Management Strategies for Recurrent Mechanical Mitral Valve Thrombosis: A Challenging Case of a Young Patient on Acenocoumarin, Presenting with Low Cardiac Output Syndrome
by Said Khallikane1*, Mehdi Didi2, Mehdi Nabil2, Hicham Kbiri2, Ayoub Dahioui3, Reda Mounir4, Hamid Jalal5, Noureddine Atmani6, Abdelmajid Bouzerda7, Abdessamad Abdou8, Issam Serghini9, Ayoub Belhadj10, Younes Aissaoui10, Youssef Qamouss11
1Former Anesthesiologist, Professor of Anesthesia-Intensive Medicine, Cardiothoracic Anesthesiologist, Cardiothoracic Anesthesia-Cardiovascular ICU, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco, Ex-Former anesthesiologist-intensivist and Head of Anesthesia-ICU Department in HASSAN II Military Hospital, Layun 70000, Morocco
2Anesthesiologist-Intensivist, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco, Morocco
3Cardiothoracic and Vascular Surgery, trainee resident in Cardiac Surgery, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco, Morocco
4Cardiothoracic and Vascular Surgeon, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco, Morocco
5Cardiologist, Cardio-echocardiographic, Professor of Cardiology, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco, Morocco
6Cardiothoracic and Vascular Surgeon, Professor of Cardiac Surgery, mini-invasive Cardiac Surgery, Interventional Cardiovascular Surgery, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco, Morocco
7Cardiologist, Interventional Cardiologist, Head of the Cardiology Department, Premier Medical-Surgical Center,
Professor of High Medical Education in Cardiology-Cardiovascular ICU- Interventional Cardiology, University Cadi Ayyad, Faculty of Medicine and Pharmacy of Marrakech 4000, Kingdom of Morocco, Morocco
8Head of Cardiothoracic and Vascular Surgeon, Professor of Cardiac Surgery, mini-invasive Cardiac Surgery, Interventional Cardiovascular Surgery, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco, Morocco
9Former Anesthesiologist-Intensivist. Professor of High Medical Education in Anesthesiology-Critical Care, at Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco, Morocco
10Head of Surgical ICU, Professor of High Medical Education in Anesthesiology-Critical Care, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco, Morocco
11Chief of Anesthesiology-ICU-Emergency Department, Anesthesiologist-Intensivist, Professor of High Medical Education Grade C in Anesthesiology-Intensive Medicine, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco, Morocco
*Corresponding author: Said Khallikane, Former Anesthesiologist, Professor of Anesthesia-Intensive Medicine, Cardiothoracic Anesthesiologist, Cardiothoracic Anesthesia-Cardiovascular ICU, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco, Ex-Former anesthesiologist-intensivist and Head of Anesthesia-ICU Department in HASSAN II Military Hospital, Layun 70000, Morocco
Received Date: 16 November 2024
Accepted Date: 18 November 2024
Published Date: 19 November 2024
Citation: Khallikane S, Didi M, Nabil M, Kbiri H, Dahioui A, et al (2024) Management Strategies for Recurrent Mechanical Mitral Valve Thrombosis: A Challenging Case of a Young Patient on Acenocoumarin, Presenting with Low Cardiac Output Syndrome. Ann Case Report. 9: 2077. https://doi.org/10.29011/2574-7754.102077
Abstract
Mechanical mitral valve thrombosis is a serious complication following valve replacement, characterized by thrombus formation due to exposure to low-pressure blood flow in the left atrium. Various risk factors, including valve design, inadequate preoperative anticoagulation, and abnormal loading conditions, contribute to its occurrence. We illustrate the management of this challenging complication with a literature review through the case of a 22-year-old female patient who underwent mechanical mitral valve replacement and tricuspid valve repair for severe rheumatic mitral stenosis complicated by atrial fibrillation and mild pulmonary hypertension. One-year post-surgery, she developed subacute heart failure symptoms after dental surgery, and echocardiography revealed thrombus on her mechanical valve. Although she was treated with anticoagulation and showed symptom improvement, she returned a year later with acute congestive heart failure. Severe mitral regurgitation due to valve obstruction from thrombi caused shock. Urgent surgery revealed valve thrombosis with an easy weaning from cardiopulmonary bypass and a transient improvement in hemodynamic parameters before she developed septic shock originating from the lungs and subsequently passed away. Valve thrombosis has multiple causes and nonspecific symptoms. Anticoagulation is crucial for prevention, with surgery as a last resort. Comprehensive management is necessary. Integrated approach with anticoagulation, thrombolytic therapy, and surgery. Education and tailored treatment plans are crucial. Future studies should focus on novel anticoagulants.
Keywords: Mechanical mitral valve thrombosis; Patient management; Anticoagulation; Hemodynamic compromise; Surgical intervention; Thrombectomy; Valve replacement; Thrombolytic therapy.
Introduction
Valve replacements have become increasingly popular as a medical practice, given that they are the definitive therapy for valvular heart diseases. The number of implants is estimated to continually increase with an estimated growth of over 1 million annually. However, despite its increasing popularity, prosthetic valve thrombosis remains a life-threatening complication. The significantly high risk of death, recurrent thromboembolic events, and treatment requirements in a substantial portion of patients clearly emphasize the need for and significance of this review of literature. Owing to the drastic impact of mechanical mitral valve thrombosis, it is highly important to focus on how to effectively manage, monitor, prevent, and treat patients suffering from this condition to improve outcomes, quality of life, and survival [1]. The aim of this international literature review, through this clinical case will be to achieve a deep understanding of the etiology, pathogenesis, and causality of mechanical thrombus formation, whereas the subsequent essay will focus on clinical and physiological aspects as well as the management of mitral mechanical valve thrombosis. Every year, patients in the United States alone require about 95,000 valve replacements due to acquired valvular heart diseases. Of these, 66% of prosthetic valves are of mechanical composition. Prosthetic mechanical valves replacements (MVRs) can stay in the body for more than 10 years from the time of initial replacement. Over their lifetime, they are subject to subacute or nonfatal complications, which include Prosthetic Valve Thrombosis [2]. As different imaging modalities and concepts have been developed, we can find that patients suffering from this condition range in varying degrees with levels of urgency and acuity. It is clear to see that the management of MVR thrombosis requires a strong understanding of the condition from both a clinical and physiological standpoint to discover the most efficacious and safe ways to treat it. The main essay on the etiology, pathogenesis, and causality of MVR thrombosis will be followed by a conclusive essay that details every possible medical, interventional, and surgical management possibility. More recent advances may also be included in the essay. After the initiation of oral Anticoagulation (OAC) to enhance effective, safe, and hospital strategies, the same patient journey from the inception of their first OAC is followed [1,3].
