Metabolic Surgery in South Africa: Baseline Demographic Description and Three-Year Outcomes for Patients Receiving GBP and BPD-DS Surgeries
by Fiona Anastasia van Vollenstee1,2*, Gregory Ronald Tintinger2, Maria-Teresa van der Merwe1,2
1The Centre of Excellence of the South African Society for Surgery, Obesity and Metabolism at the Netcare Waterfall City Hospital Unit, Waterfall City, Johannesburg, South Africa
2Department of Internal Medicine, Steve Biko Academic Hospital, Faculty of Health Sciences, School of Medicine, University of Pretoria, Pretoria, South Africa
*Corresponding author: Fiona A van Vollenstee, Department of Internal Medicine, Steve Biko Academic Hospital, Faculty of Health Sciences, School of Medicine, University of Pretoria, Pretoria, South Africa
Received Date: 21 October 2024
Accepted Date: 02 November 2024
Published Date: 05 November 2024
Citation: van Vollenstee FA, Tintinger GR, van der Merwe MT (2024) Metabolic Surgery in South Africa: Baseline Demographic Description and Three-Year Outcomes for Patients Receiving GBP and BPD-DS Surgeries. J Surg 9: 11175 https://doi.org/10.29011/2575-9760.11175
Abstract
Background: Considering increasing rates of obesity and patients undergoing metabolic surgery, Southern Africa data concerning outcomes are limited. SASSO routinely collects baseline and follow-up data from patients undergoing metabolic surgery procedures and aim to compare data between patients receiving either a GBP or BPD/DS surgery, at baseline and at 3-year follow-up and provide possible future insight in individualized patient surgery selections.
Methods: Retrospective analysis was conducted of available data including clinical and biochemical parameters, during a five-year period, pre-COVID-19 pandemic timeframe. Cohorts were stratified according to type of surgery, presence of T2DM and sex and compared between baseline and 3-year post surgery follow-up.
Results: Loss to follow-up in three years were 75%. Overall weight loss was higher in the BPD/DS vs GPB group (47% vs 28%). Overall reduction in co-morbidities for BPD/DS surgery was demonstrated, however the BPD/DS cohort showed a greater reduction overall and significantly lowered Fasting-Glucose (F-Glucose). Direct correlation was observed between clinical parameters (weight, BMI, waist-, neck- and hip circumference) and number of co-morbidities. BPD/DS cohort presented with a greater reduction and remission in co-morbidities, irrespective of glycemic control and gender.
Conclusion: Demographic landscape of obesity and associated comorbidities especially T2DM is high in South Africa. Metabolic surgery is a solution to improve patient outcomes and curb these costs. SASSO presents demographic data that could be used to assist in assigning a specific surgery type, on an individualized basis, to improve outcomes including sustained remission of co-morbidities.
Highlights
1) Surgery type can be assigned to improve desired patient outcomes
2) Sex and T2DM have an effect on assigning surgery type
3) Other comorbidities present at baseline might affect surgery selection
Introduction
The increase in Non-Communicable Diseases (NCDs) in South Africa is of growing concern. Obesity prevalence prediction rates for the population by 2025 are high for both females (47.7%) and males (25.6%) [1]. Rich diversity in ethnicity, with variation in nutritional habits and transition to Western type diets, contributes to these predictions and adds to overall increased obesity rates [2,3]. Considering sociopolitical factors and resource availability, South Africa has a unique demographic profiling for obesity prevalence, as well as state and private funders resource availability for the management of the disease and associated co-morbidities [4]. In 2019, The Fourth IFSO Global Registry Report described amalgamated metabolic surgery data from 51 countries including 394 431 patients with surgeries performed from 2014 onward[5], however data from sub-Saharan Africa, and even the African continent is sparce. The Centre of Excellence of the South African Society for Surgery, Obesity and Metabolism (SASSO) performs more than 65% of all metabolic surgeries in the country [4] and routinely collects data concerning baseline patient characteristics, clinical outcomes, morbidity and mortality associated with metabolic surgeries performed. Despite existing high-volume centers in other countries like Egypt, large scale published outcome data has to emerge, making the data from our unit in Gauteng, with current experience of over 3500 surgeries in advanced techniques such as laparoscopic Roux-en-Y Gastric Bypass (GBP), Single Anastomosis Duodenal-Ileal Bypass With Sleeve (SADI-S) and Biliopancreatic Diversion with Duodenal Switch (BPD/DS) over a period of 10 years, valuable to report on.
Obesity is associated with many co-morbidities including Type 2 Diabetes Mellitus (T2DM), cardiovascular disease, psychiatric conditions, muscle-skeletal disorders, metabolic disease syndromes and obstructive sleep apnea, where the interrelationships also fuel the progression states of diseases. T2DM considered an epidemic on its own, is considered a chronic metabolic disease associated with its own complications, morbidities, and mortality. Correlations between T2DM and other comorbidities including hypertension, dyslipidemia [6,7], and NASH have been described [6,8-9]. Resolution of these co-morbidities have been demonstrated after metabolic surgery irrespective of preoperative Body Mass Index (BMI) [10]. The type of surgery performed, however, offers different dynamics, both in terms of the anatomical, physiological and hormonal alterations which could result in differences in clinical outcomes, post-surgical required care, as well as differing rates of resolution of co-morbidities. Defining predictive clinical parameters, especially the use of baseline BMI for assigning specific procedures or surgery type, will be ever changing, as more scientific information becomes available [11]. Comparing baseline and clinical outcomes of different types of surgeries will continue to enhance our understanding of this difficult scientific field. Current literature supports the use of metabolic surgery as a successful intervention for one of the costliest complications of obesity, T2DM [12,13]. The associated healthcare cost has escalated globally [14,15]. Currently, 463 million adults (20-79 years) have been diagnosed with diabetes, with projections reaching up to 700 million by 2045, with 79% of cases being reported from lower income countries, like South Africa and 50% of cases remaining undiagnosed, highlighting the expected current and future health economic burden of this co-morbidity on an already challenged healthcare system [16].
The aim of this study was to perform a direct comparison of clinical and biochemical parameters, nutritional status and comorbidity resolution between patients receiving either a GBP or BPD/DS surgery, at baseline and at 3-year follow-up. Despite the baseline demographic profile, including nutritional wellbeing, together with expected resolution of T2DM during long term follow up, will become paramount in the future selection of the type of metabolic surgery offered to the patients as part of an individualized or patient-centered approach.
Methods
Retrospective analysis was conducted of available data collected, during a 5-year interval period before the Covid-19 pandemic commenced, from the patients undergoing either GBP or BPD/ DS surgery at the center. Prior written informed consent was obtained from all patients. Ethical approval was obtained from Netcare Limited Committee and Health Sciences Research Ethics Committee of the University of Pretoria (205/2018). Available clinical records, blood biochemistry results and radiological investigations at baseline and at 3-year follow-up visits were evaluated. Metabolic surgery was indicated when: a) BMI > 35 kg/m2 with two or more co-morbidities present at baseline, or b) a BMI > 40 irrespective of the presence of co-morbidities. The presence of T2DM favored a BPD/DS surgery due to the higher rate of remissions previously documented [12,17-20]. Clinical parameters that were considered for analysis included: weight (kg), waist and hip circumferences (cm), blood pressure (both Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP) (mmHg) and heart rate (pulse/min), these were measured and recorded, as previously described [4,20]. Percentage body fat was measured with a GE Lunar Prodigy® scanner (serial number 77060GA) for patients weighing <180 kg. Biochemical parameters with reference ranges that were considered for analysis included: glucose (F-Glucose) (3.9-6.0 mmol/l), HbA1c (4.06.0%), triglycerides (F-TG) (0.5-1.6 mmol/L), Low-Density Lipoprotein (LDL) cholesterol (1.6-2.9 mmol/l), High-Density Lipoprotein (HDL) cholesterol (1.0-1.6), Alanine Transaminase (ALT) (<50 U/l), aspartate transaminase (AST) (<38 U/l), gammaglutamyl transferase (GGT) (<60.0 U/l), C-Reactive Protein (CRP) (<5 mg/l), uric acid (0.16-0.36 mmol/l), red cell folate (3171894 nmol/l), vitamin D (>20 ng/ ml with upper limit: 50-60 ng/ ml),vitamin A (300-800 µg/l), vitamin E (5.5-15 mg/l), vitamin B12 (107-418 g/l), calcium (2.15-2.50 mmol/l), ferritin (30-400 ng/l) and albumin (35-52 g/l). All samples were collected after standard recommended fasting period and tests were performed on serum.
