N-Chlorotaurine (NCT) as an Effective and Biocompatible Antiseptic for Human Graft Tissue Decontamination: An In Vitro Comparison with Vancomycin
by Armin Runer1,2, Valentin Leitner3, Friedemann Schneider 1, Peter Kaiser 1,4, Mariette Widner1, Anke Luger1, Werner Schmölz1, Rohit Arora1, Markus Nagl3*
1Department of Orthopedics and Traumatology, Medical University of Innsbruck, Innsbruck, Austria
2Department of Sports Orthopedics, Technical University of Munich, Munich, Germany
3Institute of Hygiene and Medical Microbiology, Medical University of Innsbruck, Innsbruck, Austria
4Sportklinik Arlberg, St. Anton am Arlberg, Austria
*Corresponding author: Markus Nagl, Institute of Hygiene and Medical Microbiology, Medical University of Innsbruck, Innsbruck, Austria; E-mail: m.nagl@i-med.ac.at
Received Date: 20 August 2025
Accepted Date: 26 August 2025
Published Date: 28 August 2025
Citation: Runer A, Leitner V, Schneider F, Kaiser P, Widner M, et al. (2025) N-Chlorotaurine (NCT) as an Effective and Biocompatible Antiseptic for Human Graft Tissue Decontamination: An In Vitro Comparison with Vancomycin. Adv Biochem Biotechnol 10: 10128 https://doi.org/10.29011/2574-7258.010128
Abstract
Aims: To assess the in vitro microbicidal efficacy of the endogenous antiseptic N-Chlorotaurine (NCT) on human musculoskeletal graft tissues contaminated with clinically relevant pathogens in comparison to the antibiotic vancomycin.
Methods and Results: Human-derived bone fragments from femoral head and carpal bones, as well as anterior cruciate ligament tissue, were artificially contaminated by immersion for 10 seconds in suspensions of Staphylococcus aureus, Escherichia coli, or Candida albicans. Samples were then incubated in various antiseptic solutions. 1% NCT required 15 minutes at 37 °C to achieve significant microbial reduction, while 5% NCT was microbicidal within 5 minutes with mean log10 reductions of 0.9 CFU/mL for S. aureus, 1.6 CFU/mL for C. albicans, and >2.5 CFU/mL for E. coli (p < 0.01). Complete eradication (>2.5 log10 CFU/mL reduction) was observed with 5% NCT after 15 minutes at 37 °C, and with 5% NCT plus 0.1% ammonium chloride already after 5 minutes at both 20 °C and 37 °C. No differences in antimicrobial efficacy were observed between bone and ligament grafts. Vancomycin (1%) eliminated S. aureus, but exhibited no relevant activity against E. coli or C. albicans, consistent with its known antibacterial spectrum.
Conclusion: N-Chlorotaurine (NCT), particularly in combination with ammonium chloride, is highly effective as a rapid and broad-spectrum antiseptic for human graft decontamination. Compared to vancomycin, NCT offers a broader antimicrobial spectrum.
Impact Statement:The findings of this study support the potential of NCT as an effective, endogenous, and well-tolerated antiseptic for the rapid decontamination of biologically sensitive graft tissues.
Keywords: Anterior Cruciate Ligament; ACL; Graft Decontamination; N-Chlorotaurine; NCT; Septic Arthritis; Staphylococcus Aureus; Vancomycin
Introduction
Postoperative Septic Arthritis (PSA) is one of the most serious and feared complications following orthopaedic and trauma surgical procedures. Although relatively rare, PSA carries significant consequences, both in terms of patient morbidity and healthcare system burden. The condition often necessitates multiple revision surgeries, prolonged hospital stays, and extended courses of intravenous antibiotic therapy, contributing to substantially elevated treatment costs and reduced quality of life for affected patients [1]. The predominant causative organisms in PSA are staphylococci, particularly Staphylococcus epidermidis and Staphylococcus aureus [2]. Among the most frequently performed procedures in orthopaedic surgery are anterior cruciate ligament reconstruction (ACLR) and primary Total Knee Arthroplasty (TKA). Although the reported incidence rates of PSA following ACLR (0.14–1.8%) [3-5] and TKA (0.5–4%) [2] are comparatively low, the sheer volume of procedures performed annually translates into a substantial number of infections with devastating consequences [6,7]. As such, prevention of PSA remains a central goal in orthopaedic practice. A variety of preoperative and intraoperative measures are routinely implemented to minimize the risk of infection. Previous investigations have explored various antiseptic and antibiotic agents for decontaminating contaminated grafts. Studies have shown that chlorhexidine and povidoneiodine are effective in reducing bacterial load [8], although complete eradication of pathogens is not consistently achieved [9]. Povidone-iodine has demonstrated efficacy in decontaminating bone fragments inadvertently dropped onto the operating room floor [10], and its utility has been reinforced in systematic reviews [11]. Gentamicin and rifampicin have also shown success in eliminating susceptible bacteria following prolonged incubation [9]. In the context of ACLR, soaking of autografts in vancomycin is nowadays standard of care, showing a significant reduction of PSA [12,13]. While antiseptics are generally superior in terms of rapid and broad-spectrum microbicidal activity, their effectiveness can be compromised by organic material due to consumption of active compounds, and cytotoxicity may limit their use at higher concentrations.