Case Report
We present the clinical case of a 22-year-old female patient who underwent surgery for mitral stenosis complicated by atrial fibrillation and pulmonary hypertension. She received a mechanical valve and tricuspid valve repair. A year later, she experienced worsening symptoms. Examination revealed a thrombus on the mechanical valve. She was managed in the cardiac intensive care unit and received aspirin and heparin. Post-treatment, there was improvement in the transvalvular gradient and partial regression of the thrombus. She was discharged on acenocoumarin with regular monitoring. However, after one year (2 years post-surgery), she arrived at the emergency room with a more severe episode of acute global congestive heart failure. Upon clinical examination, crackles were detected in the lungs, along with mild hypoxia (O2 saturation 93%), and absence of fever or lower limb swelling. Blood pressure measured at 90/60 mmHg, with tachycardic atrial fibrillation (120 bpm) and a respiratory rate of 30/min. Chest X-ray revealed cardiomegaly and bilateral pulmonary edema. (Figure 1) Blood tests showed leukocytosis, elevated NT-proBNP levels (800), and moderate metabolic acidosis. Echocardiography documented near-complete blockage of the mechanical mitral valve leaflets with high transvalvular gradient and significant regurgitation. After initial stabilization with norepinephrine, heparin, diuretics, and dual antiplatelet therapy, her condition deteriorated on the fifth day with shock, hypoxia, and confusion, raising concerns about possible systemic embolisms, including secondary cerebral embolism, which was ruled out by a brain CT scan. (Figure 2) In a situation of mitral valve blood clot causing cardiogenic shock, norepinephrine dosage was increased to 15 mcg/kg/min and dobutamine started at 5 mcg/kg/min. TransThoracic Echocardiography (TTE)showed severe pulmonary hypertension, distended left atrium, high transvalvular gradient, severe mitral regurgitation, and hyperkinetic Left Ventricle (LV). Epinephrine was given, dobutamine stopped, and patient moved to operating room with equipment for perfusion and transfusion, and received vitamin K, exacyl, Vancomycin, and heating blanket.
General anesthesia induced: 250 µg fentanyl, 0.3 mg/kg etomidate, and 1.2 mg/kg rocuronium. Anesthesia maintained with sevoflurane (MAC = 1.0). Analgesia maintained: 250 µg fentanyl boluses every 30–40 min; blood gas analysis 10 min after intubation. TEE probe inserted using video laryngoscope. Patient stable with low-dose vasoactive support before cardiopulmonary bypass. Doppler ultrasound in Scarpa triangle to study femoral vein and artery, found to be patent but small in size, especially femoral artery (8 mm). TEE performed pre-sternotomy to define anesthesia and surgical strategy, evaluate lesions, and address hemodynamic instability before CPB. Bicaval view used to monitor venous cannula progression. (video 1) Upon opening Scarpa triangle, common femoral artery was smaller than available venous cannulae. Central cannulation was decided upon, with TEE monitoring throughout. LV appeared small with limited opening of one valve leaflet and complete blockage of the other. Mitral valve apparatus identified with thrombi. Anterior and inferior walls of LV retained kinetics. Oblique plane revealed LV inflow chamber and outflow tract. Right ventricle was dilated with normal aortic ring. Leaking annuloplasty seen in right atrium. Left atrium, tricuspid valve, and right ventricle were thrombus-free. In mid-esophageal view, St. Jude mechanical valve had thickened ring with thrombi on both sides. (Figure 3) Pulsed Doppler TEE at the mechanical mitral valve in the mid-esophageal view, at a perpendicular plane of 60 degrees, showing a holosystolic retrograde mitral flow velocity of 1.3 m/s. (video 2) Slight probe rotation revealed left atrium dilation, thickened leaflets, limited leaflet movement at heart crux, and blockage at atrium-ventricle junction. Hyper-echoic thrombi in left ventricle were partly obscured. (Figure 3b) TEE in 2D mode, transducer at 45° counter clockwise rotation, shows dilated left atrium with obstructed mechanical valve due to multiple thrombi at leaflets, ring, and core. Leaflet opening beneath appendage is absent and limited on opposite side. Ring appears thickened with fibrinous thrombus. Reverberation echoes obscure left ventricle walls except for anterior and inferior walls with preserved kinetic activity (Video 3).
2D TEE in a mid-esophageal view at 105-to-135-degree angle, showing thrombi on atrial and ventricular side of mechanical valve, a thicken appearance of valve ring and multiple thrombi (Video 4).
TEE in colour Doppler mode centered on the thrombosed mechanical mitral valve, showing significant mitral regurgitation reaching the depth of the left atrium. (Video 5) At 105 to 145 degrees, 2D TEE in a mid-esophageal view, The septum and mitral valve had thrombi on both sides and a thickened valve ring. Multiple thrombi were on the ventricular side. Mean transvalvular gradient was 27.85 mm Hg with VTI of 109.4 cm, indicating stenosis. Severe mitral regurgitation was observed with a gradient of 28.80 mm Hg.
(Figure 4,5) (Video 6,7) At 110-130°, a biatrial view displayed the interatrial septum, with short-axis cuts on the left atrium, aorta, pulmonary pathway, and aortic root, right atrium, left atrium, and pulmonary artery. At 30 cm, the aortic root appeared different at an angle. At 45°, sinuses were analysed. The 135° cut explored the ascending aorta. In the esophageal view, a counter clockwise rotation revealed a horn-shaped left auricle without thrombus. Doppler showed biphasic flow and negative flow during ventricular systole. The left superior pulmonary vein (LSPV) was observed above the left atrium at 0 and 45°, with continuous red flow. Pulsed Doppler showed positive S and D waves and absence A wave during atrial systole. A visible spur was not a neoformation. (video 8,9).
The aorta was analysed in TEE at three levels: ascending, horizontal, and descending. Various views allowed detailed analysis of caliber and wall condition. Probe rotation enabled visualization of the circular section of the aorta. A sternum incision was made on the previous scar. 500 µg fentanyl was given prior to sternotomy. Repeated doses of 0.2 mg/kg rocuronium were administered based on TOF values.
Sternotomy done with electric saw; thoracic cavity dissected. Vancomycin given, propofol and fentanyl-maintained anesthesia. CPB established with cannulas in aorta and veins. Heparin injected; ACT monitored. Normothermic CPB initiated, ventilation reduced during bypass, and cessation of ventilation during full-flow CPB (Figure 6). Endotracheal sevoflurane stopped, Diprivan AIVOC continued with fentanyl reinjection via CPB circuit. Antegrade cardioplegia started using trocar in aortic root. Mechanical mitral valve had total thrombosis with fresh and old thrombi filling valve ring. Leaflets blocked (Figure 7) After thorough washing and cleaning of the valve with saline, multiple ventricular thrombi were removed, successfully restoring full function to the mechanical valve. (Figure 8,9).