Co-morbidities were diagnosed using database formatted criteria of single entry standard clinical and biochemical parameters prior to surgery (Baseline) and evaluated for remission on follow-up (3 years post-surgery). Hypertension remission was considered with blood pressure (BP) ≤130/85 mmHg (Karotkoff sounds 1 and 4), T2DM remission with normalization of F-Glucose (3.96.0 mmol/l) and HbA1c (4.0-6.0%.) and dyslipidaemia remission with normalization of F-TG (0.5-1.6 mmol/l), LDL cholesterol (1.6-2.9 mmol/l) and HDL cholesterol (1.0-1.6mmol/l). Diagnosis of non-alcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) was considered using clinical findings, serum liver function tests (ALT, AST and GGT) and liver scans as previously described Hounsfield density.
Cohorts were identified according to 1) surgical procedure (GBP vs BPD/DS) and further stratified according to; 2) presence of T2DM (non-T2DM vs T2DM); and 3) sex (Male vs female) and finally compared over time intervals, baseline assessment and 3-year post surgery follow-up. Data analyses were performed using XLSTAT (by Addinsoft) with results reported as calculated means ± standard error of the mean (SEM). Unpaired Student’s t-test was used to compare means of different cohorts (e.g., GBP vs BPD/DS) at respective time intervals (Baseline vs 3-year followup), p-values of <0.05 were considered significant.
Results
A total of 979 surgeries were performed by a single surgeon with support from the same the same multidisciplinary team, during a 5-year period before the Covid-19 pandemic. Cohorts identified according to surgical procedure included 649 (66%) GBP patients vs 330 (34%) BPD/DS patients, presence of T2DM were found among 325 (33%) patients vs 654 (67%) patients classified as nonT2DM and the sex distribution included 268 (27%) males and 711 (73%) females. Of the non-T2DM patients, 486 (74%) underwent GBP vs 168 (26%) BPD/DS and of the T2DM patients, 163 (50%) GBP vs 162 (50%) BPD/DS. Of the male patients, 133 (50%) underwent GBP vs 135 (50%) BPD/DS compared to the females, 516 (73%) GBP vs 195 (27%) a BPD/DS.
Total cohort GBP vs BPD/DS – Baseline and 3-year-post surgical follow-up
Comparative clinical and biochemical parameters for total patient cohort undergoing either GBP or BPD/DS are presented in Table 1. Significant differences (p-value<0.0001) for baseline clinical parameters were documented for the BPD/DS cohort, demonstrating increased weight, BMI, neck-, waist- and hipcircumference, systolic blood pressure, heart rate and the total number of documented co-morbidities. At 3-years post-surgical follow-up, no significant differences were observed among clinical measurements except for lower blood pressure (both systolic and diastolic) and lower heart rate (p-value<0.01). A much higher % weight loss was observed in the BPD/DS (47.1%) vs GBP (28.1%) cohort from baseline to 3-years post-surgical followup. Hypertension resolution was demonstrated in the BPD/DS cohort. Significantly higher mean fasting glucose and HbA1c were observed among the BPD/DS patients, demonstrating the higher T2DM prevalence. Hyperlipidemia was present in both cohorts at baseline, however at 3-years post-surgical follow-up, the BPD/ DS cohort demonstrated significant reduction of hyperlipidemia, shown in Table 1.
|
Table 1: Baseline and Clinical outcome of
total cohort receiving laparoscopic Roux-en-Y gastric bypass (GBP) and
biliopancreatic diversion with duodenal switch (BPD/DS) surgery over a 5-year
interval period. |
||||||
|
Total cohort at baseline (n=979) |
Total cohort at 3 y follow-up (n=248) |
|||||
|
Parameter |
GBP |
BPD/DS |
GPB |
BPD/DS |
||
|
(n=649) |
(n=330) |
(n=188) |
(n=60) |
|||
|
Weight (kg) |
117.83±0.67 |
161.43±1.67 |
### |
84.7±1.17 |
85.34±1.84 |
P= NS |
|
BMI (kg/m2) |
42.13±0.16 |
55.95±0.45 |
### |
29.8±0.31 |
29.23±0.44 |
P=NS |
|
Neck (cm) |
45.04±0.2 |
52.67±0.36 |
### |
36.15±0.29 |
36.24±0.45 |
P=NS |
|
Waist (cm) |
121.27±0.52 |
147.83±1 |
### |
96.27±0.84 |
96.43±1.35 |
P=NS |
|
Hip (cm) |
127.53±0.46 |
150.28±0.99 |
### |
102.89±0.78 |
101.39±1.35 |
P=NS |
|
BP (S) (mmHg) |
143.37±0.61 |
148.63±0.97 |
### |
129.85±1.09 |
124.79±1.53 |
## |
|
BP (D) (mmHg) |
85.89±0.44 |
86.42±0.71 |
P=NS |
77.96±0.65 |
74.09±1.3 |
## |
|
HR (Pulse/min) |
77.6±0.48 |
81.94±0.68 |
### |
67.65±0.68 |
63.49±1.29 |
## |
|
F-Glucose (mmol/l) |
5.7±0.07 |
6.49±0.14 |
### |
4.93±0.06 |
4.49±0.06 |
### |
|
RR: 3.9-6.0 |
||||||
|
HbA1c (%) |
5.72±0.04 |
6.31±0.08 |
### |
5.24±0.05 |
4.44±0.05 |
### |
|
RR: 4.0-6.0 |
||||||
|
F-TG (mmol/l) |
1.72±0.04 |
1.79±0.06 |
P=NS |
1.12±0.04 |
0.82±0.05 |
### |
|
RR: 0.5-1.6 |
||||||
|
F-HDL chol (mmol/l) |
1.22±0.01 |
1.07±0.02 |
### |
1.61±0.03 |
1.22±0.04 |
### |
|
RR: 1.0-1.6 |
||||||
|
F-LDL chol (mmol/l) |
3.38±0.04 |
3.18±0.05 |
## |
2.88±0.13 |
1.63±0.06 |
### |
|
RR: 1.6-2.9 |
||||||
|
GGT (U/l) |
34.71±1.39 |
40.6±2.11 |
# |
23.01±3.52 |
16.3±1.49 |
P=NS |
|
RR: <60 |
||||||
|
ALT (U/l) |
31.18±0.81 |
34.51±1.33 |
# |
26.65±0.93 |
35.84±2.51 |
## |
|
RR: <50 |
||||||
|
AST (U/l) |
24.85±0.47 |
27.78±1.11 |
# |
25.48±0.59 |
31.21±1.71 |
## |
|
RR: <38 |
||||||
|
CRP (mg/l) |
9.09±0.3 |
14.96±0.68 |
### |
3.23±0.99 |
1.73±0.4 |
P=NS |
|
RR: <5 |
||||||
|
Uric Acid (mmol/l) |
0.39±0.01 |
0.55±0.12 |
P=NS |
0.3±0.01 |
0.26±0.01 |
## |
|
RR: 0.16-0.36 |
||||||
|
Red Cell Folate (nmol/L) |
1562.61±29.12 |
1586.53±33.83 |
P=NS |
2000.44±47.65 |
1974.89±102.45 |
P=NS |
|
RR: 317-1894 |
||||||
|
Vitamin D (ng/ml) |
22.49±0.62 |
20.28±0.66 |
