Multiple Mucosal Bleedings in a Patient Receiving Nintedanib
by Silvia Salvatori1,2, Carmelina Petruzziello1, Salvatore Cocola1, Irene Marafini1,2, Giovanni Monteleone1,2
1Gastroenterology Unit, Policlinico Universitario Tor Vergata, Rome, Italy
2Department of Systems Medicine, University of Rome Tor Vergata, Italy
*Corresponding author: Giovanni Monteleone, Department of Systems Medicine, University of Rome “TOR VERGATA”, Via Montpellier, 1 - 00133 Rome, Italy
Received Date: 05 June 2025
Accepted Date: 09 June 2025
Published Date: 11 June 2025
Citation: Salvatori S, Petruzziello C, Cocola S, Marafini I, Monteleone G (2025) Multiple Mucosal Bleedings in a Patient Receiving Nintedanib. 10: 2317. https://doi.org/10.29011/2574-7754.102317
Abstract
Nintedanib, an inhibitor of growth factor receptors, is recommended by current international guidelines for the treatment of idiopathic pulmonary fibrosis (IPF), a disabling disease characterized by a progressive development of fibrosis and loss of lung function. Unfortunately, however, the use of nintedanib can be accompanied by the occurrence of several adverse events, which can lead to drug discontinuation.
This case report presents a patient receiving nintedanib (300 mg/day) for IPF and initially developing epistaxis requiring a reduction of the drug dosage (200 mg/day). A couple of months later, the patient was admitted to the hospital for a single episode of melena, due to a duodenal ulcer, which was successfully treated with injective and thermal therapy, followed by proton pump inhibitors. A couple of days later the patient experienced several episodes of rectal bleeding, and a colonoscopy evidenced diffuse mucosal congestion, fragility, and bleeding on contact with the instrument. Histologically, there was no evidence for inflammatory bowel disease. Systemic steroids and a reduction of nintedanib dosage (100 mg/day) led to a fast resolution of the rectal bleeding. This highlights the need for careful monitoring of IPF patients on nintedanib due to the risk of developing multiple mucosal bleedings.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic and disabling disease of unknown etiology, which usually manifests in the sixth or seventh decade of life, and is characterized by a progressive alveolar epithelial cell injury and dysregulated repair. Pathogenically, IPF is marked by an uncontrolled proliferation of lung fibroblasts, which differentiate into myofibroblasts thus leading to an excessive synthesis of extracellular matrix (ECM) proteins in the interstitial space and progressive development of fibrosis and loss of lung function [1].
Nintedanib is a small molecule that targets multiple tyrosine kinases and inhibits fibroblast growth factor receptor (FGFR)-1, vascular endothelial growth factor receptor (VEGFR)-2, and platelet-derived growth factor receptors (PDGFR)-α and β [2]. Although nintedanib was originally designed for cancer treatment, it displays antifibrotic activity and, therefore, has been later on used in the management of IPF [1,3]. In the phase 3 trials (INPULSIS-1 and 2), nintedanib at 300 mg/day significantly reduced the annual rate of decline in forced vital capacity and acute exacerbations compared with placebo, determining a slowing of disease progression4. In the same trials, gastrointestinal disorders were the most frequent adverse events in the nintedanib group with diarrhea being the most frequently reported [4]. Here we describe a patient who experienced multiple mucosal bleedings while receiving nintedanib for IPF.
Case Report
A 81-year-old Caucasian man, with benign prostatic hypertrophy, a previous cholecystectomy for symptomatic lithiasis, Helicobacter pylori-induced gastritis treated with success with quadruple therapy, and IPF diagnosed in 2024 by high-resolution chest computed tomography and bronchoscopy, complicated by a left-sided shift of mediastinal structures, and treated with nintedanib (200 mg/day) was admitted to the Emergency Room at the University Hospital Tor Vergata of Rome (Italy) for a recent and single episode of melena, nausea, and vomiting. Nintedanib therapy was started in October 2024 at 300 mg a day, but it was reduced to 200 mg a day within 3 months from the start of treatment for the occurrence of epistaxis. In the last 3 months, the patient has had several episodes of abdominal pain, for which he underwent a Virtual Colonoscopy that resulted negative.