Antibiotics, in contrast, exhibit lower cytotoxicity and more favorable tissue tolerability, but their limited antimicrobial spectrum and the emergence of resistance remain significant limitations. Most of these agents are synthetic, carrying a risk of allergic reactions. Given these constraints, the exploration of novel, well-tolerated, and effective antimicrobial agents is warranted. N-chlorotaurine (NCT; Cl-HN-CH₂-CH₂-SO₃H), an endogenous long-lived oxidant produced by activated human granulocytes and monocytes, presents a promising alternative [14-16]. Chemically stable as a sodium salt (Cl-HN-CH₂-CH₂-SO₃⁻Na⁺) [14,17], NCT exhibits broad-spectrum antimicrobial activity against bacteria, viruses, fungi, and protozoa [14,16]. Its tolerability profile is favorable, even at high concentrations (typically 1%, equivalent to 55 mM), allowing application to sensitive tissues such as the eye, skin ulcers, ear, and bladder [14]. Systemic administration at lower concentrations has also proven safe in animal models [18,19]. Topical application of high concentrations overcomes chlorine consumption and maintains activity even in the presence of high organic load [20]. Moreover, this allows a significant amount of transhalogenation reactions (chlorine transfer to other amino compounds in equilibrium). Some of these, particularly with small amino molecules like ammonium, lead to formation of stronger active chloramines so that as a net effect, NCT is enhanced but not decreased in the presence of organic load such as exudates and several body fluids (e.g. [21,22]). This may even lead to a higher antimicrobial activity of NCT compared to highly reactive active halogen compounds in such environments [21]. Considering its unique combination of broad-spectrum efficacy, endogenous origin, low toxicity, resistance evasion, and potential anti-inflammatory properties, NCT emerges as a compelling candidate for graft decontamination. The aim of the present study was to assess the in vitro antimicrobial efficacy of NCT, with and without ammonium chloride as a potentiator, on human musculoskeletal graft tissues artificially contaminated with clinically relevant pathogens. Its performance was compared to vancomycin, a standard antibiotic frequently used in current decontamination protocols, with the aim of identifying an effective and biologically compatible alternative for clinical application in orthopaedic surgery.
Materials and Methods
Bacterial and Fungal Strains
Staphylococcus aureus ATCC 6538, Escherichia coli ATCC 11229, and Candida albicans CBS 5982 were used as representatives for Gram-positive and Gram-negative bacteria and fungi. Bacteria and yeast, deep frozen at minus 80°C for storage, were thawed and grown on Mueller–Hinton agar plates (Oxoid Ltd., Oxoid, UK) for use. Overnight cultures from the plates were grown at 37 °C in tryptic soy broth (Merck, Darmstadt, Germany) to 3–5 × 109 Colony Forming Units (CFU)/mL for bacteria and to 1 × 107 CFU/ mL for yeasts, respectively, assessed by quantitative cultures.
N-Chlorotaurine and other Chemicals
Pure NCT, lot 2021-04-27, was prepared as crystalline sodium salt (Cl–HN–CH2–CH2–SO3Na; molecular weight, 181.57 [14]) in pharmaceutical quality and dissolved in distilled water to the desired concentration of 0.1% - 5% (5.5 – 275 mM). Distilled water was used as a solvent in this study since aqueous solutions of NCT possess the highest stability, which is important for practical use.
Ammonium chloride (reagent grade) was from Merck (Darmstadt, Germany). Vancomycin hydrochloride (Vancocin 1g vials, Ch.-B: 216047, Astro Pharma, Vienna, Austria) was dissolved in distilled water to 1%.
Bone- and Anterior Cruciate Ligaments Samples
Human musculoskeletal graft tissues were obtained from surgical explants following written informed consent, in accordance with the Declaration of Helsinki and with approval from the Ethics Committee of the Medical University of Innsbruck (approval number 1065/2021, dated 26 May 2021). Cancellous bone samples were harvested from femoral heads and carpal bones, while ACL grafts were collected from ligament reconstruction procedures. All specimens were processed under sterile conditions. Bone samples and ACL grafts were sectioned into standardized fragments measuring approximately 5 × 5 mm using sterile surgical instruments. Following preparation, all tissue samples were stored at −20°C until the time of experimental use to preserve structural and biological integrity.
Contamination and Decontamination of Bone Samples with NCT
Bacterial suspensions were prepared by cultivating strains in Tryptic Soy Broth (TSB) overnight. They were subsequently diluted 1:100 in sterile 0.9% sodium chloride solution (10 µL of bacteria plus 990 µL saline), followed by a further 1:10 dilution (0.3 mL plus 2.7 mL to achieve a final concentration of approximately 3–5 × 10⁶ Colony-Forming Units (CFU)/mL. Bone samples were submerged in 3 mL of the bacterial suspension for 10 seconds to simulate intraoperative contamination. Immediately thereafter, samples were transferred into 5 mL of the NCT test solution or sterile distilled water (control group). Incubation was performed for either 5 or 15 minutes at temperatures of 20 °C, 37 °C, or 45 °C using a calibrated thermostatted water bath (E 100 Ecoline, Staredition; Lauda, Königshofen, Germany). Following incubation, antimicrobial activity of NCT was stopped by immersing each sample in 2 mL of an inactivation buffer containing 1% methionine and 1% histidine in a sterile 15 mL Falcon® tube. To facilitate microbial detachment, samples were vortexed three times for 3 seconds each, followed by ultrasonic treatment for 1 minute in a water bath sonicator (Bandelin Sonorex RK 102H, 35 kHz, 120/480 W; Bandelin electronic, Berlin, Germany), and then vortexed again under the same conditions. The resulting supernatants were collected, and 50 µL aliquots were plated in duplicate onto Mueller-Hinton agar using an automated spiral plater (WASP 2; Don Whitley Scientific, Shipley, United Kingdom). Plates were incubated at 37 °C for 24 hours, with a detection threshold of 10 CFU/mL based on cumulative plating volume of both plates. In cases where no bacterial growth or very low CFU counts were observed, plates were incubated for up to five days to assess for the presence of bacteria bacteria attenuated but not killed by the treatment.
Contamination and Decontamination of ACL grafts with NCT
Bacterial suspensions for ACL graft contamination were prepared as described for the bone samples. C. albicans was cultured overnight and diluted 1:100 in sterile 0.9% sodium chloride solution to achieve a final concentration of 1 × 10⁵ CFU/mL. The ACL pieces were immersed for 10 seconds in 3 mL of the respective microbial suspension (bacterial or fungal) to simulate clinical contamination. Immediately following exposure, samples were transferred into 5 mL of either NCT test solution, 1% vancomycin (10 mg/mL), or sterile distilled water (control group). Incubation was performed for 5 or 15 minutes at either 20 °C or 37 °C. To inactivate residual antiseptic activity, samples were then immersed in 2 mL of an inactivation solution containing 1% methionine and 1% histidine, placed in 15 mL Falcon® sterile polypropylene tubes. Subsequent processing—including vortexing, ultrasonic treatment, and quantitative microbiological cultures were preformed as described above.
