Medical Management and Outcome in Ectopic Pregnancy: Review of Outcome at Zulekha Hospital Sharjha
by Nora Sharafli*, Kaveeta Ramesh Kumar
Specialist Obstetrician and Gynecologist, Zulekha Hospital, Sharjah UAE
*Corresponding author: Nora Sharafali, Specialist Obstetrician and Gynecologist, Zulekha Hospital, Sharjah, UAE
Received Date: 13 July 2025
Accepted Date: 21 July 2025
Published Date: 23 July 2025
Citation: Sharafli N, Kaveeta RK (2025) Medical Management and outcome in Ectopic Pregnancy: Review of outcome at Zulekha Hospital Sharjha. Gynecol Obstet Open Acc 9: 240. https://doi.org/10.29011/2577-2236.100240
Abstract
Background and Objective: Ectopic pregnancy (EP) remains a significant cause of maternal morbidity and mortality, accounting for approximately 1–2% of all pregnancies and up to 10% of pregnancy-related deaths globally. This study aimed to evaluate the success rate, complications, and factors influencing the outcome of medical management of EP using methotrexate (MTX). Methods: A retrospective observational study was conducted at Zulekha Hospital, Sharjah, over three years (2021–2024). Medical records of patients diagnosed with EP and managed medically with MTX were reviewed. Data collected included demographics, clinical presentation, initial serum β-hCG levels, ectopic mass size and location, MTX dosage, number of doses, complications, and treatment outcomes. Results: A total of 40 patients with unruptured ectopic pregnancy were included. The mean initial serum β-hCG level was 1938.6 IU/L. Medical management with single-dose MTX was successful in 77.5%. 17.5% required a second dose, and 20.0% ultimately required surgical intervention. All patients requiring surgery failed medical therapy (p < 0.0001), while all patients not requiring surgery achieved successful resolution. No statistically significant association was found between clinical symptoms, history of EP, previous miscarriage, abdominal surgery, or presence of pelvic free fluid and treatment success (p > 0.05). Multivariable logistic regression identified the need for more than one dose of MTX (p=0.03) and surgical intervention (p=0.01) as independent predictors of treatment failure. Conclusion: Medical management with MTX is an effective and safe treatment option for patients with EP. Early diagnosis, patient selection, and close follow-up are critical to improving success rates and minimizing complications.
Keywords: Ectopic pregnancy, methotrexate, treatment outcome, gynecologic emergency
Introduction
Ectopic pregnancy (EP) is a life-threatening condition characterized by the implantation of a fertilized ovum outside the uterine cavity, most commonly in the fallopian tube. It accounts for approximately 1–2% of all pregnancies and remains a leading cause of first-trimester maternal morbidity and mortality [1]. Early diagnosis through transvaginal ultrasonography and serum β-human chorionic gonadotropin (hCG) measurement has enabled prompt treatment and better outcomes. Management of EP includes three main approaches: surgical, medical, and expectant. Methotrexate (MTX) therapy, introduced in the 1980s as a safer alternative to surgical intervention [2], is a folic acid antagonist that disrupts DNA synthesis and cell replication. It primarily targets rapidly dividing cells, such as trophoblasts [3]. MTX has been shown to effectively resolve EP, including less common rectal and retroperitoneal cases [4,5], while avoiding the risks associated with surgery and anesthesia. Nevertheless, treatment failure can occur, sometimes necessitating urgent surgical intervention [6].
Literature highlights prominent knowledge gaps. While the use of MTX for treating EP has become increasingly common [7]. there remains uncertainty regarding which pre-treatment patient and EP characteristics best predict suitability for MTX therapy [7]. Identifying predictors of treatment success and failure is essential to guide management decisions.
Therefore, this study reviewed all EP cases, analyzed the outcomes of medical management with MTX in patients with EP, and evaluated the clinical and laboratory factors influencing treatment success.
Methods
This is a retrospective cohort study conducted at the Department of Obstetrics and Gynaecology, Zulekha Hospital, over three years (August 1, 2021 – August 31, 2024). A total of 40 patients diagnosed with unruptured ectopic pregnancy were included in the study. All patients were hemodynamically stable and met the defined inclusion and exclusion criteria for MTX therapy. Ethical approval was obtained before data collection, and all patient identifiers were anonymized.