# |
48.48±1.56 |
34.53±1.85 |
### |
|
RR: >20 (SU: 50-60) |
||||||
|
Vitamin A (µg/l) |
596.24±8.07 |
543.11±9.09 |
### |
618.07±13.09 |
406.19±19.05 |
### |
|
RR: 300-800 |
||||||
|
Vitamin E (mg/l) |
13.43±0.19 |
12.61±0.25 |
## |
12.19±0.2 |
8.1 |
### |
|
RR: 5.5-18 |
||||||
|
Vitamin B12 (g/l) |
338.87±7.22 |
313.09±8.63 |
# |
330.89±13.56 |
479.36 |
### |
|
RR: 107-418 |
||||||
|
Calcium (mmol/l) |
2.32±0.01 |
2.31±0.01 |
P=NS |
2.28±0.01 |
2.21 |
### |
|
RR: 2.15-2.50 |
||||||
|
Ferritin (ng/l) |
141.35±6.19 |
176.24±10.72 |
## |
133.79±8.81 |
231.34 |
## |
|
RR: 30-400 |
||||||
|
Albumin (g/l) |
39.8±0.14 |
38.83±0.2 |
### |
40.5±0.23 |
38.71 |
## |
|
RR: 35-52 |
||||||
|
Values
expressed in mean ± SEM |
||||||
|
p#
< 0.05; p## < 0.01; p### < 0.0001; Unpaired t-test |
||||||
|
p-value
presenting non-significant (P=NS) |
||||||
Non-T2DM vs T2DM - Baseline and 3-year-post surgical follow-up
Comparative cohort results for non-T2DM and T2DM at baseline and 3-year-post surgical follow-up are presented in Tables 2 and 3. Clinical parameters for both non-T2DM and T2DM were significantly higher (p-value<0.0001 for all indices) in the BPD/DS patients, indicated in Table 2, however no significance differences were observed at 3-years follow-up between the surgical types demonstrating a higher decrease in respective indices. Considering BMI, the BPD/DS cohorts reduced within non-T2DM 48.8% and within T2DM 46.5% while 30.6% and 26.7% reductions were observed respectively for the GBP cohorts, presented in Table 3. The BPD/DS patients demonstrated a significant decrease in and HbA1c among both Non-T2DM and T2DM cohorts with 20% and 62.9% reduction respectively (p-value< 0.0001). However, the BPD/DS patients demonstrated a greater HbA1c reduction in the non-T2DM cohort (20%) vs the GBP surgery group of the T2DM cohort (17%). Similarly, a significant difference in reduced serum F-Glucose were observed among BPD/ DS patients at 3-years follow-up within both non-T2DM (p-value<0.01) and T2DM (p-value<0.0001) cohorts. The BPD/DS patients demonstrated a 49% reduction in F-Glucose among Non-T2DM cohort and 58.2 % for the T2DM cohort vs 30% and 44% respectively for the GBP patients, thus demonstrating the superiority of BPD/DS surgery procedure to counter T2DM and gain glycemic control. Both Non-T2DM and T2DM groups demonstrated a significant decrease in cholesterol (p-value<0.0001) and improvement in lipid profile (F-TG, F-HDL, and F-LDL) at 3- year-post surgical follow-up, shown in Table 3.
Table 2: Baseline data of Non-T2DM and T2DM
patients receiving laparoscopic Roux-en-Y gastric bypass (GBP) and
biliopancreatic diversion with duodenal switch (BPD/DS) surgery over a 5-year
interval period. | ||||||||
| Total
Non-T2DM cohort at baseline (n=654) | Total
T2DM cohort at baseline (n=325) | ||||||
Parameter | GBP | BPD/DS | | GPB | BPD/DS
(n=162) | GPB | BPD/DS | |
(n=486) | (n=168) | (n=163) | ||||||
Weight (kg) | 116.87±0.75 | 161.98±2.22 | ### | 120.66±1.41 | 160.85±2.51 | * | P=NS | ### |
BMI (kg/m2) | 42.04±0.18 | 56.79±0.57 | ### | 42.37±0.34 | 55.02±0.69 | P=NS | 0.05 | ### |
Neck (cm) | 44.18±0.22 | 51.23±0.49 | ### | 47.57±0.43 | 54.21±0.5 | *** | ¤¤¤ | ### |
Waist (cm) | 119.69±0.59 | 146.2±1.39 | ### | 125.91±1.03 | 149.55±1.44 | P=NS | P=NS | ### |
Hip (cm) | 127.82±0.52 | 152.53±1.36 | ### | 126.67±0.97 | 147.91±1.41 | P=NS | ¤ | ### |
BP (S) (mmHg) | 142.18±0.67 | 146.56±1.3 | ## | 146.89±1.33 | 150.82±1.43 | ** | ¤ | P=NS |
BP (D) (mmHg) | 85.09±0.5 | 85.67±1.03 | P=NS | 88.22±0.9 | 87.22±0.96 | ** | P=NS | P=NS |
HR (Pulse/ min) | 77.03±0.53 | 81.69±0.99 | ### | 79.29±1.05 | 82.21±0.94 | 0.05 | P=NS | # |
F-Glucose
(mmol/l) RR: 3.9-6.0 | 5.09±0.02 | 5.24±0.04 | ## | 7.45±0.23 | 7.82±0.26 | *** | ¤¤¤ | P=NS |
HbA1c (%) RR: 4.0-6.0 | 5.4±0.02 | 5.54±0.03 | ## | 6.66±0.12 | 7.11±0.14 | *** | ¤¤¤ | # |
F-TG (mmol/l) RR: 0.5-1.6 | 1.56±0.04 | 1.47±0.07 | P=NS | 2.18±0.12 | 2.13±0.11 | *** | ¤¤¤ | P=NS |
F-HDL chol (mmol/l) RR: 1.0-1.6 | 1.25±0.01 | 1.13±0.02 | ### | 1.11±0.02 | 1.01±0.02 | *** | ¤¤¤ | ## |
F-LDL chol (mmol/l) RR: 1.6-2.9 | 3.42±0.04 | 3.27±0.07 | P=NS | 3.27±0.07 | 3.09±0.08 | P=NS | P=NS | P=NS |
GGT (U/l) RR: <60 | 30.72±1.23 | 33.56±1.9 | P=NS | 46.47±3.99 | 47.98±3.76 | ** | ¤¤ | P=NS |
ALT (U/l) RR: <50 | 28.79±0.86 | 31.12±1.27 | P=NS | 38.24±1.84 | 38.07±2.35 | *** | ¤¤ | P=NS |
AST (U/l) RR: <38 | 23.38±0.47 | 25.35±0.91 | P=NS | 29.18±1.17 | 30.33±2.04 | *** | ¤ | P=NS |
CRP (mg/l) RR: <5 | 8.74±0.32 | 15.81±1.05 | ### | 10.12±0.7 | 14.06±0.85 | P=NS | P=NS | ## |
Uric Acid (mmol/l) RR: 0.16-0.36 | 0.37±0.01 | 0.66±0.23 | P=NS | 0.44±0.04 | 0.42±0.01 | P=NS | P=NS | P=NS |
Red Cell Folate (nmol/L) RR: 317-1894 | 1523.36±33.25 | 1561.15±47.22 | P=NS | 1689.38±58.97 | 1658.66±47.95 | * | ¤ | P=NS |
Vitamin
D (ng/ml) RR: >20 (SU: 50-60) | 23.06±0.79 | 21.42±1.12 | P=NS | 20.65±0.73 | 19.12±0.67 | * | P=NS | P=NS |
Vitamin
A (µg/l)
RR: 300-800 | 598.53±9.13 | 528.45±11.47 | ### | 588.59±17.28 | 557.78±14.05 | P=NS | P=NS | P=NS |
Vitamin
E (mg/l) RR: 5.5-18 | 13.19±0.2 | 12.03±0.25 | ## | 14.23±0.46 | 13.19±0.42 | * | ¤ | P=NS |
Vitamin
B12 (g/l) RR: 107-418 | 340.53±8.49 | 322.72±13.61 | P=NS | 333.59±13.49 | 303.15±10.49 | P=NS | P=NS | P=NS |
Calcium (mmol/l) RR:2.15-2.50 | 2.31±0.01 | 2.31±0.01 | P=NS | 2.32±0.01 | 2.32±0.01 | P=NS | P=NS | P=NS |
Ferritin (ng/l) RR: 30-400 | 122.97±5.57 | 162.33±13.95 | ## | 195.16±17.46 | 190.84±16.34 | ** | P=NS | P=NS |
Albumin (g/l) RR: 35-52 | 39.8±0.17 | 38.51±0.24 | ### | 39.81±0.24 | 39.16±0.31 | P=NS | P=NS | P=NS |