Statistics
All results are presented as mean values with standard deviations. Student’s unpaired t test for comparison of two groups and oneway analysis of variance (ANOVA) and Dunnett’s multiple comparisons tests for more than two groups were done for comparison of test samples and controls. P values < 0.05 were considered significant. Values of log10 reduction in CFU/mL were calculated by subtracting the values of the test samples from the values of controls. Standard deviations of these reduction values were calculated by the square root of (SD12/n1 + SD22/n2), where SD1 and n1 are the standard deviation and number of experiments of the test sample and SD2 and n2 are the standard deviation and number of experiments of the control sample.
Results
Decontamination of Bone Samples: In preliminary tests using S. aureus ATCC 6538, immersion of bone fragments for 10 seconds in suspensions containing 3–5 × 10⁵ CFU/mL yielded reproducible contamination levels of approximately 3–4 log10 CFU/mL following recovery via vortexing and ultrasonication in 0.9% saline. This protocol was subsequently applied to all experiments.
Activity Against Staphylococcus aureus: Incubation of contaminated hip samples in 1% NCT (55 mM) for 15 minutes at 20 °C resulted only in minimal bacterial reduction (<1 log10). At 37 °C, a significant decrease in S. aureus CFU/mL was observed compared to 0.9% NaCl controls (NCT: 1.38 ± 0.11 log10, Control:
3.06 ± 0.03 log10, p = 0.002) corresponding to a mean reduction of 1.68 ± 0.08 log10. In two pilot experiments with carpus samples, the reduction in CFU/mL at 37 °C in 1% NCT was lower with 0.40 ± 0.05 (p = 0.015). To increase the bactericidal activity, the concentration of NCT was elevated to 5% (275 mM) and the incubation temperature kept at 37 °C. Actually, there was no more difference between hip and carpus samples, and after 15 min incubation, a reduction of viable counts of S. aureus below the detection limit of 1 log10 could be reached (Figure 1). Killing was significant, too, already after 5 min incubation (Figure 1). The addition of 0.1% NH4Cl markedly enhanced the microbicidal efficacy of NCT. Even low concentrations of NCT (0.1%) in combination with 0.1% NH4Cl significantly reduced S. aureus viability (Figure 2). Higher concentrations of both agents correlated with increased bacterial killing. Near-complete eradication was achieved with 1% NCT + 0.1% NH4Cl after 15 minutes at 37 °C, while further elevation to 2% NCT enabled similar effects within 5 minutes. Complete eradication of S. aureus was consistently achieved using 5% NCT + 0.1% NH4Cl within 5 minutes at 37 °C (Figure 3).
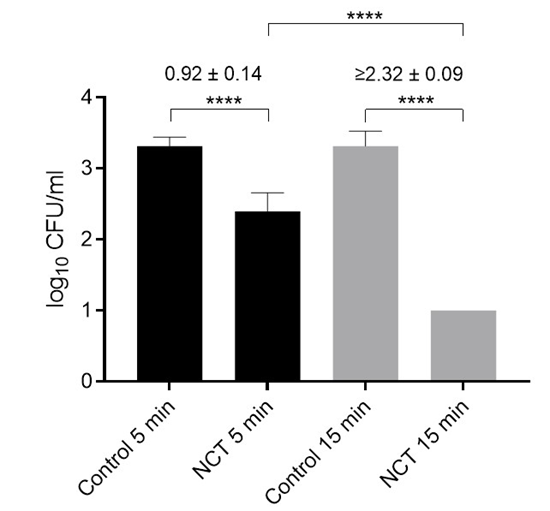
Figure 1: Viable counts of S. aureus ATCC 6538 in artificially contaminated bone samples after treatment with distilled water (controls) or 5% NCT for 5 and 15 min at 37 °C. Values of log10 reduction in CFU/mL compared to the controls are indicated. Mean values and SD of 4 independent experiments for 5 min (1 femoral head sample each, 3 carpus samples each) and of 6 independent experiments for 15 min (3 hip and carpus samples each). **** p < 0.0001 versus controls. Detection limit 1 log10 CFU/mL.
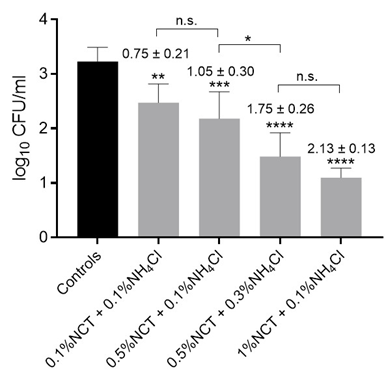
Figure 2: Viable counts of S. aureus ATCC 6538 in artificially contaminated bone samples after treatment with distilled water (controls) or different concentrations of NCT plus ammonium chloride (NH4Cl) for 15 min at 37 °C. Values of log10 reduction in CFU/mL compared to the controls are indicated. Mean values and SD of 3 independent experiments (12 summarized for controls). * p < 0.05; ** p < 0.01, *** p < 0.001, **** p < 0.0001 versus controls. n.s. not significant. Detection limit 1 log10 CFU/mL.
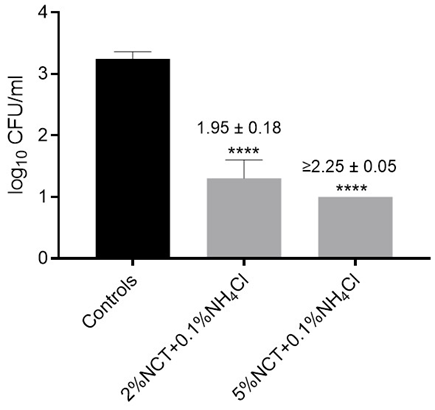
Figure 3: Viable counts of S. aureus ATCC 6538 in artificially contaminated bone samples after treatment with distilled water (controls) or 2% and 5% NCT each plus 0.1% ammonium chloride (NH4Cl) for 5 min at 37 °C. Values of log10 reduction in CFU/mL compared to the controls are indicated. Mean values and SD of 3 independent experiments (6 summarized for controls). **** p < 0.0001 versus controls. Detection limit 1 log10 CFU/mL.