Inclusion Criteria
- Patients diagnosed with EP confirmed by ultrasound or other imaging modalities.
- Patients received an injection of MTX.
- Patients who underwent surgical management (e.g., salpingectomy).
- Diagnosis of pregnancy of unknown location
- Diagnosis of scar pregnancy
- Expectant management
Exclusion Criteria
Management Protocol
Upon confirmation of the diagnosis, baseline information including age, parity, obstetric history, presenting symptoms (e.g., amenorrhea, abdominal pain, vaginal bleeding), and relevant medical/surgical history was collected. A complete physical and pelvic examination was performed. Initial laboratory investigations included complete blood count, blood grouping and Rh typing, liver and renal function tests, and baseline serum β-hCG levels.
All patients received a single intramuscular dose of MTX at 50 mg/m² body surface area (BSA) on day 1. Serum β-hCG levels were measured on day 4 and day 7 post-treatment. If the decline in β-hCG from day 4 to day 7 was ≥15%, the treatment was considered successful, and β-hCG was monitored weekly until it reached non-pregnant levels. If the decrease was <15%, a second dose of methotrexate was administered. In cases where β-hCG failed to decline adequately after two doses or clinical deterioration occurred, surgical management was performed.
Statistical Analysis
Data was recorded using a pre-designed proforma and analysed using IBM SPSS Statistics version 21. Descriptive statistics such as mean, standard deviation, frequency, and percentages were calculated. Categorical variables were compared using the Chi-square test where appropriate, and continuous variables were analysed using independent t-tests. Multivariable logistic regression was performed to identify independent predictors of treatment success. A p-value <0.05 was considered statistically significant.
Results
Baseline Characteristics
Out of 40 patients included in the study, 7.5% were UAE nationals, while 92.5% were non-UAE nationals. Among the study participants, 35.0% were nulliparous, whereas 65.0% were multiparous. A history of previous miscarriage requiring dilation and curettage (D&C) was reported by 25.0%. 10.0% had a previous history of ectopic pregnancy, and 90.0% were experiencing their first ectopic event. Previous abdominal surgery was reported by 45.0%, while 55.0% had no such history (Figure 1).
Clinical and Demographic Characteristics
Of the 40 patients included in the study, abdominal pain was the most common presenting symptom, observed in 62.5%, while 37.5% reported no pain. Vaginal bleeding was noted at 72.5% at the time of the presentation, and absent in the remaining 27.5%. On clinical examinations, all patients had a soft abdomen. Abdominal tenderness was detected in 30.0%, with 67.5% showing no tenderness.
Regarding blood type, 2.5% were Rh-negative and appropriately received anti-D immunoglobulin. The remaining 97.5% were Rh-positive. Transvaginal ultrasonography revealed the presence of an adnexal mass in 100%. Pelvic free fluid was identified in 57.5%, while 42.5% showed no such finding. Right-sided ectopic pregnancies were more common in 62.5% compared to left-sided (37.5%).
The mean serum β-hCG level was 1938.6 IU/L on Day 1, rose slightly to 2504.2 IU/L by Day 4, and declined to 1544.6 IU/L on Day 7 (Table 1). The calculated mean body surface area (BSA) was 5.61 m², although this unusually high value may indicate a data input error. The average MTX dose administered was 86.4 mg, aligning with standard weight- or BSA-based dosing protocols (Figure 2).
Treatment Outcomes and Interventions
80.0% were treated successfully with a single dose of MTX. 17.5% required more than one dose, indicating a potentially more resistant disease course. Treatment outcome data were missing for 2.5%. Overall, medical management was successful in 77.5%, while 20.0% experienced treatment failure. Surgical intervention was required in 20.0%, all of whom were categorized as medical treatment failures. Conversely, among the patients who did not require surgery, all achieved successful resolution with medical therapy. This association between surgical intervention and treatment failure was statistically significant (p < 0.0001; Figure 3).