Values
expressed in mean ± SEM | ||||||||
p#
< 0.05; p## < 0.01; p### < 0.0001; Unpaired t-test LRYGB vs BPD/DS | ||||||||
p*
< 0.05; p** < 0.01; p*** < 0.0001; Unpaired t-test LRYGB Non-T2DM vs
LRYGB T2DM | ||||||||
p¤ <
0.05; p¤¤ <
0.01; p¤¤¤ <
0.0001; Unpaired t-test BPD/DS Non-T2DM vs BPD/DS T2DM | ||||||||
p-value
presenting non-significant (P=NS) | ||||||||
Table 3: Clinical outcomes at 3-year
post-surgery of Non-T2DM and T2DM patients receiving laparoscopic Roux-en-Y
gastric bypass (GBP) and biliopancreatic diversion with duodenal switch
(BPD/DS) surgery over a 5-year period. | ||||||||
| Total Non-T2DM cohort at 3-y follow-up (n=93) | Total T2DM cohort at 3-y follow-up (n=89) | ||||||
Parameter | GBP | BPD/DS (n=32) | | GPB | BPD/DS | GPB | BPD/DS | |
(n=127) | (n=61) | (n=28) | ||||||
Weight (kg) | 82.52±1.39 | 84.03±2.44 | P=NS | 89.25±2.08 | 86.8±2.79 | ** | 0.46 | P=NS |
BMI (kg/m2) | 29.18±0.37 | 29.06±0.57 | P=NS | 31.06±0.55 | 29.43±0.67 | ** | 0.68 | P=NS |
Neck (cm) | 35.21±0.32 | 35.37±0.62 | P=NS | 38.37±0.52 | 37.06±0.62 | *** | 0.06 | P=NS |
Waist (cm) | 94.31±1.01 | 94.29±1.97 | P=NS | 100.42±1.41 | 98.77±1.78 | ** | 0.1 | P=NS |
Hip (cm) | 101.73±0.95 | 102.4±1.85 | P=NS | 105.54±1.31 | 100.13±2 | * | 0.41 | # |
BP (S) (mmHg) | 127.63±1.29 | 123.11±2.08 | P=NS | 134.57±1.88 | 126.69±2.25 | ** | 0.25 | # |
BP (D) (mmHg) | 77.38±0.81 | 72.36±1.91 | ## | 79.19±1.08 | 76.03±1.72 | P=NS | 0.16 | P=NS |
HR (Pulse/ min) | 68.28±0.85 | 64.03±1.73 | # | 66.31±1.1 | 62.88±1.97 | P=NS | 0.66 | P=NS |
F-Glucose
(mmol/l) RR: 3.9-6.0 | 4.7±0.04 | 4.39±0.06 | ## | 5.39±0.15 | 4.61±0.11 | *** | 0.09 | ### |
HbA1c (%) RR: 4.0-6.0 | 5.1±0.03 | 4.43±0.07 | ### | 5.53±0.14 | 4.45±0.09 | ** | 0.91 | ### |
F-TG (mmol/l) RR: 0.5-1.6 | 1.08±0.05 | 0.75±0.04 | ### | 1.2±0.07 | 0.89±0.09 | P=NS | 0.16 | ## |
F-HDL chol (mmol/l) RR: 1.0-1.6 | 1.68±0.04 | 1.26±0.06 | ### | 1.46±0.04 | 1.19±0.06 | *** | 0.42 | ## |
F-LDL chol (mmol/l) RR: 1.6-2.9 | 2.72±0.07 | 1.46±0.07 | ### | 3.21±0.37 | 1.81±0.08 | P=NS | ¤¤ | ## |
GGT (U/l) RR: <60 | 25.11±5.14 | 16.6±2.39 | P=NS | 18.57±1.44 | 15.93±1.57 | P=NS | 0.82 | P=NS |
ALT (U/l) RR: <50 | 26.5±1.16 | 35.57±3.58 | # | 26.97±1.57 | 36.18±3.51 | P=NS | 0.91 | # |
AST (U/l) RR: <38 | 25.52±0.7 | 30.26±1.96 | # | 25.4±1.08 | 32.39±2.99 | P=NS | 0.54 | # |
CRP (mg/l) RR: <5 | 3.48±1.44 | 2.36±0.76 | P=NS | 2.7±0.35 | 1.07±0.05 | P=NS | 0.1 | ### |
Uric Acid (mmol/l) RR: 0.16-0.36 | 0.3±0.01 | 0.27±0.01 | # | 0.31±0.01 | 0.26±0.01 | P=NS | 0.56 | ## |
Red Cell Folate (nmol/L) RR: 317-1894 | 1899.62±59.68 | 1835.58±139.19 | | 2220.09±69.81 | 1921.04±154.14 | ** | 0.68 | P=NS |
Vitamin
D (ng/ml) RR: >20 (SU: 50-60) | 48.15±2.02 | 33.69±2.1 | ### | 49.19±2.34 | 35.55±3.23 | P=NS | 0.62 | ## |
Vitamin
A (µg/l) RR: 300-800 | 619.48±16.94 | 376.51±24.29 | ### | 615.25±10.07 | 441.81±29.06 | P=NS | 0.09 | ### |
Vitamin
E (mg/l) RR: 5.5-18 | 12.07±0.25 | 8.18±0.28 | ### | 12.45±0.3 | 8±0.45 | P=NS | 0.71 | ### |
Vitamin
B12 (g/l) RR: 107-418 | 328.27±18.1 | 518.87±36.06 | ### | 336.28±18.38 | 435.9±34.37 | P=NS | 0.1 | ## |
Calcium (mmol/l) RR:2.15-2.50 | 2.29±0.01 | 2.2±0.01 | ### | 2.27±0.03 | 2.21±0.02 | P=NS | 0.59 | 0.05 |
Ferritin (ng/l) RR: 30-400 | 125.72±10.08 | 225±28.75 | ## | 150.43±17.12 | 238.73±43.46 | P=NS | 0.79 | P=NS |
Albumin (g/l) RR: 35-52 | 40.65±0.29 | 37.14±0.88 | P=NS | 40.17±0.36 | 38.2±0.27 | P=NS | 0.42 | # |
Values
expressed in mean ± SEM | ||||||||
p#
< 0.05; p## < 0.01; p### < 0.0001; Unpaired t-test LRYGB vs BPD/DS | ||||||||
p*
< 0.05; p** < 0.01; p*** < 0.0001; Unpaired t-test LRYGB Non-T2DM vs
LRYGB T2DM | ||||||||
p¤ <
0.05; p¤¤ <
0.01; p¤¤¤ <
0.0001; Unpaired t-test BPD/DS Non-T2DM vs BPD/DS T | ||||||||
p-value
presenting non-significant (P=NS) | ||||||||
Male vs. Female - Baseline and 3-year-post surgical follow-up
Comparative cohort results for males and females at baseline and 3-year-post surgical follow-up is presented in Tables 4 and 5. Significant differences at baseline, comparing surgery types, for most of the clinical indices were observed in both male and female cohorts (p-value<0.0001), however these differences dissipated at 3 years follow-up. The BPD/DS cohort had significantly lower levels of fasting glucose for males (p-value<0.0001) and females (p-value<0.01), and HbA1c for both genders (p-value<0.0001) at 3-years post-surgery follow-up. Cholesterol profiles were high for all cohorts at baseline. At 3-years follow up, the lipid profile (F-TG, F-HDL and F-LDL) were all within normal ranges for all cohorts, but the BPD/DS showed significant reduction for male (p-value<0.01) and female (p-value<0.0001) cohorts. Serum Vitamin D levels were low across all cohorts at baseline but showed a non-significant increase at 3-years follow-up. The GBP cohorts had significantly higher vitamin D levels at 3-years follow-up for both genders. Vitamin A and E were significantly lower in the BPD/DS cohorts at baseline and at 3-years. Vitamin B12 in the BPD/DS cohorts for both gender groups were significantly higher compared to the GBP patients. Calcium levels were significantly lower in the BPD/DS group for both genders at 3-years. Increase in ferritin levels were higher among women however both BPD/DS cohorts showed significantly higher levels compared to GBP patients. Albumin levels remained normal from baseline to 3-years, although both genders in the BPD/DS cohorts had significantly lower levels (p-value<0.01), presented in Table 4 and 5.
