- Erdozain AM, Peñagarikano O (2020) Oxytocin as treatment for social cognition, not there yet. Front Psychiatry 10: 930.
- Lord C, Risi S, Lambrecht L, Cook EH Jr., Leventhal BL, et al. (2000) The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30: 205-223.
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, et al. (2012) Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Manual (Part I): Modules 1-4.
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, et al. (2009) The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res 168: 242-249.
- Sato J, Safar K, Vogan VM, Taylor MJ (2023) nctional connectivity changes during working memory in autism spectrum disorder: A twoyear longitudinal MEG study. NeuroImage Clin 37: 103364.
- Oostenveld R, Fries P, Maris E, Schoffelen JM (2011) FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011: 156869.
- Nolte G (2003) The magnetic lead field theorem in the quasi-static approximation and its use for magnetoencephalography forward calculation in realistic volume conductors. Phys Med Biol 48: 3637-3652.
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, et al. (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI singlesubject brain. NeuroImage 15: 273-289.
- Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, et al. (2011) Unbiased average age- appropriate atlases for pediatric studies. NeuroImage 54: 313-327.
- van Veen B, van Drongelen W, Yuchtman M, Suzuki A (1997) Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng 44: 867-880.
- Stam CJ, Nolte G, Daffertshofer A (2007) Phase lag index: Assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum Brain Mapp 28: 11781193.
- Safar K, Wong SM, Leung RC, Dunkley BT, et al. (2018) Increased functional connectivity during emotional face processing in children with autism spectrum disorder. Front Hum Neurosci 12: 408.
- Zalesky A, Cocchi L, Fornito A, Murray MM, Bullmore ED (2012) Connectivity differences in brain networks. NeuroImage 60: 1055-1062.
- Zalesky A, Fornito A, Bullmore ET (2010) Network-based statistic:identifying differences in brain networks. NeuroImage 53: 1197-1207.
- Jensen O, Kaiser J, Lachaux JP (2007) Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci 30: 317-324.
- Mennella R, Leung RC, Taylor MJ, Dunkley BT (2017) Disconnection from others in autism might be more than just a feeling: Reduced functional connectivity during socio-emotional processing. Mol Autism 8: 7.
- Kuusikko S, Haapsamo H, Jansson-Verkasalo E, Hurtig T, Mattila M, et al. (2009) Emotion recognition in children and adolescents with autism spectrum disorders. J Autism Dev Disord 39: 938-945.
- Eriksson J, Vogel EK, Lansner A, Bergström F, Nyberg L (2015) Neurocognitive architecture of working memory. Neuron 88: 33-46.
- Owen AM, McMillan KM, Laird AR, Bullmore E (2005) N-back working memory paradigm: a meta- analysis of normative functional neuroimaging studies. Hum Brain Mapp 25: 46-59.
- Baribeau D, Vorstman J, Anagnostou E (2022) Novel treatments in autism spectrum disorder. Curr Opin Psychatry. Curr Opin Psychatry 35: 101-110.
- Korisky A, Gordon I, Goldstein A (2022) Oxytocin impacts top-down and bottom-up social perception in adolescents with ASD: a MEG study of neural connectivity. Mol Autism 13: 36.
- Fries P (2015) Rhythms for Cognition: Communication through Coherence. Neuron 88: 220- 225.
- Le J, Zhang L, Zhoa W, Zhu S, Lan C, et al. (2022) Infrequent intranasal oxytocin followed by positive social interaction improves symptoms in autistic children: A pilot randomized clinical trial. Psychother Psychosom 91: 335-347.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.