Intraoperative assessment showed replacement unnecessary. Adequate oxygen delivery and extraction confirmed. Hematocrit: 20, transfused 2 units of red blood cells. MAP maintained at 65-75 mm Hg. Left atrium closed, DE bubbling for venting cardiac chambers. Aorta unclamped, continuous venting, electrodes placed, spontaneous defibrillation. Clamping duration: 90 mins. Heart loaded; ventilation resumed after TEE control. Weaned from CPB with gradual pump flow reduction and resumed ventilation. Cardiac function restored under dobutamine at 5 µg/kg/min and norepinephrine at 2 µg/kg/min, initial sinus rhythm: 80 bpm. Right atrial DE cannulation performed, blood retrieved from reservoir via aortic cannula due to hypovolemia observed on TEE. Heparin antagonized with protamine in a 1:1 ratio, aortic cannula removed. Hemostasis checked before closing thoracic cage. Drains inserted before closing sternum with steel wires. Skin closed with absorbable sutures. CPB duration: 125 mins. Post-CPB TEE under dobutamine at 4 µg/kg/min and norepinephrine at 2 µg/kg/min showed good mitral valve functionality, good LV contractility, improved subaortic velocities, and high filling pressures. (Video 10) (Figure 10).
The patient was intubated, ventilated, hemodynamically stable, and transferred to cardiovascular intensive care. Vital parameters, oxygenation, and cardiac function were continuously monitored. A blood gas test after weaning from cardiopulmonary bypass (CPB) hours showed compensated lactic metabolic acidosis with a lactate level of 4.5 mmol/L, a pH of 7.36, HCO3 of 21,5 mEq/L, BE(S) of -4, PaCO2 of 38 mmHg, Ht of 20%, and PaO2 of 134 mmHg on 45% FiO2. There was a slight false hyponatremia at 131 mmol/L, corrected to 138 mmol/L in view of hyperglycemia, potassium level at 2.4 mmol/L, and hypocalcemia at 0.88 mmol/L (Figure 11).
Transfusion of 2 units of packed red blood cells, with correction of potassium levels and calcium supplementation, was administered. A blood gas test at 2 hours post-op showed compensated lactic metabolic acidosis with a lactate level of 4.5 mmol/L, a pH of 7.37, HCO3 of 21 mEq/L, BE of -5, PaCO2 of 34 mmHg, Ht of 25%, and PaO2 of 157 mmHg on 40% FiO2. There was a slight false hyponatremia at 132 mmol/L, corrected to 137 mmol/L in view of hyperglycemia, potassium level at 3.5 mmol/L, and slight hypocalcemia at 0.99 mmol/L (Figure 12). Repeated TEE showed reassuring results regarding the function of the mechanical valve. in 2D and Doppler. (Figure 13,14). On postoperative day 4, high-dose heparin (HBP) was given. The next day, the patient showed signs of instability, including low blood pressure and decreased urine output. They also had a fever, increased heart rate, and increased oxygen needs. TTE showed no issues with the heart valves or pericardial effusion. Blood cultures were taken and antibiotics started for pneumonia. Chest X-ray and CT scan confirmed pneumonia. (Figure 15) Despite aggressive treatment, the patient developed refractory septic shock with a pulmonary origin and passed away on postoperative day 3.
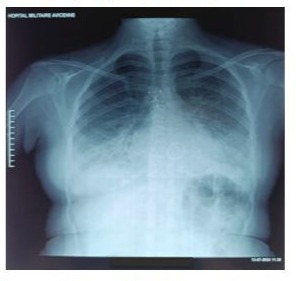
Figure 1: Preoperative frontal chest X-ray showing bilateral basal opacities with blunting of both pleural recesses, a normal-appearing cardiac silhouette, sternal wire sutures, and the projection of the mechanical valve ring.
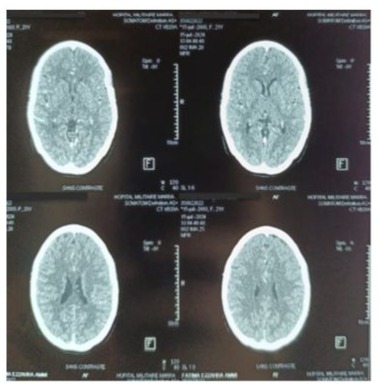
Figure 2:Contrast brain CT scan in axial slices, performed to investigate delayed thromboembolic complications, returned without abnormalities.
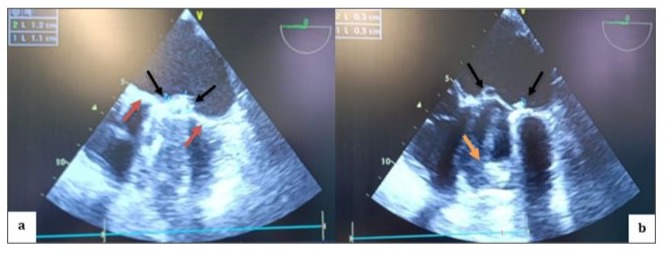
Figure 3: TEE in mid-esophageal view, transducer at 0 degrees, showing a thickened appearance of the St. Jude mechanical valve ring in an anatomical anti-position (a) (red arrow) with multiple thrombi on the atrial (a; b) (black arrow) and ventricular (b) (orange arrow) sides of both the internal and external leaflets.
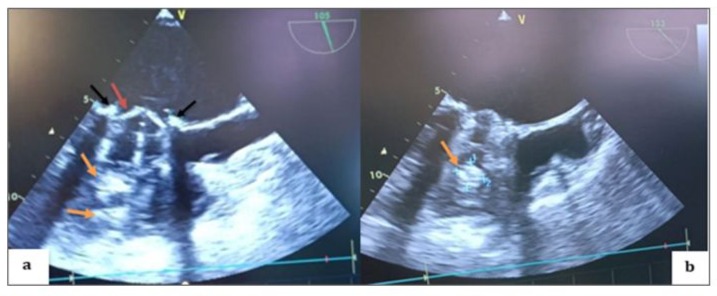
Figure 4: TEE 2D in the mid-esophageal view, transducer at 105 degrees (a; b), showing thrombi on the atrial side of both the internal and external leaflets of the mechanical valve on the aortic valve side as well as on the opposite side (black arrows), with a thickened appearance of the mechanical valve ring (red arrow) and multiple thrombi on the ventricular side (orange arrows).