Activity Against Escherichia coli: Testing with E. coli ATCC 11229 revealed an even higher susceptibility to NCT. Nearcomplete eradication was achieved with 1% NCT after 15 minutes at 37 °C. Full reduction to below the detection limit occurred with 2% NCT + 0.1% NH4Cl after 5 minutes under the same conditions (Figure 4).
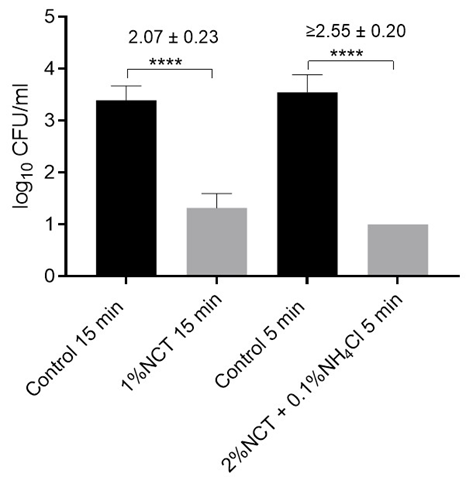
Figure 4: Viable counts of E. coli ATCC 11229 in artificially contaminated bone samples after treatment with distilled water (controls) or 1% NCT for 15 min or 2% NCT plus 0.1% ammonium chloride (NH4Cl) for 5 min at 37 °C. Values of log10 reduction in CFU/mL compared to the controls are indicated. Mean values and SD of 3 independent experiments. **** p < 0.0001 versus controls. Detection limit 1 log10 CFU/mL.
Impact of Temperature and Ultrasonication: To assess potential enhancement of NCT efficacy by thermal augmentation or mechanical disruption, additional experiments were conducted with S. aureus as the test organism. Treatment with 1% NCT at 45 °C for 5 minutes resulted in a non-significant reduction of 0.47 ± 0.17 log10 CFU/mL (p = 0.054), while 15 minutes of exposure yielded a significant reduction of 1.73 ± 0.36 log10 (p = 0.009). However, this was not statistically different from the results obtained at 37 °C. Ultrasonication (Bandelin Sonorex RK 102H, 35 kHz) applied during the 5-minute NCT incubation at 20 °C did not enhance antimicrobial activity (reduction: 0.06 ± 0.12 log10 CFU/ mL; p = 0.66), indicating no added benefit under these conditions.
Decontamination of Anterior Cruciate Ligament samples
Experiments using contaminated cruciate ligament fragments demonstrated a pattern of antimicrobial efficacy comparable to that observed in bone samples.
Activity Against Staphylococcus aureus: For S. aureus ATCC 6538, significant CFU/mL reductions were achieved with either 1% NCT for 15 minutes or 5% NCT for 5 minutes at 37 °C. Complete eradication to the detection limit was observed following exposure to 5% NCT for 15 minutes or with the addition of 0.1% ammonium chloride to NCT for 5 minutes at 37 °C (Figure 5A).
Activity Against Escherichia coli: For E. coli, susceptibility appeared slightly higher. A statistically significant reduction in CFU/mL was already observed with 1% NCT after 5 minutes, and complete eradication was achieved with 5% NCT after 5 minutes at 37 °C (Figure 5B).
Activity Against Candida albicans: In assays using C. albicans CBS 5982, 5% NCT in combination with 0.1% NH4Cl resulted in complete fungal eradication within 5 minutes at 37 °C. A significant but incomplete reduction in CFU was observed with 5% NCT alone, whereas 1% NCT had no measurable effect under identical conditions (Figure 5C).
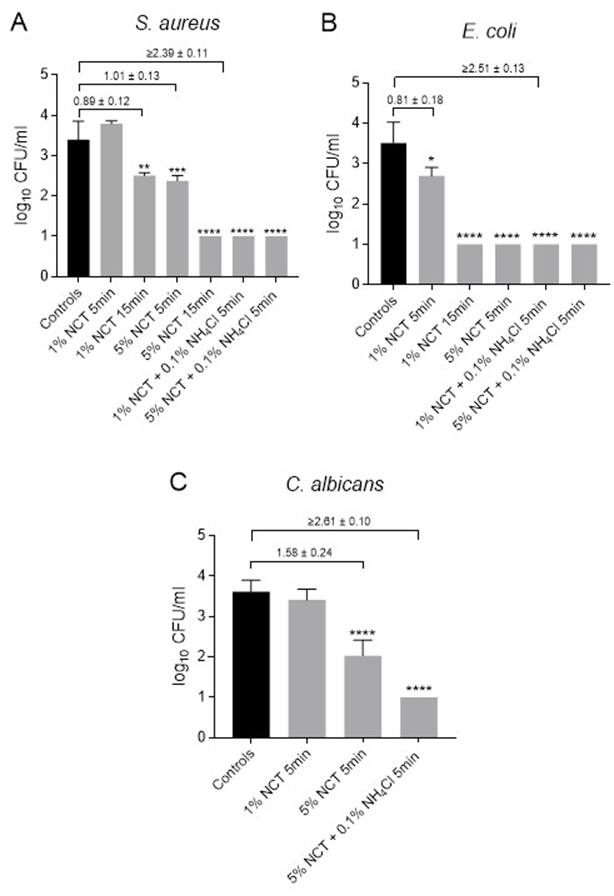
Figure 5: Viable counts of S. aureus ATCC 6538 (A), E. coli ATCC 11229 (B), and C. albicans CBS 5982 (C) in artificially contaminated anterior cruciate ligament samples after treatment with distilled water (controls) or 1% - 5% NCT or 1% - 5% NCT plus 0.1% ammonium chloride (NH4Cl) for 5 min or 15 min at 37 °C. Values of log10 reduction in CFU/mL compared to the controls are indicated. Mean values and SD of 3 independent experiments (9-18 summarized for controls). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 versus controls. Detection limit 1 log10 CFU/mL.