Table 4: Baseline data of Male and Female
patients receiving laparoscopic Roux-en-Y gastric bypass (GBP) and
biliopancreatic diversion with duodenal switch (BPD/DS) surgery over a 5-year
interval period. | ||||||||
| Total Male cohort at baseline (n=268) | Total Female cohort at baseline (n=711) | ||||||
Parameter | GBP | BPD/DS | | GPB | BPD/DS
(n=195) | GPB | BPD/DS | |
(n=133) | (n=135) | (n=516) | ||||||
Weight (kg) | 135.15±1.57 | 180.37±2.77 | ### | 113.32±0.16 | 148.59±1.51 | *** | ¤¤ | ### |
BMI (kg/m2) | 43.09±0.38 | 57.05±0.83 | ### | 41.87±0.18 | 55.17±0.49 | ** | 0.05 | ### |
Neck (cm) | 51.46±0.4 | 58.26±0.42 | ### | 43.41±0.18 | 48.86±0.33 | *** | ¤¤¤ | ### |
Waist (cm) | 135.22±1.11 | 160.85±1.5 | ### | 117.64±0.49 | 139±0.92 | *** | ¤¤¤ | ### |
Hip (cm) | 123.55±1 | 145.55±1.69 | ### | 128.56±0.51 | 153.49±1.15 | *** | ¤¤ | ### |
BP (S) (mmHg) | 150.24±1.43 | 148.07±1.53 | P=NS | 141.59±0.65 | 149.01±1.25 | *** | P=NS | ### |
BP (D) (mmHg) | 91.82±0.97 | 88.1±1.16 | # | 84.34±0.47 | 85.28±0.88 | *** | 0.05 | P=NS |
HR (Pulse/ min) | 77.31±1.06 | 80.93±1.06 | # | 77.68±0.53 | 82.63±0.9 | 0.75 | P=NS | ### |
Number of Co-morbidities | 6.54±0.14 | 7.6±0.16 | ### | 6.22±0.09 | 7.48±0.15 | 0.05 | P=NS | ### |
Dexa % Fat (kg/m2) | 49.01±0.64 | 51.56±0.59 | ## | 51.2±0.26 | 52.51±0.43 | ** | P=NS | ## |
F-Glucose
(mmol/l) RR: 3.9-6.0 | 6.58±0.25 | 6.93±0.26 | P=NS | 5.47±0.06 | 6.19±0.16 | *** | ¤ | ### |
HbA1c (%) RR: 4.0-6.0 | 6.1±0.12 | 6.55±0.15 | # | 5.62±0.04 | 6.14±0.1 | ** | ¤ | ### |
F-TG (mmol/l) RR: 0.5-1.6 | 2.22±0.12 | 2.02±0.12 | P=NS | 1.59±0.04 | 1.64±0.07 | *** | ¤¤ | P=NS |
F-HDL chol (mmol/l) RR: 1.0-1.6 | 1.01±0.03 | 0.99±0.03 | P=NS | 1.27±0.01 | 1.13±0.02 | *** | ¤¤¤ | ### |
F-LDL chol (mmol/l) RR: 1.6-2.9 | 3.23±0.07 | 3.03±0.09 | P=NS | 3.42±0.04 | 3.28±0.07 | * | ¤ | P=NS |
GGT (U/l) RR: <60 | 47.24±4.37 | 45.5±3.85 | P=NS | 31.47±1.3 | 37.31±2.38 | ** | P=NS | # |
ALT (U/l) RR: <50 | 48.25±2.27 | 40.7±1.79 | ## | 26.77±0.72 | 30.37±1.81 | *** | ¤¤¤ | P=NS |
AST (U/l) RR: <38 | 31.14±1.16 | 30.07±1.3 | P=NS | 23.22±0.48 | 26.25±1.63 | *** | P=NS | P=NS |
CRP (mg/l) RR: <5 | 6.29±0.52 | 12.07±1.18 | ### | 9.81±0.35 | 16.92±0.78 | *** | ¤¤ | ### |
Uric Acid (mmol/l) RR: 0.16-0.36 | 0.51±0.05 | 0.49±0.03 | P=NS | 0.36±0.01 | 0.58±0.2 | ** | P=NS | P=NS |
Red Cell Folate (nmol/L) RR: 317-1894 | 1598.34±58.86 | 1570.23±51.53 | P=NS | 1553.59±33.33 | 1597.73±44.85 | 0.54 | P=NS | P=NS |
Vitamin
D (ng/ml) RR: >20 (SU: 50-60) | 23.27±2.33 | 18.74±0.55 | P=NS | 22.28±0.49 | 21.33±1.03 | 0.68 | ¤ | P=NS |
Vitamin
A (µg/l) RR: 300-800 | 645.96±19.61 | 572.48±16.08 | ## | 582.75±8.65 | 522.71±10.4 | ** | ¤¤ | ### |
Vitamin
E (mg/l) RR: 5.5-18 | 13.54±0.48 | 12.75±0.42 | P=NS | 13.4±0.2 | 12.51±0.3 | 0.8 | P=NS | # |
Vitamin
B12 (g/l) RR: 107-418 | 338.2±15.19 | 314.73±12.87 | P=NS | 339.04±8.2 | 311.94±11.6 | 0.96 | P=NS | 0.05 |
Calcium (mmol/l) RR: 2.15-2.50 | 2.3±0.01 | 2.31±0.01 | P=NS | 2.32±0.01 | 2.32±0.01 | 0.2 | 0.31 | P=NS |
Ferritin (ng/l) RR: 30-400 | 313.65±20.51 | 276.91±20.18 | P=NS | 96.46±3.82 | 106.94±8.72 | *** | ¤¤¤ | P=NS |
Albumin (g/l) RR: 35-52 | 41.53±0.27 | 39.8±0.34 | ### | 39.35±0.15 | 38.16±0.22 | *** | ¤¤¤ | ### |
Values
expressed in mean ± SEM | ||||||||
p#
< 0.05; p## < 0.01; p### < 0.0001; Unpaired t-test LRYGB vs BPD/DS | ||||||||
p*
< 0.05; p** < 0.01; p*** < 0.0001; Unpaired t-test LRYGB male vs
LRYGB Female | ||||||||
p¤ <
0.05; p¤¤ <
0.01; p¤¤¤ <
0.0001; Unpaired t-test BPD/DS male vs BPD/DS Female | ||||||||
p-value
presenting non-significant (P=NS) | ||||||||
Table 5: Clinical outcomes at 3-year
post-surgery of Male and Female patients receiving laparoscopic Roux-en-Y
gastric bypass (GBP) and biliopancreatic diversion with duodenal switch
(BPD/DS) surgery over a 5-year interval period. | ||||||||
| Total
Male cohort at 3-y follow-up (n=71) | Total
Female cohort at 3-yrs follow-up (n=177) | ||||||
Parameter | GBP | BPD/DS | | GPB | BPD/DS | GBP | BPD/DS | |
(n=44) | (n=27) | (n=144) | (n=33) | |||||
Weight (kg) | 101.28±2.24 | 95.33±2.16 | P=NS | 79.48±1.09 | 77.45±2.06 | *** | ¤¤¤ | P=NS |
BMI (kg/m2) | 31.94±0.57 | 29.94±0.57 | # | 29.11±0.35 | 28.72±0.62 | *** | P=NS | P=NS |
Neck (cm) | 41.4±0.4 | 38.83±0.44 | ### | 34.42±0.22 | 33.97±0.47 | *** | ¤¤¤ | P=NS |
Waist (cm) | 106.13±0.6 | 102.89±1.3 | P=NS | 93.1±0.85 | 91.54±1.79 | *** | ¤¤¤ | P=NS |
Hip (cm) | 105.86±1.42 | 104.61±1.53 | P=NS | 101.98±0.91 | 99±1.98 | * | ¤ | P=NS |
BP (S) (mmHg) | 135.2±1.89 | 129.13±2.13 | # | 128.16±1.27 | 121.37±2.02 | ** | ¤ | # |
BP (D) (mmHg) | 81.61±1.13 | 77.1±1.55 | # | 76.81±0.76 | 71.71±1.92 | ** | ¤ | ## |
HR (Pulse/ min) | 66.1±1.45 | 63.17±2.03 | P=NS | 68.14±0.77 | 63.74±1.7 | P=NS | P=NS | # |
F-Glucose