Figure 5: TEE Doppler with the transducer at 105 degrees showing a very high mean transvalvular gradient of 27.85 mm Hg on continuous Doppler, with a calculated valve area of 109.4 cm² (a) in favor of stenosis, and severe thrombotic regurgitation with a mean gradient of 28.80 mm Hg (b) in favor of severe mitral regurgitation.

Figure 6: Flow of the CPB pump at 3.7 L/min based on 2.4 L/min/m² in normothermia to obtain a MAP of 65 to 75 mmHg, SvO₂ above 75%, SaO₂ at 100%, with good venous return from the superior and inferior venous cannulas and an antegrade injection pressure in the aortic root measured at 194 mmHg.
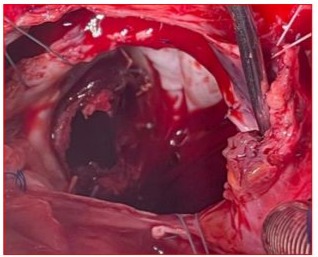
Figure 7 : Surgical image after right arteriotomy and expanded transseptal approach at the roof of the left atrium showing the appearance of the St. Jude mechanical valve completely thrombosed, encompassing the ring, both leaflets, and the core.
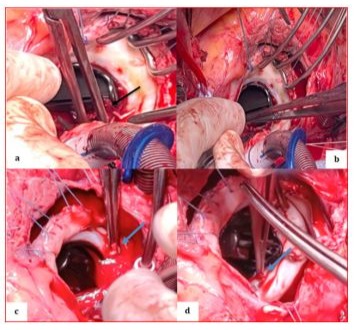
Figure 8: Perioperative image showing the St. Jude mechanical valve partially cleaned in its initial anatomical anti-position during the extraction of remaining thrombi using forceps on the atrial side (a) (black arrow), the ventricular side (b) on the internal and external leaflets, and at the level of the coated ring (c, d) (blue arrows).
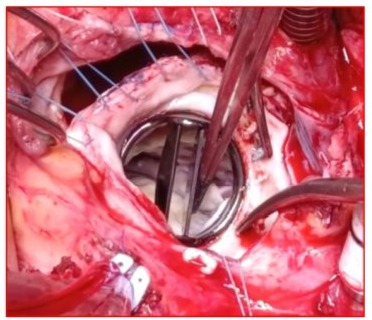
Figure 9: Surgical image showing a clean appearance of the St. Jude Medical mitral valve after being completely cleared of thrombi following saline washing, returned to its anatomical position, and opened freely around its pivot with both the anterior and posterior leaflets.
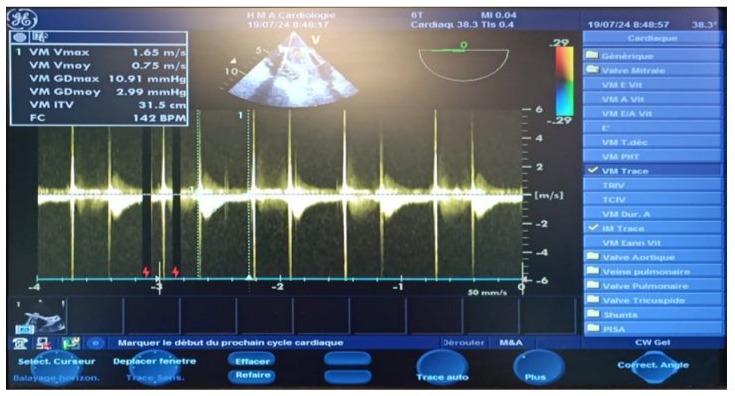
Figure 10: Postoperative continuous Doppler TEE on the mechanical mitral valve after weaning from CPB, showing a low mean gradient measured at 3 mm Hg following cleaning of the valve.
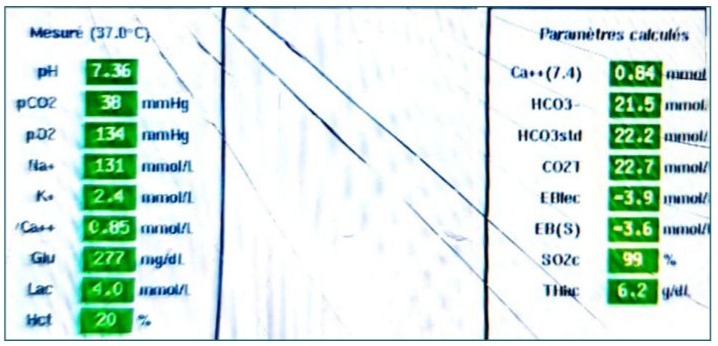
Figure 11:Arterial blood gas analysis performed after weaning from CPB, showing compensated lactic metabolic acidosis with normocapnia under hyperventilation and a standard bicarbonate deficit of -3.6 mmol/L.
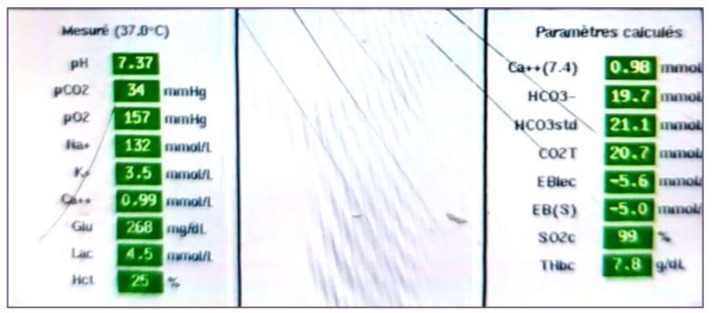
Figure 12: Arterial blood gas analysis performed 2 hours after weaning from CPB, showing compensated lactic metabolic acidosis with hypocapnia under hyperventilation and a standard bicarbonate deficit of -5 mmol/L.
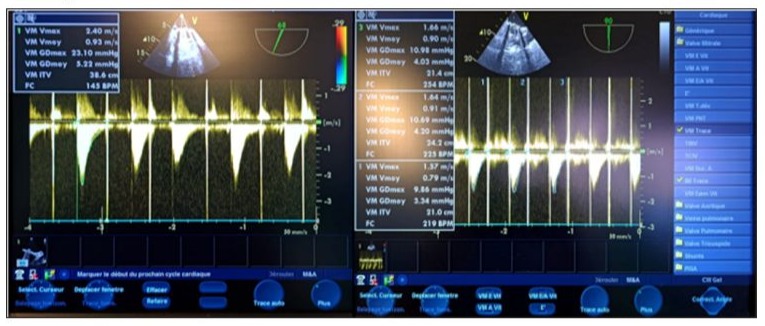
Figure 13: Postoperative continuous Doppler TEE on the mechanical mitral valve 1 hour after weaning from CPB, showing a mean gradient of approximately 4 mm Hg, with a mitral valve area of around 22 mmHg.