Impact of Temperature and Ultrasonication: Subsequent experiments were conducted at room temperature (20 °C) to simulate less favorable antimicrobial conditions. In this setting, 1% NCT combined with 0.1% NH4Cl achieved complete eradication of E. coli (≥2.44 ± 0.16 log10 reduction, p < 0.0001), but only a partial reduction in S. aureus (1.45 ± 0.10 log10, p < 0.001) after 5 minutes of incubation. However, treatment with 5% NCT plus 0.1% NH4Cl at 20 °C resulted in complete eradication of both S. aureus and C. albicans within 5 minutes.
Activity of Vancomycin
Vancomycin, used at a concentration of 1%, (10 mg/mL), completely eliminated S. aureus from ligament samples (≥3.00 ± 0.07 log10 reduction, p < 0.0001), as expected based on its antimicrobial spectrum. However, it had negligible effects on E. coli (0.30 ± 0.08 log10 reduction, p = 0.028) and C. albicans (0.16 ± 0.07 log10 reduction, p = 0.070), although the reduction for E. coli reached statistical significance (Student’s unpaired t-test, n = 3 for all comparisons). It is important to note that vancomycin is not inactivated by methionine/histidine neutralization. As a result, residual activity persisted after plating, effectively extending the exposure time to the complete eradication of S. aureus, even with only 5 minutes of nominal incubation.
Discussion
The most significant finding of this study is that NCT, particularly in combination with ammonium chloride, demonstrates high efficacy as a rapid, broad-spectrum antiseptic for the decontamination of human musculoskeletal grafts contaminated with pathogens relevant to Postoperative Septic Arthritis (PSA). In direct comparison to vancomycin, NCT exhibited a broader antimicrobial spectrum, effectively eliminating not only S. aureus but also E. coli and C. albicans. Current strategies for chemical graft decontamination typically rely on either antibiotics or antiseptics [11]. Antibiotics such as vancomycin are generally well tolerated and provide prolonged activity on graft surfaces but are limited by their narrow antimicrobial spectrum and potential for resistance development. In contrast, antiseptics offer rapid, broad-spectrum activity, encompassing bacteria, fungi, and viruses, with minimal risk of resistance. However, their use can be restricted by concerns over cytotoxicity and shorter residual activity.Vancomycin has gained popularity for intraoperative use in ACL reconstruction, where wrapping autografts in vancomycin-soaked sterile gauze has led to significant reductions in PSA [23,24]. Vancomycin exhibits limited cytotoxicity toward chondrocytes, with adverse effects only reported at supratherapeutic concentrations ≥5 mg/mL [25-26]. Antiseptics from the classes of tensides (e.g., chlorhexidine) and active halogen compounds (e.g., povidoneiodine) have demonstrated reliable efficacy in decontaminating grafts contaminated intraoperatively or dropped in the operating theatre environment [8-11,27-30]. However, concerns regarding their cytotoxicity persist, particularly with regard to their effects on cells and tissues essential for graft viability and integration. While in vitro cytotoxicity data commonly derived from monolayer cell cultures can overestimate clinical toxicity, especially in structured tissues, which often tolerate higher concentrations [31-32], the need for better-tolerated and equally effective alternatives remains justified. In this context, NCT emerges as a promising candidate for orthopaedic use, not only for graft decontamination but also for intraoperative field irrigation and wound management. As an endogenous compound with mild oxidative potential, NCT is well tolerated even at high concentrations (1%, 55 mM) in vivo, including on mucosal surfaces and in the respiratory tract [14,16].
Inhalation studies have shown that concentrations up to 5% can be administered with minimal irritation in animal models, supporting its safety profile for broader clinical application [33-34]. Nevertheless, the relatively lower reactivity of NCT compared to more aggressive antiseptics (e.g., iodine, hypochlorous acid) translates to slower microbicidal activity [14,35]. The present study specifically addressed this limitation by identifying optimized concentrations and synergistic formulations that allow for effective and rapid graft decontamination, such as NCT combined with ammonium chloride. No significant difference in the microbicidal efficacy of NCT was observed between bone and anterior cruciate ligament samples. This indicates that immersion-based application of NCT ensures effective penetration into various graft tissues. Consistent with previous reports, S. aureus and C. albicans exhibited slightly lower susceptibility to NCT compared to E. coli [14]. Bacteria are generally more susceptible than fungi to NCT, but killing of the latter is particularly enhanced in the presence of body fluids due to transhalogenation reactions (see below). This phenomenon may account for the similar susceptibility of S. aureus and C. albicans observed under the conditions of this study. Although 1% NCT (55 mM) is established as a standard concentration for the treatment of infections at various anatomical sites [16,18], it appears insufficient for rapid eradication of pathogens from musculoskeletal grafts ex vivo. Increasing the incubation temperature from 20 °C to 37 °C and elevating the NCT concentration to 5% (275 mM) resulted in a significant improvement in antimicrobial activity after 5 minutes; however, complete eradication was consistently achieved only after 15 minutes. Whether full bacterial eradication is essential for the prevention of postoperative septic arthritis remains uncertain. Beyond direct reduction of colony-forming units (CFU), postantiseptic effects, particularly relevant for mild oxidants like NCT, may contribute to clinical protection by inhibiting regrowth or reducing bacterial virulence following sublethal exposure [3638]. For example, a highly virulent strain of S. aureus treated with NCT for only one minute without a measurable reduction in viable cell counts was no longer capable of inducing lethal septicaemia in mice following intraperitoneal inoculation [38]. Nonetheless, irreversible inactivation of all contaminating pathogens within a short incubation period remains the clinical ideal. The present data show that moderate increases in incubation temperature to 45 °C and the application of mild ultrasonication had no additional microbicidal benefit. Given the potential risk of thermal or mechanical damage to graft integrity, further escalation of these physical measures is not advisable in clinical practice.In contrast, complete eradication of all tested microorganisms including S. aureus, E. coli, and C. albicans was consistently achieved through