Influence of Patient History and Symptoms on Treatment Success
Among patients with a history of miscarriage treated by dilation and curettage, 22.6% achieved medical resolution, while 37.5% experienced treatment failure. In contrast, 24 of 29 patients without such a history, 77.4% succeeded, and 62.5% failed. The difference was not statistically significant (p=0.329; Table 2).
Four patients had a history of EP; 6.5% achieved treatment success, and 25.0% failed. Among patients without previous EP, 93.5% succeeded and 75.0% failed, with no significant difference observed (p=0.18). Similarly, prior abdominal surgery did not significantly influence treatment outcome (p=0.26), although a slightly higher failure rate was noted among those with previous surgical history (Tables 3 and 4).
Symptomatology at presentation also showed no significant association with treatment success. Among patients with abdominal pain, 64.5% were treated successfully, and 50.0% failed. Among those without pain, 35.5% succeeded and 50.0% failed (p=0.36). Vaginal bleeding showed a similar pattern; 21 of 28 patients
67.7% with bleeding succeeded, and 87.5% failed, while 10 of 11 without bleeding 32.3% succeeded and 12.5% failed (p=0.262). The presence of pelvic free fluid also did not correlate significantly with treatment outcomes (p=0.50; Tables 5, 6, and 7).
Among the patients who required more than one dose of methotrexate, 20.0% achieved treatment success, and 12.5% failed. In contrast, of the patients who received a single dose, 80.0% were successful, while 87.5% experienced treatment failure. There was no statistically significant association between requiring multiple doses and treatment outcome (p=0.538; Tables 8 and 9).
Predictors of Methotrexate Treatment Failure