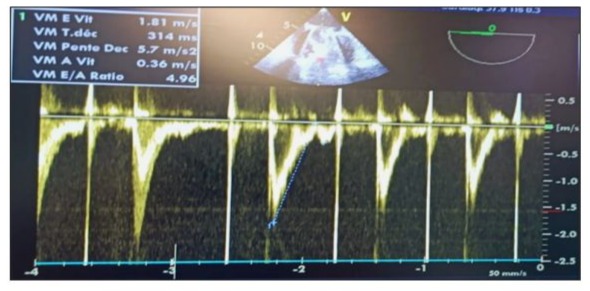
Figure 14: Postoperative pulsed Doppler TEE after weaning from CPB on the mechanical mitral valve showing an antegrade mitral flow velocity of the E wave at 2 m/s (>1.5 m/s) and an E/A ratio of 4.96, indicating elevated filling pressures in the absence of an A wave due to atrial fibrillation.
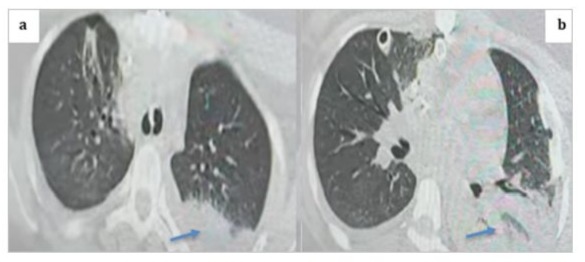
Figure 15: Thoracic CT scan in the parenchymal window with axial cuts showing a posterior basal left alveolar consolidation syndrome consistent with pneumonia, given the clinical context of the patient (blue arrows).
Video 1: ETT in bicaval view to visualize the right atrium in contact with the probe, the superior vena cava containing the central venous catheter, and the inferior vena cava.
Video 2: Pulsed Doppler TEE at the mechanical mitral valve in the mid-esophageal view, with the transducer at 60 degrees perpendicular to the flow, showing a holosystolic retrograde mitral flow velocity of 1.3 m/s.
Video 3 : TEE in 2D mode in the mid-esophageal view, with the transducer at approximately 45 degrees in a counterclockwise rotation of the cutting plane over the left cavities, showing a dilated left atrium, a mechanical valve obstructed by multiple mobile thrombi at the leaflets, the ring, and the core, with absence of leaflet opening beneath the appendage and limited opening of the posteromedial leaflet on the opposite side, where the ring appeared thickened and filled with hyper-echoic fibrinous thrombus, with multiple reverberation echoes obscuring the lateral and septal walls of the left ventricle, while preserving the kinetic activity of the anterior and inferior walls of the left ventricle.
Video 4: TEE in 2D mode in the mid-esophageal four-chamber view, at 35 cm from the dental arches with a slight counterclockwise rotation, centered on the left cavities, showing a dilated left atrium in contact with the probe, with thickened leaflets and very limited movement of the leaflet on the side of the heart cross, blockage of the leaflet at the junction of the lateral wall of the left atrium and left ventricle, associated with multiple hyper-echoic thrombi in the left ventricle slightly obscured by reverberation echoes.
Video 5: TEE in 2D mode in the mid-esophageal view, with the transducer at 105 degrees, showing a thickened appearance of the mechanical valve ring, a mobile thrombus on the atrial side of the anterior mechanical leaflet on the aortic valve side, with incomplete opening and closure during diastole and systole, respectively, and complete blockage of the contralateral leaflet with multiple thrombi on the ventricular side.
Video 6: TEE in the mid-esophageal view, with the transducer at 135 degrees, in color Doppler mode centered on the thrombosed mechanical mitral valve, showing significant mitral regurgitation reaching the depth of the left atrium.
Video 7: TEE in 2D mode in the mid-esophageal view, at an oblique angle of 145 degrees, representing the equivalent of the long-axis view of the left cavities in transthoracic imaging, showing a thickened appearance of the mechanical valve ring beneath the aorta and on the opposite side, a mobile thrombus on the atrial side of the mechanical leaflet on the aortic side, with incomplete opening and closure during diastole and systole, respectively, and complete blockage of the contralateral leaflet with multiple hyper-echoic thrombi on their ventricular sides.
Video 8: Continuous Doppler TEE at the mechanical mitral valve showing high-velocity negative flow with a significant mean transvalvular gradient during diastole, along with a significant positive holosystolic regurgitant flow during systole of high velocity, indicating stenosis and significant valve leakage due to obstructive thrombosis.
Video 9: ETT in high esophageal view, a counterclockwise rotation and a cut plane between 45 and 60 degrees revealed a horn-shaped left auricle with a pointed bottom, free of thrombus.
Video 10: ETT in high esophageal view, a counterclockwise rotation and a cut plane between 35 and 60 degrees revealed in color doppler red flow during the systole and blue flow during the diastole due to the elevated filling pressure. Continuous-wave Doppler displayed a biphasic flow in absence ECG P wave but elevation of filling pressure, and a negative flow during ventricular systole, and a negative flow during ventricular systole. The left superior pulmonary vein (LSPV) was observed above the left atrium in a high esophageal view at 0 and 45 degrees, facilitated by color Doppler, with a continuous red flow as it approached the probe. Pulsed Doppler showed a briphasic flow, including a positive S wave at 55 cm/s, a positive D wave at 37 cm/s, and an absence A wave during atrial systole. Between the LSPV and auricle, a spur was visible, not to be confused with a neoformation.
biphasic flow in absence ECG P wave but elevation of filling pressure, and a negative flow during ventricular systole
Discussion
Thrombosis is the most feared complication of mechanical mitral valve replacement. The mitral position of a mechanical prosthesis exposes the thrombogenic foreign material to the low-pressure left atrial blood flow. Multiple risk factors contribute to the occurrence of thrombosis, including valve design, inadequate preoperative anticoagulation, and abnormal loading conditions in the atrium, such as atrial fibrillation. Our understanding is shifting toward the prediction of thrombotic events according to the patients' risk profile. Selected cases of mechanical mitral valve thrombosis can be managed medically with thrombolytic therapy, although surgery remains the gold standard. Excessive anticoagulation due to paravalvular leakage does not play a major risk in most instances. This complex interplay of clinical, surgical, design, and procedural considerations of mechanical mitral valve thrombosis is elaborated in this review [4,5].