the addition of ammonium chloride (NH4Cl) to 5% NCT. This combination resulted in total microbial kill within 5 minutes, even at room temperature (20 °C). The enhanced efficacy can be attributed to the well-characterized chemical reaction of transhalogenation (or transchlorination), in which NCT transfers its active chlorine to NH4Cl, forming monochloramine (NH2Cl). This chloramine is more lipophilic and exhibits superior membrane penetration compared to NCT alone, thereby accelerating intracellular oxidative damage [14,16,37,39]. Importantly, this reaction mimics physiological processes occurring during natural inflammation, where neutrophil-derived NCT interacts with ammonium ions present in body fluids to generate chloramines in situ. Therefore, the addition of NH4Cl does not compromise the endogenous nature of the antiseptic formulation, maintaining its biocompatibility and physiological relevance [39-41]. While the addition of NH4Cl to NCT potentially increases the cytotoxic potential of the formulation slightly [34,42], this effect is mitigated by the rapid degradation of chloramines following graft implantation. Furthermore, clinical and preclinical data indicate that the combination of 0.1% NCT with 0.1% NH4Cl is well tolerated, even at sensitive anatomical sites like the human eye [43]. Additionally, recent biomechanical investigations confirm the safety of this antiseptic combination: neither 1–5% NCT nor 5% NCT with 0.1% NH4Cl affected maximal tendon load to failure or construct stiffness in treated ACL grafts during biomechanical testing [44]. From a cytological perspective, the susceptibility of chondrocytes and synoviocytes to NCT is comparable to that of other cell types in tissues where NCT has been safely applied [32,45], including the respiratory tract [34]. Furthermore, intra-articular administration of NCT has been evaluated in murine models of septic arthritis, where it was well tolerated and demonstrated therapeutic efficacy [4647]. These findings provide a strong foundation for future clinical translation, not only for intraoperative graft decontamination but also for potential therapeutic use in the topical treatment of infectious arthritis, an area that warrants further investigation. In considering the clinical application of NCT solutions for graft decontamination in routine practice, it is important to acknowledge the limitation of its chemical stability. 1% NCT solutions require refrigerated storage (2–8 °C) to maintain efficacy over extended periods of 6–12 months [17]. The addition of ammonium chloride, while enhancing microbicidal activity, further reduces solution stability [48]. This constraint is particularly relevant for higher NCT concentrations, where freshly prepared solutions from aliquoted and stored portions of the crystalline compound may be more practical. Therefore, the optimal balance between concentration, formulation, and shelf-life remains to be defined, and further work is required to identify the most clinically feasible and microbiologically effective preparations.
When comparing NCT to vancomycin, it is essential to consider their fundamentally different mechanisms and kinetics of action. Vancomycin exhibits selective activity predominantly against Gram-positive organisms, while NCT demonstrates a broader antimicrobial spectrum, including efficacy against Gram-negative bacteria and fungal species. Unlike vancomycin, which requires several hours to achieve substantial bactericidal effects [49], NCT acts rapidly, with antimicrobial activity evident within minutes. The prolonged activity of vancomycin observed in the present experimental setup can therefore be attributed to its chemical stability and resistance to inactivation by neutralizing agents such as methionine or histidine. Consequently, its antimicrobial effect extended beyond the nominal 5-minute incubation period and continued throughout the plating and incubation phases. This sustained presence may more accurately reflect in vivo conditions, where tissue and graft exposure to vancomycin often persists over extended periods. The experimental design was therefore intentionally selected to simulate such clinical scenarios, particularly in the context of graft decontamination protocols. Nevertheless, there is limited evidence regarding the persistence of vancomycin on ACL grafts following implantation and subsequent continuous irrigation or joint lavage. In summary, NCT demonstrated potent bactericidal and fungicidal activity in vitro against clinically relevant pathogens in artificially contaminated human bone and cruciate ligament grafts. At a high concentration of 5%, and particularly when combined with 0.1% ammonium chloride, complete eradication of S. aureus, E. coli, and C. albicans was consistently achieved within 5 minutes of exposure. These findings support the potential of NCT as an effective, endogenous, and well-tolerated antiseptic for the rapid decontamination of biologically sensitive graft tissues. Moreover, this data may pave the way for further exploration of NCT in orthopaedics as a safe, endogenous-based alternative for broader intraoperative antiseptic use. Further translational and clinical studies are needed to confirm its efficacy, safety, and practicality in the orthopaedic surgical setting.
Conclusion
N-Chlorotaurine (NCT), particularly in combination with ammonium chloride, is highly effective as a rapid and broadspectrum antiseptic for human graft decontamination. Compared to vancomycin, NCT offers a broader antimicrobial spectrum, effectively eliminating Gram-positive bacteria, Gram-negative organisms, and fungal species. These findings support the potential of NCT as an effective, endogenous, and well-tolerated antiseptic for the rapid decontamination of biologically sensitive graft tissues.
Author Contributions: Conceptualization, A.R., R.A., M.N., W.S.; methodology, M.N., A.R.; validation, M.N., ; formal analysis, V.L., M.N.; investigation, V.L., P.K., A.M., F.S., A.L.; resources, M.N., R.A. ; data curation, M.N., V.L.; writing—original draft preparation, V.L., M.N., A.R.; writing—review and editing, M.N., A.R., P.K., R.A., F.S., W.S., M.W., A.L.; visualization, V.L., M.N.; supervision, M.N., A.R., R.A.; project administration, M.W., M.N., R.A. All authors have read and agreed to the published version of the manuscript.
Funding: This research received external public funding by the “Tyrolean Science Fund” (“Tiroler Wissenschaftsförderung”); contract number: F.18814.
Ethics statement: The research was conducted in accordance with the Declaration of Helsinki Ethics Committee of the Medical University of Innsbruck, approval number 1065/2021, 26.5.2021. Patients provided written informed consent to the use of the bone and cruciate ligament samples.