Multivariable logistic regression analysis revealed that several clinical and historical factors were not significantly associated with MTX treatment success, including maternal age (OR 1.11; 95% CI: 0.88–1.40; p=0.40), gestational age at diagnosis (OR 1.22; 95% CI: 0.71–2.08; p=0.46), initial serum β-hCG levels (OR 1.00; p=0.33), presence of pelvic free fluid (OR 3.3; 95% CI: 0.4–28.5; p=0.27), history of ectopic pregnancy (OR 4.1; 95% CI: 0.4–37.6; p=0.22), and abdominal pain (OR 2.0; 95% CI: 0.2–19.1; p=0.53; Table 10). However, two factors emerged as significant independent predictors of treatment failure. Requiring more than one dose of MTX was significantly associated with failure (OR 16.5; 95% CI: 1.2–224.3; p=0.03), suggesting early drug resistance. Additionally, surgical intervention was a strong predictor of failed medical therapy (OR 33.1; 95% CI: 2.1–523.2; p=0.01).
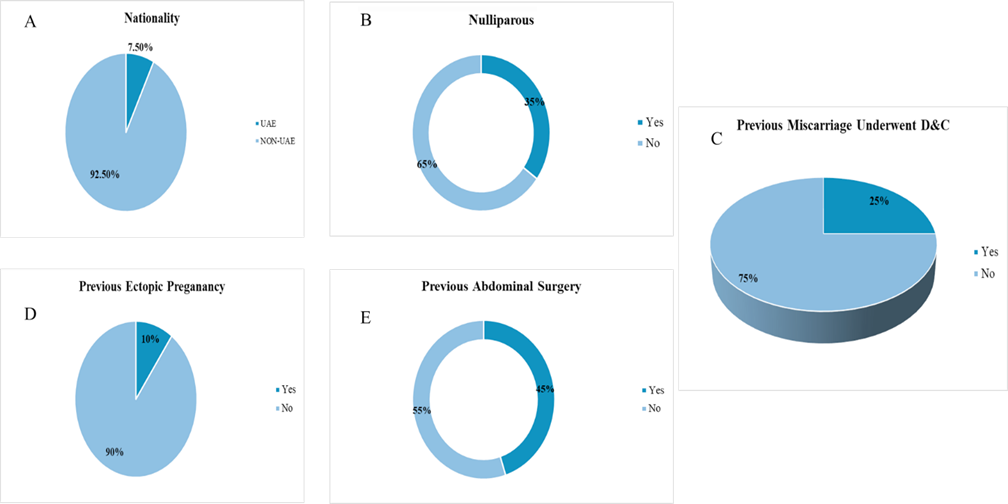
Figure 1: Baseline Characteristics
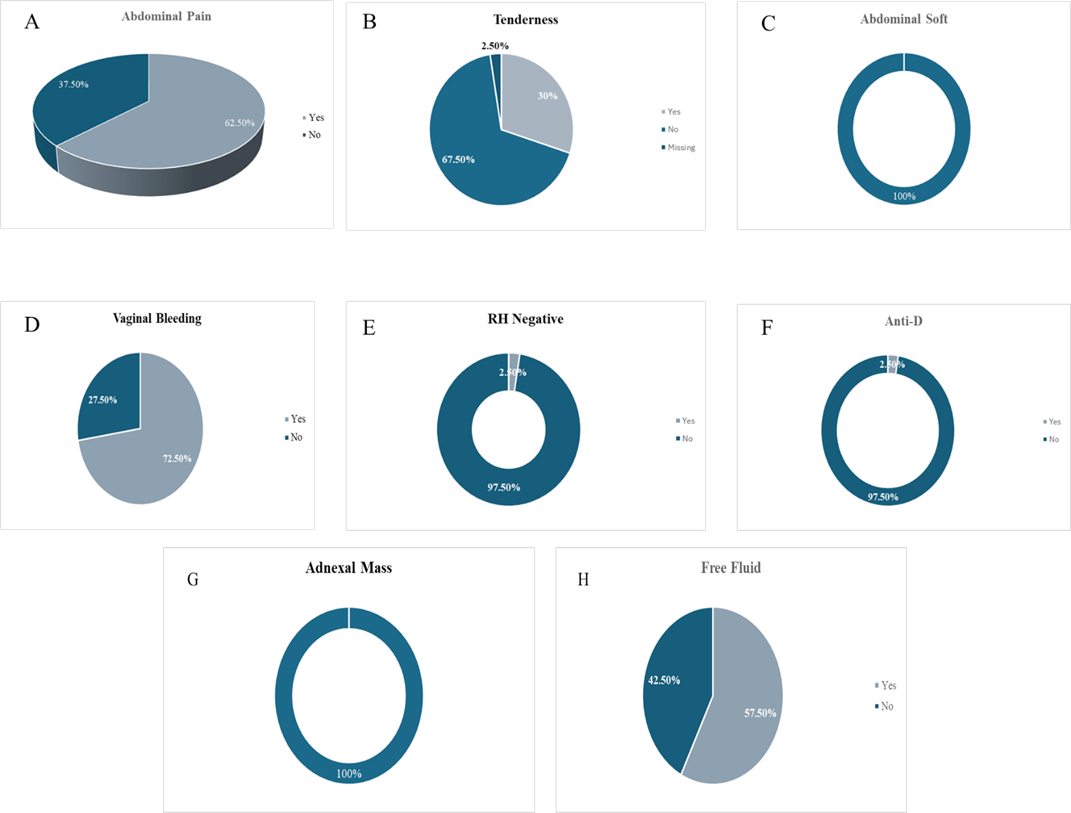
Figure 2: Clinical Findings
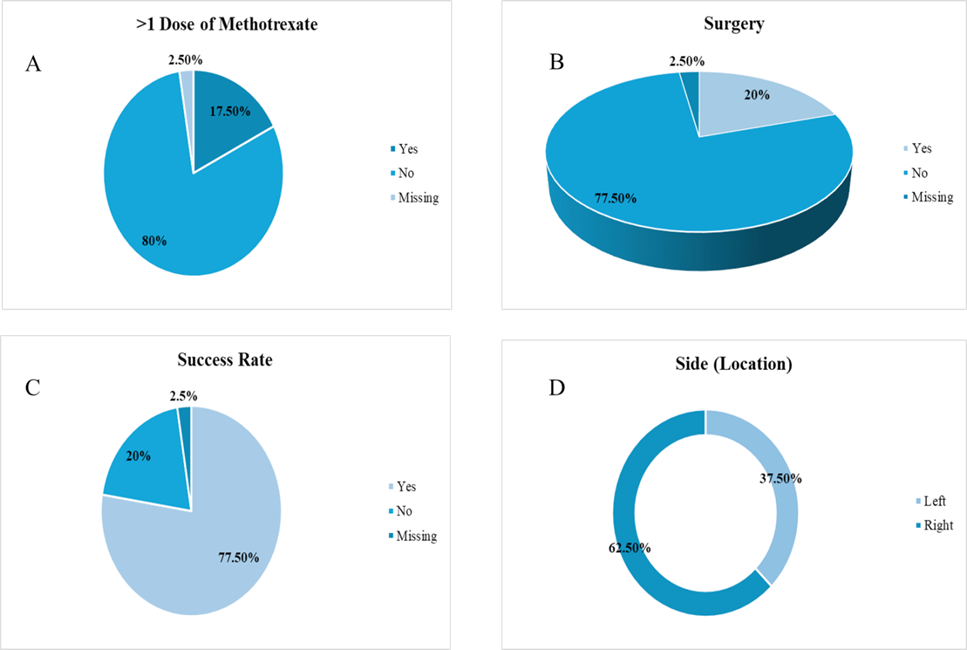
Figure 3: Treatment Outcomes and Interventions
|
Descriptive Statistics |
|||||
|
N |
Mean |
Std. Deviation |
Minimum |
Maximum |
|
|
BHCG1 |
40 |
1938.640 |
3128.3320 |
21.0 |
18887.0 |
|
BHCG 4 |
37 |
2504.189 |
3709.6809 |
14.0 |
15742.0 |
|
BHCG 7 |
33 |
1544.61 |
2146.406 |
0 |
7994 |
|
BSA (m^2) |
39 |
5.6126 |
24.05843 |
1.40 |
152.00 |
|
MTX dose (mg) |
40 |
86.410 |
9.1044 |
71.5 |
112.5 |
Table 1: BHCG, BSA, and MTX dose
|
Suc |
cess |
Total |
p-value |
|||
|
Yes |
No |
|||||
|
Yes |
Count |
7 |
3 |
10 |
0.329107 |
|
|
% within Success |
22.6% |
37.5% |
25.6% |
|||
|
No |
Count |
24 |
5 |
29 |
||
|
% within Success |
77.4% |
62.5% |
74.4% |
Table 2: Previous miscarriage and underwent D & C treatment.
|
Success |
Total |
p-value |
|||
|
Yes |
No |
||||
|
Yes |
Count |
13 |
5 |
18 |
0.26 |
|
% within Success |
41.9% |
62.5% |
46.2% |
||
|
No |
Count |
18 |
3 |
21 |
|
|
% within Success |
58.1% |
37.5% |
53.8% |
|
Success |
Total |
p-value |
|||
|
Yes |
No |
||||
|
Yes |
Count |
2 |
2 |
4 |
0.18 |
|
% within Success |
6.5% |
25.0% |
10.3% |
||
|
No |