At the entry (May 2025), the patient had severe anemia (hemoglobin 7.3 g/dl). Since a direct and indirect Coomb test was positive, he was transfused with concentrated red blood cells and treated with intravenous methylprednisolone 1 mg/kg/day. He was taking neither anti-coagulants nor nonsteroidal anti-inflammatory drugs. An upper endoscopy showed a Forrest IIa ulcer of the duodenal bulb, which was successfully treated with injective and thermal therapy, followed by proton pump inhibitor therapy in continuous infusion (8 mg/hour) for 72 hours.
Physical examination findings and vital signs were unremarkable. No further significant abnormalities were noted on physical exam and lab tests. Specifically, PT, PTT, fibrinogen, and thrombin time were normal. No pathogens were detected in the stool culture. During the hospitalization, the patient experienced abdominal pain and several episodes of rectal bleeding but not diarrhea. Ileocolonoscopy showed diffuse mucosal congestion, fragility, and bleeding on contact with the instrument but no ulcers/erosions. Histological evaluation of biopsy colonic samples showed a nonspecific inflammation in the lamina propria with a preserved glandular profile while in the rectal biopsy samples, there were some atrophic and dysmorphic glands (Figure 1).
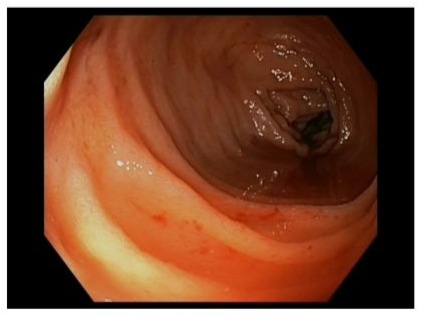
Figure 1: Endoscopic picture showing colonic mucosa characterized by diffuse congestion and fragility, and blood into the lumen.
One week later, while the patient was on methylprednisolone (50 mg/day orally), we documented a partial improvement of the rectal bleeding and resolution of the other gastrointestinal symptoms. Therefore, nintedanib dosage was further reduced to 100 mg/day. Ten days later, the patient achieved clinical remission. Discussion IPF is a chronic and ultimately fatal disease, with a median survival of only 2-3 years after diagnosis [5]. The cause of IPF is unknown, but several environmental factors (e.g. cigarette smoking, wood dust, metals, biological pollutants, and infectious agents) have been involved in IPF development [6]. Additionally, an increased risk of IPF has been reported in patients with obstructive sleep apnea and patients with diabetes mellitus [7-8]. Due to the progressive accumulation of fibrosis in the third space, the first manifestation of the disease often involves a respiratory gas exchange disorder between the lungs and the blood, detectable by assessing the reduced diffusing capacity of the lungs for carbon monoxide (DLCO) [9]. Optimal management of patients with IPF comprises maintaining physical fitness by practicing physical activity, ensuring adequate nutritional status, and appropriate treatment of comorbidities (e.g. obstructive sleep apnoea, pulmonary hypertension) [9]. The IPF-associated fibrogenesis is not therapeutically revertible but advances in IPF pathogenesis have facilitated the advent of novel and promising therapeutics. One such drug is nintedanib, an antifibrotic compound binding growth factor receptors that have been implicated in the progression of IPF. Additionally, nintedanib has been approved for the treatment of interstitial lung diseases (ILDs) associated with systemic sclerosis, and for progressive fibrosing ILDs [10,11]. Since the registrative studies, nintedanib has been associated with the occurrence of several adverse events, including cardiovascular and gastrointestinal disorders, which can lead to discontinuation of the study medication [4]. The case report illustrated in this article supports and expands on these data and shows that nintedanib treatment can cause various forms of mucosal bleeding that occur at different time points from the start of the treatment. Specifically, the patient experienced initially epistaxis, and this is in line with post-marketing data indicating that epistaxis is the most frequent bleeding adverse event in nintedanib-treated patients [12]. Despite a reduction of the drug dosage from 300 to 200 mg/day, which was accompanied by a persistent control of the epistaxis, a couple of months later the patient experienced melena due to a duodenal ulcer and later rectal bleeding due to mild-to-moderate colitis, the later requiring systemic steroids and further reduction of nintedanib dosage. In our patient, the absence of colonic erosions/ulcers and the histologic findings of the mucosal specimens excluded the diagnosis of IBD, and this is in line with data published by other authors. For instance, Odah and colleagues described 3 IPF patients on nintedanib and complaining of diarrhoea and other gastrointestinal symptoms associated with mild-to-moderate or no endoscopic evidence of colonic lesions. In all 3 cases, nintedanib discontinuation, together with steroid treatment, was needed to obtain a complete resolution of symptoms [13]. Miyaguchi and collaborators described one IPF patient developing proctitis and diarrhoea while taking nintedanib (300 mg/day). The reduction of nintedanib to 100 mg a day was accompanied by improvement of the patient’s symptoms and colonoscopy findings [14].