(mmol/l) RR: 3.9-6.0 | 5.27±0.1 | 4.54±0.08 | ### | 4.83±0.07 | 4.45±0.09 | ** | P=NS | ## |
HbA1c (%) RR: 4.0-6.0 | 5.22±0.12 | 4.47±0.08 | ### | 5.25±0.06 | 4.42±0.08 | P=NS | P=NS | ### |
F-TG (mmol/l) RR: 0.5-1.6 | 1.24±0.11 | 0.83±0.09 | ## | 1.08±0.04 | 0.81±0.04 | P=NS | P=NS | ### |
F-HDL chol (mmol/l) RR: 1.0-1.6 | 1.36±0.05 | 1.13±0.05 | ## | 1.69±0.03 | 1.3±0.06 | *** | ¤ | ### |
F-LDL chol (mmol/l) RR: 1.6-2.9 | 3.36±0.49 | 1.51±0.08 | ## | 2.73±0.07 | 1.71±0.08 | P=NS | P=NS | ### |
GGT (U/l) RR: <60 | 21.95±1.37 | 16.48±1.51 | # | 23.31±4.53 | 16.17±2.37 | P=NS | P=NS | P=NS |
ALT (U/l) RR: <50 | 32.98±1.99 | 34.63±3.16 | P=NS | 24.77±1.01 | 36.75±3.72 | ** | P=NS | ## |
AST (U/l) RR: <38 | 27.75±1.03 | 32±2.57 | P=NS | 24.81±0.69 | 30.61±2.31 | * | P=NS | # |
CRP (mg/l) RR: <5 | 1.9±0.35 | 2.74±0.94 | P=NS | 3.64±1.29 | 1.04±0.04 | P=NS | P=NS | # |
Uric Acid (mmol/l) RR: 0.16-0.36 | 0.38±0.01 | 0.3±0.01 | ### | 0.28±0.01 | 0.23±0.01 | *** | ¤¤¤ | ### |
Red Cell Folate (nmol/L) RR: 317-1894 | 2098.33±88 | 1877.17±156.32 | P=NS | 1969.27±56.14 | 1872.95±137.9 | P=NS | 0.98 | P=NS |
Vitamin
D (ng/ml) RR: >20 (SU: 50-60) | 46.53±2.35 | 35.16±2.86 | ## | 49.09±1.91 | 33.98±2.43 | P=NS | 0.75 | ### |
Vitamin
A (µg/l) RR: 300-800 | 647.28±23.31 | 402.7±28.7 | ### | 609.41±15.46 | 409.32±25.84 | P=NS | 0.86 | ### |
Vitamin
E (mg/l) RR: 5.5-18 | 11.54±0.36 | 7.51±0.22 | ### | 12.39±0.23 | 8.59±0.4 | P=NS | ¤ | ### |
Vitamin
B12 (g/l) RR: 107-418 | 326.24±17.16 | 456.59±33.75 | ## | 332.31±16.94 | 497.57±36.92 | P=NS | 0.43 | ### |
Calcium (mmol/l) RR:2.15-2.50 | 2.32±0.02 | 2.21±0.02 | ### | 2.27±0.02 | 2.2±0.01 | 0.05 | 0.71 | ## |
Ferritin (ng/l) RR: 30-400 | 195.01±22.49 | 299.43±46.87 | 0.05 | 115.01±8.71 | 172.97±19.66 | ** | ¤ | ## |
Albumin (g/l) RR: 35-52 | 42.28±0.44 | 39.76±0.88 | # | 39.96±0.25 | 37.86±0.75 | *** | 0.1 | # |
Values
expressed in mean ± SEM | ||||||||
p#
< 0.05; p## < 0.01; p### < 0.0001; Unpaired t-test LRYGB vs BPD/DS | ||||||||
p*
< 0.05; p** < 0.01; p*** < 0.0001; Unpaired t-test LRYGB male vs LRYGB
Female | ||||||||
p¤ <
0.05; p¤¤ <
0.01; p¤¤¤ <
0.0001; Unpaired t-test BPD/DS male vs BPD/DS Female | ||||||||
p-value
presenting non-significant (P=NS) | ||||||||
Comorbidities at Baseline and 3-years Post-Surgical Follow-Up
BPD/DS patients presented with more documented co-morbidities at baseline compared to GBP indicated as the bars graph in Figure 1. The number of comorbidities significantly decreased in both groups, but to a greater degree in the BPD/DS patients with 62.9% vs 50.5% for GBP patients presented as the line graphs in Figure 1. These co-morbidity reductions was consistently illustrated in all BPD/DS surgery groups, irrespective of glycemic control, within the T2DM (61.7%) and Non-T2DM (65.9%) cohorts and across both genders with comorbidity reductions observed both among males (62%) and females (66%).
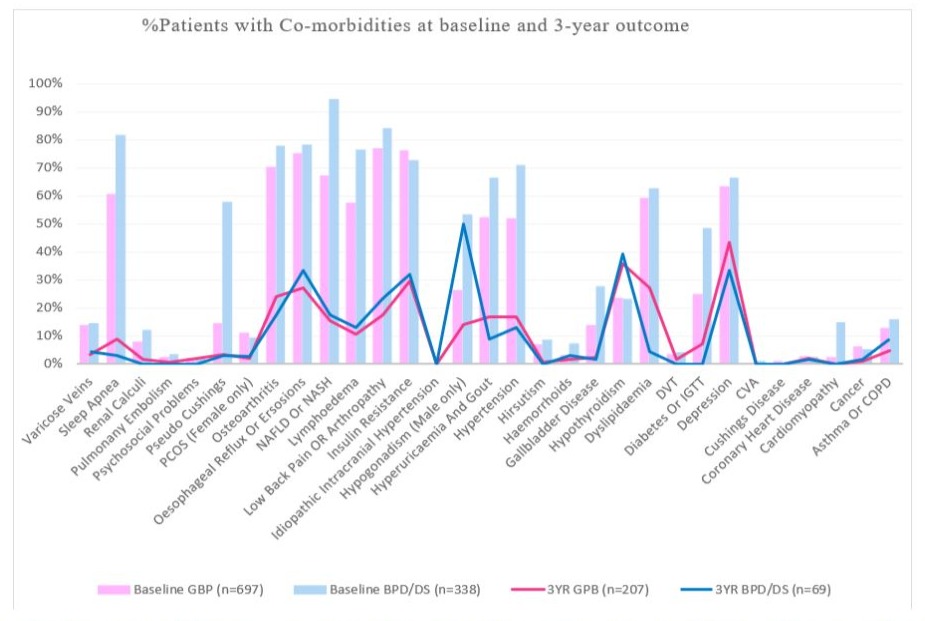
Figure 1: Percentage of patients presenting with respective co-morbidities at baseline and 3-year outcomes after receiving either a laborascopic Roux-en-Y gastric bypass (GBP) or biliopancreatic diversion with duodenal switch (BPD/DS) surgeries.
Discussion
This study outlines the demographics of patients diagnosed with obesity that received either a GBP or a BPD/DS surgery at a SASSO Center, over a five-year period. Clinical outcome data is presented at a 3 year-post surgical follow-up period, in addition further stratification was done according to the presence of T2DM and according to gender. Comparing patient baseline characteristics and follow-up outcomes for different surgery types, could provide valuable information for future assignment of surgery based on patient profile. Overall weight loss was higher in the BPD/DS vs GPB group (47% vs 28%), favoring this surgery type for this outcome with patients achieving an average reduction in BMI of 26.72 vs 12.33 for GBP patients. In this study, patients who underwent BPD/DS surgery presented with significantly increased weight, BMI, neck-, waist- and hip circumferences, compared to the GBP cohort. BPD/ DS, is associated with a higher degree of malnutrition and would be the preferred choice of surgery where a greater need for more weight loss is required in order to obtain a BMI considered as essential for permanent remission of diabetes, obstructive sleep apnea and several other co-morbidities, replacing the need for chronic medication, CPAP devices and care [12,13,21]. Despite the more invasive nature of the BPD/DS procedures, no significant difference in body composition could be observed in our study at 3-year post-surgical follow-up confirming previous reports of metabolic surgery having a favorable effect on preservation of lean body mass [22,23].