Count |
29 |
6 |
35 |
|
|
% within Success |
93.5% |
75.0% |
89.7% |
Table 4: Previous abdominal surgery and treatment success
|
Success |
Total |
p-value |
|||
|
Yes |
No |
||||
|
Yes |
Count |
20 |
4 |
24 |
0.36 |
|
% within Success |
64.5% |
50.0% |
61.5% |
||
|
No |
Count |
11 |
4 |
15 |
|
|
% within Success |
35.5% |
50.0% |
38.5% |
Table 5: Abdominal pain and treatment success
|
Success |
Total |
p-value |
|||
|
Yes |
No |
||||
|
Yes |
Count |
21 |
7 |
28 |
0.262 |
|
% within Success |
67.7% |
87.5% |
71.8% |
||
|
No |
Count |
10 |
1 |
11 |
|
|
% within Success |
32.3% |
12.5% |
28.2% |
Table 6: Vaginal bleeding and treatment success
|
Success |
Total |
p-value |
|||
|
Yes |
No |
||||
|
Yes |
Count |
17 |
5 |
22 |
0.508 |
|
% within Success |
54.8% |
62.5% |
56.4% |
||
|
No |
Count |
14 |
3 |
17 |
|
|
% within Success |
45.2% |
37.5% |
43.6% |
Table 7: Free fluid and treatment success
|
Success |
Total |
p-value |
|||
|
Yes |
No |
||||
|
Yes |
Count |
6 |
1 |
7 |
0.537707 |
|
% within Success |
20.0% |
12.5% |
18.4% |
||
|
No |
Count |
24 |
7 |
31 |
|
|
% within Success |
80.0% |
87.5% |
81.6% |
Table 8: More than one dose of methotrexate and treatment success
|
Success |
Total |
p-value |
|||
|
Yes |
No |
||||
|
Yes |
Count |
0 |
8 |
8 |
<0.0001 |
|
% within Success |
0.0% |
100.0% |
20.5% |
||
|
No |
Count |
31 |
0 |
31 |
|
|
% within Success |
100.0% |
0.0% |
79.5% |
Table 9: Surgery and treatment success
|
Predictor |
B |
SE |
OR (95% CI) |
p-value |
|
Age (years) |
0.10 |
0.12 |
1.11 (0.88–1.40) |
0.40 |
|
Gestational Age (weeks) |
0.20 |
0.28 |
1.22 (0.71–2.08) |
0.46 |
|
Initial BhCG (IU/L) |
0.00 |
0.00 |
1.00 (1.00–1.00) |
0.33 |
|
More than 1 MTX dose (Yes) |
2.8 |
1.3 |
16.5 (1.2–224.3) |
0.03* |
|
Surgery Required (Yes) |
3.5 |
1.6 |
33.1 (2.1–523.2) |
0.01* |
|
Free Fluid (Yes) |
1.2 |
1.1 |
3.3 (0.4–28.5) |
0.27 |
|
Previous Ectopic Pregnancy (Yes) |
1.4 |
1.2 |
4.1 (0.4–37.6) |
0.22 |
|
Abdominal Pain (Yes) |