Mechanical mitral valve thrombosis has a multifactorial etiology. Thrombus primarily forms due to the perturbed flow dynamics at the valve site and the contact of blood with the foreign material of the valve; however, individual patient differences in thrombogenic predisposition also play a role. The predisposing factors of mechanical valve thrombosis that have been examined are, blood flow dynamics; valve-related characteristics; and other patient- and/or therapy-related factors. These themes are closely interrelated because blood flow dynamics are significantly influenced by the valve-related characteristics and the shape of the diseased heart. Understanding the etiology, pathophysiology, and underlying dynamics or causative mechanisms of this pathology is a fundamental prerequisite for appropriate clinical management. This understanding is critical; one of the pressing issues should incorporate all patient-related factors specific to the risks of recurrent thrombosis for use in stroke and thromboembolism risk stratification systems [4,6,7]. In patients with mechanical mitral valves, thrombosis presents as a clinical condition of obstructive dysfunction, poor anticoagulation, and paradoxical embolism, which varies from cardiac symptoms to stroke. Patients may present with chest tightness, shortness of breath, murmurs, or signs in stroke patients, making it challenging to differentiate from other cardio embolic diseases, infective endocarditis, mitral stenosis, tumors, or pan-vasculitis. The criteria are typically used to diagnose the patency of mitral valve replacements. The failure of leaflet motion is in the closed position and unresponsive to dobutamine stress as a therapeutic trial. Echocardiography has narrowed down the suitable diagnosis and has also successfully monitored the therapeutic results serially. Patients' history and clinical presentations are valuable sources compared to other diagnostic tools for facilitating the diagnosis of thrombotic obstructions in mechanical mitral valves to an accurate level. The specific guidelines and consensus are absent regarding individual patients' workup for detecting the underlying hemorrhagic and infectious etiology, as well as the severity of mechanical mitral valve stenosis at initial presentation. Factors contributing to the time lag of more than a month after medical treatment and appropriate redo surgery include the old age of patients, atypical presentations, and the lack of clinicians' decision-making guidelines. A functional diagnosis of this complication should not be delayed, even in the absence of typical features. A timely diagnosis ultimately favours a better prognostic approach for this severe disease. Healthcare professionals other than cardiologists, such as ischemic stroke specialists, make a critical addition to patients' multidisciplinary management [3,8]. The goal of medical management is to achieve optimal relief of symptoms, restore normal valve movement (opening and closing), and promote unobstructed blood flow. The medical management of a mitral valve in any patient, particularly those with mitral mechanical valve thrombosis, consists of the initiation of pharmacological agents. Pharmacological management of mechanical mitral valve thrombosis focuses on anticoagulation and/or thrombolytic therapies. Anticoagulation therapies are targeted to prevent clot formation and to decrease the ongoing possibility of thrombus evolution. Once the diagnosis of thrombosis is evident, the first step is to avoid the delay of anticoagulation initiation [1,4,5,9]. Heparin, warfarin, enoxaparin, and, more recently, direct oral anticoagulants are some options that may be considered for long-term anticoagulation therapy initiation. The use of all of these agents is limited by potential side effects, including bleeding complications, and as a result, frequent and mandatory monitoring is necessary to ensure safe and appropriate titration, and to prevent or monitor for potential complications including stroke, venous thromboembolism, and valve thrombosis. Thrombolytic therapy can be considered in certain patients, particularly those in whom acute thrombosis is a potentially life-threatening condition. Streptokinase or tissue plasminogen activator can be considered as a first-line treatment in this emergency setting. The support of the cardiothoracic surgeon is an important decision that will affect timing and diagnosis. Ideal candidates for this thrombolysis protocol have yet to be well defined, but the use of specific guidelines and patient factors such as age, existing heart valve conditions, and renal function may help tailor therapy and provide successful outcomes. [10-12]. Several anticoagulants have been reported for the treatment of mitral valve thrombosis. Various anticoagulants have different pharmacologic properties. Warfarin is a valuable anticoagulant. It inhibits the synthesis of vitamin K-dependent proteins, which are circulating coagulation inhibitors, namely antithrombin III, and the coagulation factors proteins C and S. This causes an antithrombotic effect by reducing the ability to form blood clots. Warfarin has a cholestatic effect, so a relatively high initial dose is required until the maximal antithrombotic effect is achieved. Even with the risk of inducing a transient hyper coagulation, warfarin should be initiated as soon as possible after confirming the diagnosis of MMVT. The anticoagulant effect can be determined clinically by wall time or through a laboratory examination to check the von K and Bloom test. Abnormal results such as wall time > 25 seconds and international normalized ratio (INR) < 2 should raise suspicions of inadequate anticoagulation therapy [13,14]. A balance between the risks and benefits of anticoagulation therapy should be maintained because the presence of sub-therapeutic INR might worsen the thrombosis. It is imperative that proper dosage timing and the INR value should be closely monitored when only pharmacological therapy is pursued. The mainstay of therapy in MMVT is anticoagulation to prevent an existing thrombus from growing, embolizing, and causing further complications. Most patients with MMVT require lifelong anticoagulation. The approach differs based on patient-related factors and their clinical presentation. Patients with risk factors previously mentioned, like atrial fibrillation, left atrial diameter > 5 cm, history of thromboembolism, low ejection fraction < 0.35, congestive heart failure, and MMVT of the mitral valve less than 10 days, as well as multiple mobile left atrial thrombi, including patients in sub-therapeutic warfarin therapy, will require lifelong anticoagulation. Currently, the use of novel anticoagulants and factor X inhibitors in MMVT of the mitral valve has not been evaluated for efficacy compared to the standard of care. Future randomized controlled trials will direct clinical management. Patient education and adherence are key factors in treatment success. Management should, however, be individualized rather than based on protocol [5]. Thrombolytic therapy is widely considered a modality for the treatment of acute mechanical mitral valve thrombosis (AMMVT). Retrospective publications suggest the possibility of using thrombolytic therapy in Valve Mechanical Anomalies (VMA) with a success rate of up to 70%, against 20% with a failed attempt [4]. Indications for thrombolytic therapy: It has been recognized that VMA is a medical emergency and patients present with symptoms within 5–10 days of the first episode. Thrombolytic therapy is the most sought-after mode of therapy. Primary therapy with thrombolytic agents such as tissue plasminogen activator, streptokinase, or recombinant human plasminogen activator is indicated if surgery is not feasible. The application of thrombolytic agents is widely used one week after the appearance of symptoms, but a decision can be made when symptoms appear. Thrombolytic agents are contraindicated in case stenosis occurs and MVT occurs post-windowing. It is understood that thrombolytic therapy is effective 48 hours after prior cardioversion and open-heart surgery for atrial fibrillation anticoagulant therapy [4]. Administration of thrombolytic therapy: The door-in-door time refers to the length of time in receiving any medication between the onset of symptoms and the emergency room. Patients exhibiting symptoms of systemic and pulmonary thromboembolism should be taken for physical examination. A surface electrocardiogram and chest radiograph are necessary following a