Acknowledgments: We are grateful to Andrea Windisch, Institute of Hygiene and Medical Microbiology, Medical University of Innsbruck, for excellent technical assistance with antimicrobial efficacy testing of NCT.
Conflict of Interest: The authors declare no conflict of interest.
Data Availability Statement: The data underlying this article are available in the article.
References
- Kapadia BH, McElroy MJ, Issa K, Johnson AJ, Bozic KJ, et al. (2014) The economic impact of periprosthetic infections following total knee arthroplasty at a specialized tertiary-care center. J Arthroplasty 29: 929-932.
- Lenguerrand E, Whitehouse MR, Beswick AD, Kunutsor SK, Foguet P, et al. (2019) National Joint Registry for England WNI and the Isle of M. Risk factors associated with revision for prosthetic joint infection following knee replacement: an observational cohort study from England and Wales. The Lancet. Infectious diseases 19: 589-600.
- Mouzopoulos G, Fotopoulos VC and Tzurbakis M, et al. (2009) Septic knee arthritis following ACL reconstruction: a systematic review. Knee Surg Sports Traumatol Arthrosc 17: 1033-1042.
- Schuster P, Schlumberger M, Mayer P, Eichinger M, Geßlein M, et al. (2020) Soaking of the graft in vancomycin dramatically reduces the incidence of postoperative septic arthritis after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 28: 2587-2591.
- Schuster P, Schulz M, Immendoerfer M, Mayer P, Schlumberger M, et al. (2015) Septic Arthritis After Arthroscopic Anterior Cruciate Ligament Reconstruction: Evaluation of an Arthroscopic Graft-Retaining Treatment Protocol. Am J Sports Med 43: 3005-3012.
- Boddapati V, Fu MC, Mayman DJ, Su EP, Sculco PK, et al. (2018) Revision Total Knee Arthroplasty for Periprosthetic Joint Infection Is Associated With Increased Postoperative Morbidity and Mortality Relative to Noninfectious Revisions. J Arthroplasty 33: 521-526.
- Lum ZC, Natsuhara KM, Shelton TJ, Giordani M, Pereira GC, et al. (2018) ortality During Total Knee Periprosthetic Joint Infection. J Arthroplasty 33: 3783-3788.
- Bruce B, Sheibani-Rad S, Appleyard D, Calfee RP, Reinert SE, et al. (2011) Are dropped osteoarticular bone fragments safely reimplantable in vivo? J Bone Joint Surg Am 93: 430-438.
- Saegeman VS, Ectors NL, Lismont D, Verduyckt B, Verhaegen J (2009) Effectiveness of antibiotics and antiseptics on coagulasenegative staphylococci for the decontamination of bone allografts. Eur J Clin Microbiol Infect Dis 28: 813-816.
- A, Hazirolan G, Çelen ZE, Köse CC, Özkurt B (2024) What if an articular bone fragment drops on the floor in the course of osteosynthesis? An experimental study. Jt Dis Relat Surg 35: 209-217.
- Mortazavi SMJ, Ghasemi MA, Khan FMY, Zarei M, Shahabinezhad A (2021) Contamination and Decontamination of Autologous Bone in the Operating Room: A Systematic Review. J Orthop Trauma 35: 65-70.
- Perez-Prieto D, Portillo ME, Torres-Claramunt R, Pelfort X, Hinarejos P, et al. (2018) Contamination occurs during ACL graft harvesting and manipulation, but it can be easily eradicated. Knee Surg Sports Traumatol Arthrosc 26: 558-562.
- Schuttler KF, Scharm A, Stein T, Heyse TJ, Lohoff M, et al. (2019) Biomechanical and microbiological effects of local vancomycin in anterior cruciate ligament (ACL) reconstruction: a porcine tendon model. Arch Orthop Trauma Surg 139: 73-78.
- Gottardi W, Nagl M (2010) N-chlorotaurine, a natural antiseptic with outstanding tolerability. Journal of Antimicrobial Chemotherapy 65: 399-409.
- Kim SH, Yum HW, Kim SH, Kim W, Kim SJ, et al. (2021) Protective Effects of Taurine Chloramine on Experimentally Induced Colitis: NFκB, STAT3, and Nrf2 as Potential Targets. Antioxidants (Basel) 10: 479.
- Marcinkiewicz J, Nagl M, Kyriakopoulos A, Walczewska M, Skóra M, et al. (2022) Current Opinion on the Therapeutic Capacity of TaurineContaining Halogen Derivatives in Infectious and Inflammatory Diseases. In: Schaffer SW, El Idrissi A and Murakami S. Taurine 12: A Conditionally Essential Amino Acid, Adv Exp Med Biol. Springer International Publishing. Cham 2022: 83-98.
- Staudinger GJ, Thomas ZM, Hooper SE, Williams JF, Robins LI (2024) Long-Term Stability and Efficacy of NCT Solutions. International Journal of Molecular Sciences 25: 8745.
- Murashevych B, Bilenkyi G, Girenko D, Bilenkyi E (2024) N-Chlorotaurine Solutions as Agents for Infusion Detoxification Therapy: Preclinical Studies. International Journal of Molecular Sciences 25: 8345.
- Murashevych B, Girenko D, Koshova I, Maslak G, Burmistrov K, et al. (2024) Broad-Purpose Solutions of N-Chlorotaurine: A Convenient Synthetic Approach and Comparative Evaluation of Stability and Antimicrobial Activity. Journal of Chemistry 2024: 8959915.
- Gottardi W, Nagl M (2013) Active halogen compounds and proteinaceous material: loss of activity of topical antiinfectives by halogen consumption. J. Pharm. Pharmacol 65: 213-218.
- Gottardi W, Klotz S, Nagl M (2014) Superior bactericidal activity of N-bromine compounds compared to their N-chlorine analogues can be reversed under protein load. J. Appl. Microbiol 116: 1427-1437.
- Gruber M, Moser I, Nagl M, Lackner M (2017) Bactericidal and fungicidal activity of N-chlorotaurine is enhanced in cystic fibrosis sputum medium. Antimicrobial Agents and Chemotherapy 61: 1-10.