Previous studies have demonstrated correlations between weight loss and improvement of costly co-morbidities [24,25]. Our data positively confirms these findings, illustrating a reduction in comorbidities for BPD/DS surgeries, but further suggest consideration of surgery type. For the BPD/DS cohort greater reduction overall and significantly lowered F-BG were pertinent. Ultimately, an improved rate of diabetes remission will by implication lead to improved prevention and decreased occurrence of cardiovascular- and metabolic disease [12,26-29]. The health economic impact for persons living with obesity will have far reaching implications in the future, as countries and governments battle the ever-increasing rise of health care expenses that obesity is affording towards the GDPs % expenditure worldwide.
The constellation of obesity, hyperlipidemia, T2DM, hypertension, pre-diabetes and obstructive sleep apnea are obesity related comorbidities that act as predictors of Non-Alcoholic Fatty Liver Disease (NAFLD) and non- alcoholic steatohepatitis (NASH) [30,31-32]. Using ALT, alone, or as a biochemical marker for liver injury have been debated, since numerous studies have demonstrated all stages of NAFLD could present in patients with normal and even low ALT values [31]. Our findings confirmed this. It is essential that clinical observation, liver sonar or hepatic scans, and even liver biopsies be considered where a high index of suspicion exists for the presence of NASH/NAFLD [33-35]. Both BPD/DS and GBP surgery types demonstrated T2DM remission at 3 years. However, the lower F-G in BPD/DS cohort at 3 years, signifies a more sustained improvement in the fasted glycemic metabolism, and will ultimately concur with higher potential remission rates that the malnutrition procedures have on glucose metabolism [36]. BPD/DS surgery cohorts demonstrated a greater reduction in BMI from baseline measurements. Supportive argument from the studies of Rubino et al., demonstrate that the clinical manifestation of T2DM can clear within days after surgery before any significant weight loss, suggesting that changes in the distal stomach, duodenum and proximal jejunum hormonal milieu are influencing T2DM mechanisms [37]. The study data illustrates that BPD/DS surgeries offers greater glycemic control in patients with T2DM with a total HbA1c and fasting glucose reduction of 62.6% and 58.2% respectively vs GBP patients with 17% and 44% respectively. Non-T2DM cohorts also demonstrated increased glycemic control with BPD/DS surgeries with a total HbA1c and fasting glucose reduction of 20% and 49% respectively vs GBP patients with 5.5% and 30% respectively. Similar results of increased glycemic control were observed in males who received BPD/DS surgeries. HbA1c and fasting glucose reduced in PBD/DS surgeries with 31.8% and 34.5% in males as well as 28% and 28.1% in females respectively vs GBP surgeries with 14.4% and 19.9% in males as well as 6.5% and 11.7% in females respectively. The BPD/DS surgeries offer increased glycemic control irrespective of T2DM and gender, although patients profiling as male with T2DM would benefit the most from a BPD/DS surgery.
The new guidelines for hypertension diagnosis by the American College of Cardiology and American Heart Association in 2017 have lowered the systolic and diastolic BP threshold to define hypertension from >140/90 mmHg (set out by 2018 European Society of Hypertension and European Society of Cardiology guidelines) to >130/80 mmHg, with cardiovascular disease, diabetes or risk of >10% of developing cardiovascular disease within 10-years [38]. Considering this threshold, all baseline measurements confirm that obesity is a definitive risk factor for hypertension. After metabolic surgery procedures hypertension subsides with the loss of excess weight, although the total cohort demonstrated blood pressure measurements below <130/80 mmHg, the BPD/DS surgery cohorts demonstrated significantly lower measurements than the GBP cohorts (p-value<0.0001).
C-Reactive Protein (CRP) have been identified as an independent risk factor for cardiovascular disease and T2DM, correlating with inflammation and metabolic syndrome [39-41]. The interrelationship between CRP and metabolic syndrome synergistically increases the cardiovascular morbidity and mortality [42]. Metabolic syndrome is characterized by abdominal obesity, diabetes, glucose intolerance, dyslipidemia, hypertension and hyperuricemia. Obesity and waist circumference are directly associated with elevated CRP levels, which have anti-inflammatory and proinflammatory properties in vitro, including activation of complement system [40,43]. In this study, elevated CRP level correlates directly with increased BMI, waist and hip circumference although no significant difference were observed between T2DM and non-T2DM cohorts, indicating that CRP could not be used as an independent risk factor for T2DM. Elevated CRP levels were observed in both female surgery type cohorts at baseline, with a significant (p-value<0.05) decrease in the female cohort with BPD/DS surgery as well as the T2DM BPD/DS (p-value<0.0001) cohorts at three years follow-up. Based on our findings, women with an elevated CRP value at baseline, BMI>40 and T2DM may require a more detailed study and profiling in the future.
Similar results were observed with uric acid indices, where significant baseline correlations were observed with BMI, independent of glycemic control. The greatest uric acid reductions at 3-year follow-up were observed in the BPD/DS surgery patients of both T2DM as well as gender profiles with Non-T2DM (59.1%) and the female (60%) cohorts. The least reduction in uric acid was observed in the GBP surgeries with the Non-T2DM (18.9%) and male (25.9%) cohorts. Contrasting literature have been published in some studies, suggesting uric acid to be a predictor for T2DM [42,44]. Our baseline data demonstrates that patients with T2DM had a tighter correlation with BMI per se, and that gender have an influence on uric acid values. A difference in dietary and alcohol intake may exist and this will need clarification in future analyses. A significant improvement in uric acid values were observed in men within the BPD/DS cohort vs GBP, suggesting that men with hyperuricemia or gout, independent of glycemic control, could benefit more from a BPD/DS procedure, also considering the benefit if other risk factors for cardiovascular disease are present.
All micro and macro nutrient elements measured were within normal limits at 3-years. This stands testimony to the fact that stringent follow-up, standardized replacement, and rapid outpatient corrective treatment can be equally effectively achieved in malnutrition procedures. Patients with a GBP had significantly higher levels of Vitamin A, D and E, Calcium (p<0.0001) as well as higher levels of ferritin and albumin (p<0.01) at 3-years, though both surgical types remained within the normal range. Higher levels were maintained irrespective of glycemic control or gender. BPD/DS patients have significantly higher levels of Vitamin B12 at 3-years post-surgery (p<0.0001), in keeping with previously published data from our center [45]. A direct correlation was observed between clinical parameters (weight, BMI, waist-, neck- and hip circumference, weight) and number of co-morbidities. The GBP cohort presented with significantly lower number of co-morbidities than BPD/DS patients at baseline, irrespective of glycemic control and gender orientation and would support a clinical decision in favor of a malnutrition procedure for patients with more co-morbidities. No significant difference in the of number of co-morbidities between the two surgery types were observed at 3-years, but as would be expected, the BPD/DS cohort did present with less co-morbidities, irrespective of glycemic control and gender. Possible limitations of this study could be the retrospective analysis and the high loss of follow-up over three years and performance of BPD/DS procedures, considering patients at a BMI exclusively above 40. In addition, the fasting insulin levels were not recorded on the single ended database and the quick index were not calculated.
Conclusion
Our study of patients requiring metabolic surgery in South Africa, over 5 years, demonstrates a better understanding of outcomes across all ethnic groups in the country. Our work is ongoing and subsequent data collection is continuing. With an ever-increasing and high prevalence of T2DM, hypertension, hyperlipidemia and sleep apnea, medical professionals will be facing a rapid increase in cardio metabolic disease. The foreseeable future may present with diminished public health care expenditure and limited human resources, more so after the COVID pandemic. Private healthcare funders will be considering the best health economic return on their investment, being the payers of metabolic surgery. In addition, funders in South Africa have a pay policy of ’one surgery per lifetime without further financial support’ for revision procedures. Decisions for primary choice of surgery type must therefore be carefully based on evidence of achieving the most durable co-morbidity remission rates. If we are to diminish our ever-increasing burden of non-communicable diseases, we will have to act with decisiveness and insight. Patients will have to be serviced in centers that can offer them the most appropriate choice of surgery at the most advanced level of care. Considering baseline demographics and outcome data can aid these decisions around surgery type as well as achieving the required remission of comorbidities associated with obesity.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent: Informed consent was obtained from all individual participants included in the study, for performing tests, collection of personal data, analysis of all data and for anonymous publication of that data.