physical examination, and laboratory tests are optional. The cardiac suitability proteins should be estimated along with other tests if a thrombolytic agent is presented. Radiological investigation should be performed after this. Choice of thrombolytic agents: There are various thrombolytic agents with benefits and drawbacks. It is clear from the wider world that the consistently concerted administration of these agents prior to surgery or before routine care results in life-threatening hemorrhaging. Doses should be based on patient weight at admission, delivery of intravenous fluids, and either unshunt or right-sided shunt. Administered either alone or with intravenous anticoagulants, thoracotomy is classically performed. Surgical therapy must be considered in cases of failure. The benefits of thrombolytic therapy can be implemented under the circumstances of monitoring such conditions as timing and medical mortality. Adjuvant therapy for the treatment of immediate and follow-up thrombolytic agents is a work in progress to increase blood pressure and oxygen supply to the patient by ensuring that the heart rate and volume are not bound to the number of medications. Adjunctive therapy may be administered to all patients to prevent ventricular enlargement. The use of thrombolytic agents is essential for the treatment of special conditions and can be considered a possible treatment strategy for thrombosis in selected patients. Therapy is an important intuition in cases of frustration and confusion. Thrombolytic agents can be administered using clock watch factors. In addition, it prevents the need for surgical interventions in the future. [15,16] Surgical management of mechanical mitral valve thrombosis should be performed promptly, especially in cases of complicating infective endocarditis, severe hemodynamic deterioration, and lack of response to thrombolytic therapy. Restoration of the preoperative valve function should be obtained if the valve appears to be structurally intact. This strategy aims to prevent recurrent thrombosis postoperatively by using high-dose anticoagulation in the presence of the coumadin-resistant hypercoagulable state of antiphospholipid syndrome, which in turn may impose the risk of bleeding from the operated sites, thus necessitating a timely return to surgery. In the presence of severe valve structural damage, especially with valve leaflet perforation and annular tissue destruction, a new prosthetic mitral valve replacement should be implanted given the inadequate surgical reconstructive options. Management of postoperative complications that might occur after repair of the infected mechanical mitral valve thrombosis, such as heart failure, thrombosis, and paravalvular leakage, should be made in a timely and adequate manner, such as early reopening of the chest, response to thrombolytic and antibiotic therapy, and/or consideration for surgical reoperation. Both the prosthetic valve and a mechanical bioprosthetic mitral valve before the exacerbation of coagulability due to prothrombotic courses, such as the use of perioperative micro vascularized and osteomyocutaneous free tissue transfer to the resected sternum, should be protected by the timely use of a stainless-steel wound vacuum system [1,3,4,17,18]. Thrombosed mitral valve prostheses that fail to respond to fibrinolytic therapy or if there is a contraindication to its use require surgery. Surgical options involve dealing with the thrombus by either thrombectomy or valve replacement. During thrombectomy, it is important to ensure complete removal of the thrombus; otherwise, any remnants are associated with a high re-thrombosis rate. Completing the thrombectomy while the patient is on bypass to allow full pressurization of the left atrium is essential. If thrombectomy is unsuccessful or if there is an experienced surgeon and it is likely to be time-consuming, then mitral valve replacement is quicker and associated with less blood loss. If valve replacement is required, it is done through the same median sternotomy and with the same anesthetic management used for the initial replacement. Thrombus almost invariably forms in the left atrial appendage when the patient is in atrial fibrillation, and this should be amputated before the start of bypass. It can then be sent later for histological analysis. Only the removal of the atrial appendage together with the atrial thrombus will guarantee a very low recurrent thromboembolic rate. If a previous prosthesis has been inserted, the aortic cross-clamp should be applied proximal to the valve while the left atrium is opened to avoid detrimental clot migration. Retrograde cardioplegia can be used to protect and empty the left side of the heart [1,3,4,19,20].
Thrombectomy may not always be successful; however, in acute total thrombosis, the removal of clots by a closed or open technique is always the first intervention to use. If thrombectomy is unsuccessful, the valve should be cut out and replaced or opened, and the chordae should be cut and cleaned with special care not to allow the prolapse of the AMV leaflet. The transaortic or atrial approach can be used for thrombectomy. In the operating room under general anesthesia and standard cardiopulmonary bypass using arterial and venous cannulation, when the valve is approached using the trans-septal or left atriotomy approach, the heart must be arrested, and the cannulas must be manipulated to expose the mitral valve. The transaortic approach, on the other hand, is a fast, safe, and easier technique to visualize all valve structures in acute cases. Plain and Doppler echocardiograms must be available just to avoid regurgitation or left ventricular outflow obstruction with the positioning of the cannulas [10,15]. The involved patient should be heparinized, and unfractionated heparin should be administered to reach an activated clotting time of 250 seconds before the aortotomy incision because, in the aortic approach, an arterial cannula is placed as usual in the ascending aorta and remains in situ during the whole procedure. The beating heart must be defibrillated when the aorta is opened using forceps meeting the descending aorta together with a good vent on the apex. Hemostasis is obtained using the device at the proximal and distal ends of the aortotomy site. Neither cannulation line is displaced after the aortotomy. Then, 3/0 prolene guides are placed around the proximal and distal sides without being closed. The valve is seen about 3 cm below the aortotomy incision and under this aortic valve. Retrograde cardioplegia can be easily and safely injected into the coronary ostia from below when the valve is open (only during the aortic cross-clamp insertion).
Conclusion
Mechanical valve thrombosis is a critical complication after mitral valve replacement. Factors like valve design, hemodynamic changes, and patient characteristics contribute to this condition. Surgical intervention and anticoagulation therapy are key for management. Individualized treatment plans are important due to the risks and benefits of anticoagulation. Advancements in imaging techniques and therapeutic strategies can improve care. Research is essential for better treatment protocols and outcomes. Overall, management includes timely diagnosis, intervention, education, and support.
Participants Consent: All participants (patient and her family) have given their explicit consent for the publication of personal data concerning themselves as part of this study. They understand that this data may include information that could identify them in the context of the research findings. They have been informed about the purpose of this publication, the type of data that will be disclosed, and the potential implications. They acknowledge that this information will be publicly accessible after publication. They confirm that their consent is voluntary and that they have the right to withdraw it at any time prior to publication.
Data availability statement: The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement: The studies involving humans were approved by the ethics committee of Avicenne Military Hospital, chaired by Colonel Major Dr. Radouane Niamane, Professor of Higher Education at the Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech, Morocco, and Head of the Rheumatology Department at Avicenne Military Hospital.The studies were conducted in accordance with the local legislation and institutional requirements. The participant provided their written informed consent to participate in this study.