The mechanism by which nintedanib induces mucosal damage remains unclear. One hypothesis is that the degradation products of the drug can directly elicit gastrointestinal mucosal damage [15]. Another, and perhaps more plausible possibility, is that the blockade of growth factor receptors (e.g. VEGFR) can promote mucosal damage and be associated with significant bleeding risks [16].
Conclusion
In conclusion, the clinical case described in this article supports data indicating the increased risk of bleeding in patients on nintedanib and suggests the possibility that various forms of mucosal bleeding can occur even in the same patient at different time points and under distinct dosages. Patients on nintedanib must be closely monitored for bleeding, and limit risk factors during treatment. Further and long-term studies are needed to ascertain whether patients who cannot discontinue nintedanib and experience peptic ulcer-related bleeding or rectal bleeding may benefit from the use of proton pump inhibitors or topically-acting steroids, respectively.
References
- Wollin L, Wex E, Pautsch A, Schnapp G, Hostettler KE, et al. (2015). Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J. 45: 1434-1445.
- Wind S, Schmid U, Freiwald M, Marzin K, Lotz R, et al. (2019). Clinical Pharmacokinetics and Pharmacodynamics of Nintedanib. Clin Pharmacokinet. 58: 1131-1147.
- Vancheri C, Failla M, Crimi N, Raghu G. (2010). Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J. 35: 496-504.
- Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, et al. (2014). Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 370: 2071-2082.
- Maher TM, Bendstrup E, Dron L, Langley J, Smith G, et al. (2021). Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir Res. J22: 197.
- Wuyts WA, Wijsenbeek M, Bondue B, Bouros D, Bresser P, et al. (2020). Idiopathic pulmonary fibrosis: best practice in monitoring and managing a relentless fibrotic disease. Respiration. 99: 73-82.
- Schiza SE, Bouloukaki I, Bolaki M, Antoniou KM. (2020). Obstructive sleep apnea in pulmonary fibrosis. Curr Opin Pulm Med. 26: 443-448.
- Wang D, Ma Y, Tong X, Zhang Y, Fan H. (2020). Diabetes mellitus contributes to idiopathic pulmonary fibrosis: a review from clinical appearance to possible pathogenesis. Front Public Health. 8: 196.
- Muri J, Durcová B, Slivka R, Vrbenská A, Makovická M, et al. (2024). Idiopathic Pulmonary Fibrosis: Review of Current Knowledge. Physiol Res. 73: 487-497.
- Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, et al. (2019). Nintedanib for Systemic Sclerosis-Associated Interstitial Lung Disease. N Engl J Med. 380: 2518-2528.
- Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, et al. (2019). Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med. 381: 1718-1727.
- Chaudhuri N, Azuma A, Sroka-Saidi K, Erhardt E, Ritter I, et al. (2024). Safety and Tolerability of Nintedanib in Patients with Fibrosing Interstitial Lung Diseases: Post-marketing Data. Adv Ther. 41: 4581-4590.
- Odah T, Karime C, Hashash JG, Farraye FA. (2024). Nintedanib-Induced Colitis Can Mimic Inflammatory Bowel Disease. ACG Case Rep J. 11: e01282.
- Miyaguchi K, Tsuzuki Y, Uemuara H, Ishizawa K, Shinomiya S, et al. (2024). Nintedanib-associated enterocolitis with intractable diarrhea: a case report. Clin J Gastroenterol. 17: 271-275.
- Kato M, Sasaki S, Nakamura T, Kurokawa K, Yamada T, et al. (2019). Gastrointestinal adverse effects of nintedanib and the associated risk factors in patients with idiopathic pulmonary fibrosis. Sci Rep. 9: 12062.
- Das A, Mahapatra S, Bandyopadhyay D, Samantha S, Chakraborty S, et al. (2021). Bleeding with vascular endothelial growth factor tyrosine kinase inhibitor: a network meta-analysis. Crit Rev Oncol Hematol. 157: 103186.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.