0.7 |
1.1 |
2.0 (0.2–19.1) |
0.53 |
Table 10: Logistic regression predictors of medical management success
Discussion
This study evaluated the clinical outcomes of medical management using MTX in patients with unruptured EP. The findings demonstrate a high overall success rate with single-dose MTX therapy, consistent with previous literature, which cites success rates between 72% [8] and 96% [9]. The 17.5% failure rate, requiring surgical intervention, underscores the importance of patient selection and close monitoring.
The clinical outcomes of MTX for the medical management of unruptured EP have been well documented in multiple studies. Success rates vary but generally range from about 55% to over 90%, influenced primarily by patient selection criteria such as initial β-hCG levels below 5,000 mIU/mL and ectopic mass size under 4 cm. For instance, one study reported a 55.3% success rate overall, with local MTX administration achieving 87.5% success compared to systemic routes [10]. Another study found a 65% success rate in small unruptured EP treated with a single dose of MTX, with an average resolution time of 32 days for single-dose therapy and 58 days for multiple doses [11]. Side effects were generally mild and infrequent. Fertility outcomes post-treatment are favorable, with tubal patency rates around 80% and subsequent intrauterine pregnancy rates ranging from 30% to nearly 90%, though recurrent ectopic pregnancy risk remains low but present [9]. A large, randomized trial also confirmed that single-dose intramuscular MTX leads to resolution in a median of 28 days, with about 29% of patients requiring surgery and 14% needing a second MTX dose, supporting the efficacy and safety of MTX in this setting [12]. Overall, MTX offers a less invasive, cost-effective alternative to surgery for selected patients with unruptured EP, emphasizing the importance of careful patient selection and close follow-up to optimize outcomes.