- Schuster P, Schlumberger M, Mayer P, Eichinger M, Gesslein M, et al. (2020) Soaking of the graft in vancomycin dramatically reduces the incidence of postoperative septic arthritis after anterior cruciate ligament reconstruction. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA 28: 2587-2591.
- Naendrup JH, Marche B, de Sa D, Koenen P, Otchwemah R, et al. (2020) Vancomycin-soaking of the graft reduces the incidence of septic arthritis following ACL reconstruction: results of a systematic review and meta-analysis. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA 28: 1005-1013.
- Grayson JE, Grant GD, Dukie S and Vertullo CJ (2011) The in vitro elution characteristics of vancomycin from tendons. Clinical orthopaedics and related research 469: 2948-2952.
- Shaw KA, Eichinger JK, Nadig N and Parada SA (2018) In Vitro Effect of Vancomycin on the Viability of Articular Chondrocytes. Journal of orthopaedic trauma 32: 148-153.
- Badran MA, Moemen DM. Hamstring graft bacterial contamination during anterior cruciate ligament reconstruction: clinical and microbiological study. Int Orthop 40: 1899-1903.
- Barbier O, Danis J, Versier G, Ollat D. When the tendon autograft is dropped accidently on the floor: A study about bacterial contamination and antiseptic efficacy. Knee 22: 380-383.
- Molina ME, Nonweiller DE, Evans JA, Delee JC (2000) Contaminated anterior cruciate ligament grafts: the efficacy of 3 sterilization agents. Arthroscopy 16: 373-378.
- Plante MJ, Li X, Scully G, Brown MA, Busconi BD, et al. (2013) Evaluation of sterilization methods following contamination of hamstring autograft during anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 21: 696-701.
- Nagl M, Nguyen VA, Gottardi W, Ulmer H, Höpfl R (2003) Tolerability and efficacy of N-chlorotaurine compared to chloramine T for treatment of chronic leg ulcers with purulent coating. British Journal of Dermatology 149: 590-597.
- Pilz M, Staats K, Assadian O, Windhager R, Holinka J (2024) Tolerability of N-chlorotaurine in comparison with routinely used antiseptics: an in vitro study on chondrocytes. Pharmacol Rep 76: 878-886.
- Arnitz R, Stein M, Bauer P, Lanthaler B, Jamnig H, et al. (2018) Tolerability of inhaled N-chlorotaurine in humans – a doubleblind randomized phase I clinical study. Therapeutic Advances in Respiratory Disease 12: 1-14.
- Geiger R, Treml B, Pinna A, Barnickel L, Prossliner H, et al. (2009) Tolerability of inhaled N-chlorotaurine in the pig model. BMC Pulmonary Medicine 9: 33.
- Hacioglu M, Oyardi O, Yilmaz F, Nagl M (2022) Comparative fungicidal activities of N-chlorotaurine and other conventional antiseptics against Candida spp. isolated from vulvovaginal candidiasis. Journal of Fungi 8: 682.
- Fuursted K, Hjort A, Knudsen L (1997) Evaluation of bactericidal activity and lag of regrowth (postantibiotic effect) of five antiseptics on nine bacterial pathogens. J. Antimicrob. Chemother 40: 221-226.
- Lackner M, Binder U, Reindl M, Gönül B, Fankhauser H, et al. (2015) N-chlorotaurine exhibits fungicidal activity against therapy-refractory Scedosporium species and Lomentospora prolificans. Antimicrobial Agents and Chemotherapy. 2015; 59: 6454-6462.
- Nagl M, Hengster P, Semenitz E, Gottardi W (1999) The postantibiotic effect of N-chlorotaurine on Staphylococcus aureus. Application in the mouse peritonitis model. J. Antimicrob. Chemother 43: 805-809.
- Grisham MB, Jefferson MM, Melton DF, Thomas EL (1984) Chlorination of endogenous amines by isolated neutrophils. Ammonia-dependent bactericidal, cytotoxic, and cytolytic activities of the chloramines. J Biol Chem 259: 10404-10413.
- Thomas EL, Grisham MB, Jefferson MM (1986) Preparation and characterization of chloramines. Methods Enzymol 132: 569-585.
- Weiss SJ (1989) Tissue destruction by neutrophils. N. Engl. J. Med 320: 365-376.
- Thomas EL, Grisham MB, Jefferson MM (1986) Cytotoxicity of chloramines. Methods Enzymol 132: 585-593.
- Teuchner B, Schmid E, Ulmer H, Gottardi W, Nagl M (2008) Tolerability of N-chlorotaurine plus ammonium chloride in the rabbit and human eye - a phase 1 clinical study. Graefe’s Archive for Clinical and Experimental Ophthalmology 246: 1723-1730.
- Runer A, Schneider F, Wawer K, Gruber K, Arora R, et al. (2025) N-chlorotaurine does not alter structural tendon properties: a comparative biomechanical study. Arch Orthop Trauma Surg 145: 223.
- Kontny E, Chorazy-Massalska M, Rudnicka W, Marcinkiewicz J, Maslinski W (2007) Comparison of taurine chloramine and taurine bromamine effects on rheumatoid arthritis synoviocytes. Amino Acids 32: 447-452.
- Marcinkiewicz J, Kontny E (2014) Taurine and inflammatory diseases. Amino Acids 46: 7-20.
- Verdrengh M, Tarkowski A (2005) Inhibition of septic arthritis by local administration of taurine chloramine, a product of activated neutrophils. J. Rheumatol 32: 1513-1517.
- Gottardi W, Arnitz R, Nagl M (2007) N-chlorotaurine and ammonium chloride: an antiseptic preparation with strong bactericidal activity. International Journal of Pharmaceutics 335: 32-40.
- Haworth CS, Sobieski MW, Scheld WM, Park TS (1990) Staphylococcus aureus ventriculitis treated with single-dose intraventricular vancomycin or daptomycin (LY146032): bacterial and antibiotic kinetics in hydrocephalic rabbits. Antimicrob. Agents Chemother 34: 245-251.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.