Statements of Ethics
A retrospective analysis study was performed from data collected from SASSO. The study was performed at Netcare Waterfall Hospital in Midrand, South Africa. Ethical approval was obtained from the Netcare Limited Ethics Committee and Faculty of Health Sciences Research Ethics Committee of the University of Pretoria (205/2018). Informed consent was obtained from each patients prior to data collection. All patients were adults and could consent for themselves.
References
- Van Vollenstee FA, van der Merwe M-T (2021) Obesity and its implications for Covid-19 pandemic in South Africa. S.Afr. J. Infect 36: 288.
- Bennett G, Bardon LA, Gibney ER (2022) A comparison of dietary patterns and factors influencing food choice among ethnic groups living in one locality: A systematic review. Nutrients 14: 941.
- Casarie S, Paola MD, Banci E (2022) Changing dietary habits: The impact of urbanization and rising socio-economic status in families from Burkina Faso in Sub-Saharan Africa. Nutrients 14: 1782e.
- Van der Merwe M-T, Fetter G, Naidoo S (2015) Baseline patient profiling and three-year outcome data after metabolic surgery at a South African center of excellence. JEMDSA 20: 115-126.
- Welbourn R, Hollyman M, Kinsman R (2019) Bariatric Surgery Worldwide: Baseline Demographic Description and one-year outcomes from the fourth IFSO Global Registry Report 2018. Obes Surg 29: 782-795.
- Al-Jameil, Khan FA, Arjumand S (2014) Associated liver enzymes with hyperlipidemic profile in teyp 2 diabetes patients. Int J Clin Exp Pathol 7: 4345-4349.
- Mooradian AD (2009) Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab 5: 150-159.
- Harris EH (2005) Elevated Liver Function Tests in Type 2 Diabetes. Clin Diabetes 23: 115-119.
- Mandal A, Bhattarai B, Kafle P (2018) Elevated Liver Enzymes in Patients with Type 2 Diabetes Mellitus and Non-alcoholic Fatty Liver Disease. Cureus 10: e3626.
- Hariri K, Guevara D, Dong M (2018) Is bariatric surgery effective for co-morbidity resolution in the super-obese patients? SOARD 16: 1261-1268.
- Mechanick JI, Apovian C, Brethauer S (2020) Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures-2019 update: Cosponsored by Ameriacan Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society of Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Association of Anesthesiologists. SOARD 16: 175-247.
- Mingrone G, Panunzi S, De Gaetano A (2021) Metabolic Surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trail. Lancet 397: 293-304.
- Panunzi S, De Gaetano A, Carnicelli A (2015) Predictors of Remission of Diabetes Mellitus in severely obese individuals undergoing bariatric surgery: Do BMI or procedure choice matter? Ann Surg 261: 461-467.
- Keating C, Neovius M, Sjöholm K (2015) Healthcare costs during 15 years after bariatric surgery for patients with different baseline glucose status. Lacet Diabetes Endocrinol 3: 855-865.
- Rubino F, Nathan DM, Eckel RH (2016) Metabolic surgery in the treatment algorithm for type 2 diabetes: A joint statement by international diabetes organizations. Diabetes Care 39: 861-877.
- Saeedi P, Petersohn I, Salpea P (2019) IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 157: 107843.
- Chumakova-Orin M, Vanetta C, Moris DP (2021) Diabetes remission after bariatric surgery. World J Diabetes 12: 1093-1101.
- Kapeluto JE, Tchernof A, Masckauchan D (2020) Ten-year remission rates in insulin-treated type 2 diabetes after biliopancreatic diversion with duodenal switch. Surg Obes Relat Dis 16: 1701-1712.
- Frenken M, Kemmet O, Frenken M (2022) Long-term remission of Type 2 Diabetes and patient survival after biliopancreatic diversion with duodenal switch. Obes Surg 32: 3340-3350.
- Van der Merwe MT, Wing JR, Celgow LH (1996) Metabolic indices in relation to body composition changes during weight loss on Dexfenfluramine in obese women from two South African Ethnic groups. Int J Obes Relat Metab Disord 20: 768-776.
- Rubino F, Shukla A, Pomp A (2014) Bariatric, metabolic, and diabetes surgery: what’s in a name? Ann Surg 259: 117-122.
- Romeijn MM, Holthuijsen DB, Leclercq KG (2021) The effect of additional protein on lean body mass preservation in post-bariatric surgery patients: a systematic review. Nutr J 20: 1-9.
- Skogar M, Holmbäck U, Hedberg J (2017) Preserved fat-free mass after gastric bypass and duodenal switch. Obes Surg 27: 1735-1740.
- Sugerman HJ, Wolfe LG, Sica D (2003) Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg 237: 751-758.
- Buchwald H, Avidor Y, Braunwald E (2004) Bariatric surgery: a systematic review and meta-analysis. JAMA 292: 1724-1737.
- Ding L, Fan Y, Li H (2020) Comparative effectiveness of bariatric surgeries in patients with obesity and type 2 diabetes mellitus: A network meta-analysis of randomized controlled trials. Obes Rev 21: e13030.
- Benetti E, Chiazza F, Patel NSA (2013) The NLRP3 inflammasone as a novel player of the intracellular crosstalk in metabolic disorders. Mediators Inflamm 2013: 678627.
- Hounkpatin H, Stuart B, Farmer A (2021) Association of type 2 diabetes remission and risk of cardiovascular disease in pre-defined subgroups. Endocrinol Diabetes Metab 4: e00280.
- Nguyen NT, Varela JE (2017) Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol 14: 160-169.
- Esler WP, Bence KK (2019) Metabolic targets in nonalcoholic fatty liver disease. Cell Mol Gastroenterol Hepatol 8: 247-267.
- Mofrad P, Contos MJ, Haque M (2003) Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 37: 1286-1292.
- Neuman MG, Cohen LB, Nanau RM (2014) Biomarkers in nonalcoholic fatty liver disease. Can J Gastroenterol Hepatol 28: 607-618.
- Ando Y, Jou JH (2021) Nonalcoholic fatty liver disease and recent guideline updates. Clin Liver Dis (Hoboken) 17: 23-28.
- Chalasani N, Younossi Z, Lavine JE (2018) The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the study of liver diseases. Hepatology 67: 328-357.
- Sanyal AJ, Friedman S, McCullough AJ (2015) Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the study of liver diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology 61: 1392-1405.
- Azim S, Kashyap SR (2016) Bariatric Surgery: Pathophysiology and outcomes. Endocrinol Metab Clin North Am 45: 905-921.
- Rubino F, Forgione A, Cummings DE (2006) The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 244: 741-749.
- Aydogan U, Doganer YC, Ebiloglu A (2019) Projection of new thresholds for hypertension to outpatient clinic patients and impact of risk factors: a cross-sectional study. Sao Paulo Med J 137: 356-362.
- Pradhan A (2007) Obesity, metabolic syndrome and type 2 diabetes: Inflammatory basis of glucose metabolic disorders. Nutr Rev 65: S152-S156.
- Sun M, Zhang L, Chen S (2015) Association of C-reactive protein and metabolic disorder in a Chinese population. Int. J. Environ. Res. Public Health 12: 8228-8242.
- Tchernof A, Nolan A, Sites CK (2002) Weight loss reduces C-reactive protein levels in obese postmenopausal women. Circulation 105: 564569.
- Lim S, Lee HK, Kimm KC (2005) C-reactive protein level as an independent risk factor for metabolic syndrome in the Korean population. Diabetes Res Clin Pract 70: 126-133.
- Lagrand WK, Visser CA, Hermens WT (1999) C-reactive protein as a cariovascular risk factor more than an epiphenomenon. Circulation 100: 96-102.
- Xu Y, Zhu J, Gao L (2013) Hyperuricemia as an Independent Predictor of Vascular Complications and Mortality in Type 2 Diabetes Patients: A Meta-Analysis. PLoS ONE 8: e78206.
- Van Vollenstee FA, Van der Merwe MT (2021) Evaluating the pharmacoeconomic impact of nutrient supplementation postoperatively on patients receiving Roux-Y Gastric bypass vs. Biliopancreatic diversion with duodenal switch. Obes Surg 31: 24342443.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.