The relationship between patient obstetric history and MTX treatment outcomes in unruptured EP is nuanced. While multiparity was more prevalent in the studied cohort-potentially reflecting cumulative reproductive risk factors like pelvic inflammatory disease or tubal damage-it did not independently predict MTX failure [11]. Similarly, parity itself, history of miscarriage requiring D&C, and prior EP showed no statistically significant association with treatment success or failure in this group. This contrasts with earlier studies identifying previous EP as a risk factor for MTX failure (odds ratio 3.12), suggesting that strict patient selection criteria (e.g., β-hCG <5,000 mIU/mL, mass size <4 cm, hemodynamic stability) in the current cohort may mitigate this risk [11]. For example, while prior EP increased failure rates in other studies, the focus on optimal candidates (e.g., low β-hCG, small mass) in this cohort likely overshadowed obstetric history as a predictor. Instead, factors like initial β-hCG levels and ectopic mass characteristics (e.g., subchorionic hematoma, embryonic presence) emerged as stronger determinants of MTX success [13]. These findings underscore that obstetric history alone may not reliably predict MTX outcomes when rigorous clinical and biochemical selection criteria are applied.
Clinically, abdominal pain and vaginal bleeding are common presenting symptoms in EP; however, these symptoms do not show statistical significance as predictors of treatment outcome. In contrast, the presence of an adnexal mass was observed in nearly all cases, and free intraperitoneal fluid was present in over half, underscoring the critical role of early and accurate transvaginal ultrasonography in diagnosing EP before rupture. Transvaginal ultrasonography demonstrates high sensitivity and specificity for detecting ectopic pregnancies, with adnexal masses-particularly noncystic masses such as tubal rings or gestational sacs-being the most reliable sonographic finding, present in approximately 89–95% of cases. Additionally, free fluid, especially when moderate to large and containing blood products or debris, supports the diagnosis, though it is less specific. The combination of TVS findings with serum β-hCG measurements further enhances diagnostic accuracy, allowing for timely identification and management. Importantly, the absence of an intrauterine gestational sac alongside these adnexal findings should prompt close monitoring, as EP cannot be excluded even if adnexal masses are not initially visualized [14,15]. Thus, reliance on clinical symptoms alone is insufficient, and transvaginal ultrasonography remains indispensable for early detection and prevention of complications such as tubal rupture.
β-hCG trends in patients treated with methotrexate for unruptured EP typically show an initial rise followed by a decline, reflecting the expected pharmacodynamic response to therapy. However, the predictive value of initial β-hCG levels and their changes over time remains controversial. While some studies emphasize that lower initial β-hCG levels (commonly below 1,362 to 5,000 mIU/mL) correlate with higher success rates of MTX treatment [16,17]. Others report that neither the baseline β-hCG nor its early temporal changes independently predict treatment success when considered alone. For example, a decline in β-hCG between days 4 and 7 post-treatment of more than 15% is generally regarded as a strong indicator of successful therapy, yet some data suggest that initial levels and early rises or falls in β-hCG do not independently determine outcomes without considering other clinical factors [17]. This indicates that β-hCG trends should be interpreted in conjunction with clinical parameters such as ectopic mass size, presence of free fluid, and patient stability rather than as sole predictors. Therefore, while β-hCG monitoring is essential for assessing response to MTX, initial levels and early changes alone may not reliably forecast treatment success.
Taken together, MTX remains a safe, effective, and fertilitypreserving treatment for unruptured EP in appropriately selected patients. However, the need for multiple doses or surgical intervention should alert clinicians to potential treatment failure, emphasizing the value of vigilant monitoring and timely decisionmaking.
The study’s limitations include a relatively small sample size and missing follow-up data in one patient. Nevertheless, the findings are consistent with prior studies and reinforce that MTX is an effective and safe first-line option in most stable EP. Future studies with larger cohorts and longer follow-up may further refine the predictors of treatment success and inform individualized care.
Conclusion
This study confirms that MTX is a highly effective first-line treatment for carefully selected, hemodynamically stable patients with unruptured EP, achieving a medical resolution rate of 77.5%. While most clinical factors, including symptom presentation, obstetric history, and presence of pelvic free fluid, were not significantly associated with treatment outcomes, the need for a second MTX dose and surgical intervention were strong independent predictors of treatment failure. These findings highlight the importance of close follow-up and early identification of patients who may not respond to single-dose therapy.