Author agreement and contribution: All authors contributed to the production of this article. They also declare that they have read and approved the final version of this manuscript.
S. KHALLIKANE: Conceptualization, Investigation, Methodology, Writing–original draft.
M. Nabil: Supervision, Writing–review and editing.
H. Kbiri: Supervision, Writing–review and editing.
M. Didi: Data curation, Formal Analysis, Writing–review and editing.
A. Dahioui: Investigation, Validation, Writing–review and editing.
R. Mounir: Investigation, Validation, Writing–review and editing.
H. Jalal: Investigation, Validation, Writing–review and editing.
N. Atmani: Investigation, Validation, Writing–review and editing.
A. Abdou: Investigation, Validation, Writing–review and editing.
A. Bouzerda: Investigation, Validation, Writing–review and editing.
I. Serghini: Funding acquisition, Writing–review and editing.
A. Belhadj: Conceptualization, Investigation, Supervision, Writing–review and editing.
Y. Aissaoui: Supervision, Guidance, Mentorship, Final review
Y. Qamouss: Proposal of the clinical case idea, drafting of the article outline, Assistance in obtaining references, Supervision, and Editing
Funding: The author(s) declare that no financial support was received from any organization for the submitted work.
Acknowledgments: The authors want to express their appreciation and gratitude to the following medical professionals involved in this investigation: members of the Perfusionists’ Team (El hilali Otmane )–for their cooperation and meritorical support; members of the Anesthesia Team (Aziz Akhiri, Atif Bouhadane, Jaafari Mohamed, Lhbib)–for their contribution in obtaining images and data for this study; operating room instrument technician team members ( El hilali Otmane)– for his cooperation and support and members of the Postoperative Care Team (Abderrazzak ech-chahed, Azeroual Hamza, aiabbir Mohamed)–for their invaluable help in collecting samples for this investigation.
Conflict of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: that they have no conflicts of interest. The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. And that there are no other relationships or activities that could appear to have influenced the submitted work.
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material: The is No Supplementary Material for this article to declare
References
- Gündüz S, Kalçık M, Gürsoy MO, and Güner A (2020) "Diagnosis, treatment & management of prosthetic valve thrombosis: the key considerations," *Expert Review of*, Taylor & Francis.
- Le NK, Chervu N, Mallick S, Vadlakonda A, KimS (2024) "Mortality and resource utilization in surgical versus transcatheter repeat mitral valve replacement: A national analysis," *Plos One,.
- Khan HS, Ijaz Z, Ali M, Saif M, Ishaq U, et al (2020) "Clinical outcomes of mechanical prosthetic valve thrombosis," *Cureus, 12: e8760.
- Soria Jiménez CE, Papolos AI, Kenigsberg BB (2023) "Management of Mechanical Prosthetic Heart Valve Thrombosis: JACC Review Topic of the Week," *Journal of the American, 81: 2115-2127.
- Serban A, Gavan D, Pepine D, and Dadarlat A (2023) "Mechanical valve thrombosis: Current management and differences between guidelines," Trends in Cardivascular Medicine, 34: 351-359.
- Botsile E and Mwita JC (2020) "Incidence and risk factors for thromboembolism and major bleeding in patients with mechanical heart valves: a tertiary hospital-based study in Botswana," Cardiovascular Journal of Africa, 31: 185-189.
- Tagliari F, Correia MG, and Amorim GD (2022)"Clinical Features and Survival Analysis of Patients after Mechanical Heart Valve Replacement, with an Emphasis on Prosthetic Valve Thrombosis," Arquivos Brasileiros, 119.
- Truchart J, Plein D, Balthazar T, and Droogmans S (2024) "Mechanical valve obstructive thrombosis in an asymptomatic patient: a gap in current recommendations," *Acta, 79: 77-78.
- Johansson I, Benz AP, Kovalova T (2024) "Outcomes of patients with a mechanical heart valve and poor anticoagulation control on warfarin," Thrombosis, 124: 613-624.
- Jolugbo P and Ariëns RAS, (2021) "Thrombus composition and efficacy of thrombolysis and thrombectomy in acute ischemic stroke," Stroke, 52: 1131-1142.
- Keller K, Hobohm L, Ebner M, and Kresoja KP (2020) "Trends in thrombolytic treatment and outcomes of acute pulmonary embolism in Germany," *European Heart*, 41: 522-529.
- Meinel TR, Wilson D, Gensicke H, and Scheitz JF, (2023) "Intravenous thrombolysis in patients with ischemic stroke and recent ingestion of direct oral anticoagulants," *JAMA, 80: 233-243.
- Galliazzo S, Bucciarelli P, Barcellona D (2024) "Practical Suggestions for an Optimal Management of Vitamin K Antagonists: Italian Federation of Centers for the Diagnosis of Thrombotic Disorders and the Surveillance" Thrombosis.
- Woller SC, Stevens SM, Kaplan D, and Wang TF (2022) "Apixaban compared with warfarin to prevent thrombosis in thrombotic antiphospholipid syndrome: a randomized trial," Blood, 6: 1661-1670.
- Gauberti, M, de Lizarrondo, S.M. and Vivien, D, (2021) Thrombolytic strategies for ischemic stroke in the thrombectomy era. Journal of Thrombosis and Haemostasis, 19:1618-1628.
- Mackman, N, Bergmeier, W, Stouffer, G.A. and Weitz, J.I, (2020) Therapeutic strategies for thrombosis: new targets and approaches. Nature reviews Drug discovery, 19:333-352.
- Özkan, M, Gündüz, S, Güner, A, Kalçık, M, Gürsoy, M.O, et al (2022) Thrombolysis or surgery in patients with obstructive mechanical valve thrombosis: the multicenter HATTUSHA study. Journal of the American College of Cardiology, 79:977-989.
- IJsselhof, R.J, Slieker, M.G, Hazekamp, M.G, Accord, R, van Wetten, H, et al (2020) Mitral valve replacement with the 15-mm mechanical valve: a 20-year multicenter experience. The Annals of Thoracic Surgery, 110:956-961.
- Bhatia S, Jain S, Sharma V, Mansuri Z (2020) "Clinical profile of patients with prosthetic heart valve thrombosis undergoing fibrinolytic therapy and NYHA class as a predictor of outcome," *International Journal of Cardiovascular Practice.
- Mansuri Z, Sharma V, Jain S, Prajapati J, and Bhatia S (2020) "Clinical profile of prosthetic heart valve thrombosis and outcome analysis of fibrinolytic therapy versus surgical management: A single-center experience," Heart India, 8: 74-79.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.