Ethical Considerations:
This study was approved by Zulekha Hospital Ethics Committee (approval no. S-01/2025).
Conflict of Interest:
The authors have no conflicts of interest related to this publication.
References
- Jurkovic D, Memtsa M, Sawyer E, Donaldson A, Jamil A, et al. (2017) Single‐dose systemic methotrexate vs expectant management for treatment of tubal ectopic pregnancy: a placebo‐controlled randomized trial. Wiley Online Library.
- Glock J L, Johnson J V, Brumsted J R (1994). Efficacy and safety of single-dose systemic methotrexate in the treatment of ectopic pregnancy. Fertil Steril 62: 716-721.
- Stika C S (2012). Methotrexate: the pharmacology behind medical treatment for ectopic pregnancy. Clin Obstet Gynaecol 55: 433-439.
- Huang X, Zhong R, Tan X, Zeng L, Jiang K, et al. (2019) Conservative management of retroperitoneal ectopic pregnancy by computed tomographic–guided methotrexate injection in the gestational sac: 2 case reports and literature review. J Minim Invasive Gynecol 26: 1187-1192.
- Le D N, Nguyen P N (2023). Rectal ectopic pregnancy managed successfully by minimally invasive treatment using local methotrexate injection and systemic methotrexate administration: an extremely rare case at Tu Du Hospital in Vietnam and literature review. J Minim Invasive Gynecol, 30: 418-423.
- Gnisci A, Rua S, Courbiere B, Cravello L, Gamerre M, et al. (2011). Plasma creatine phosphokinase level may predict successful treatment after a single injection of methotrexate for ectopic pregnancy. Fertil Steril 95: 2131-2133.
- Zhang J, Zhang Y, Gan L, Liu X.-y, et al. (2020) Predictors and clinical features of methotrexate (MTX) therapy for ectopic pregnancy. BMC Pregnancy and Childbirth 20: 1-9.
- Sendy F, AlShehri E, AlAjmi A, Bamanie E, Appani S, et al. (2015). Failure rate of single dose methotrexate in managment of ectopic pregnancy. Obstet Gynecol Int 902426.
- Stovall T G, Ling F W, Gray L A, Carson S A, Buster J E (1991). Methotrexate treatment of unruptured ectopic pregnancy: a report of 100 cases. Obstet Gynecol, 77: 749-753.
- Kim M J, Cha J H, Bae H S, Kim M K, Kim M L, et al. (2017). Therapeutic outcomes of methotrexate injection in unruptured interstitial pregnancy. Obstet Gynecol Sci 60: 571-578.
- Dhar H, Hamdi I, Rathi B (2011). Methotrexate treatment of ectopic pregnancy: experience at nizwa hospital with literature review. Oman Med J, 26: 94-98.
- Horne A W, Tong S, Moakes C A, Middleton L J, Duncan W C, et al. (2023) Combination of gefitinib and methotrexate to treat tubal ectopic pregnancy (GEM3): a multicentre, randomised, double-blind, placebocontrolled trial. The Lancet, 401: 655-663.
- Lipscomb G H, Givens V A, Meyer N L, Bran D (2004). Previous ectopic pregnancy as a predictor of failure of systemic methotrexate therapy. Fertil Steril 81: 1221-1224.
- Lee R, Dupuis C, Chen B, Smith A, Kim YH (2018). Diagnosing ectopic pregnancy in the emergency setting. Ultrasonography, 37: 78-87.
- Madani Y (2008) The use of ultrasonography in the diagnosis of ectopic pregnancy: a case report and review of the literature. Medscape J Med 10: 35.
- Pulatoglu C, Dogan O, Basbug A, Kaya A E, Yildiz A, et al. (2018) Predictive factors of methotrexate treatment success in ectopic pregnancy: A single-center tertiary study. North Clin Istanb, 5: 227-231.
- Ray A, Gaur A, Kumari S (2022) Predictors of Successful Medical Management With Methotrexate in Unruptured Tubal Ectopic Pregnancy. Cureus 14: e31923.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.