Management of Synchronous Ipsilateral Renal Cell Carcinoma and Well-Differentiated Renal Liposarcoma: A Case Report of a Rare Clinical Entity
by Uros Kojic*, Uros Nesic, Nikola Lukac, Djordje Milic, Tijana Kojic, Djordje Jakulic, Vladan Markovic, Zoran Filipovic
Urology, University Hospital Medical Center Bezanijska kosa, Belgrade, SRB
*Corresponding author: Uros Kojic, Urology, University Hospital Medical Center Bezanijska kosa, Belgrade, SRB
Received Date: 22 October 2025
Accepted Date: 27 October 2025
Published Date: 29 October 2025
Citation: Kojic U, Nesic U, Lukac N, Milic D, Kojic T, et al. (2025) Management of Synchronous Ipsilateral Renal Cell Carcinoma and Well-Differentiated Renal Liposarcoma: A Case Report of a Rare Clinical Entity. J Surg 10: 11476 https://doi.org/10.29011/2575-9760.011476
Abstract
The concurrent occurrence of Renal Cell Carcinoma (RCC) and Well-Differentiated Liposarcoma (WDLPS) within the same kidney represents an exceptionally rare clinical phenomenon. The etiology of this synchronous presentation remains unclear and poses substantial diagnostic and therapeutic challenges. RCC, which arises from the renal parenchyma, accounts for approximately 80–90% of all primary renal malignancies. Liposarcoma, a rare mesenchymal tumor derived from adipose tissue, is extremely uncommon within the kidney. The Well-Differentiated Subtype (WDLPS) represents the least aggressive form, composed predominantly of mature adipocytes with minimal cytologic atypia. This report presents a rare case of synchronous ipsilateral RCC and WDLPS, emphasizing the diagnostic complexity, management strategy, and the importance of a multidisciplinary approach to achieve optimal outcomes.
Keywords: Renal Cell Carcinoma; Synchronous Renal Neoplasms; Well-Differentiated Liposarcoma
Introduction
Renal Cell Carcinoma (RCC) is the most common primary malignancy of the kidney, while liposarcoma is a rare softtissue sarcoma typically arising in the retroperitoneum [1]. The simultaneous occurrence of both neoplasms within the same kidney is exceedingly rare, with a limited number of cases reported in the literature. The pathogenesis of such synchronous tumors remains uncertain and presents significant diagnostic and therapeutic challenges. RCC originates from the renal parenchyma, most commonly as the clear cell subtype arising from the proximal convoluted tubules. It is frequently asymptomatic and often discovered incidentally during imaging for unrelated conditions. When symptoms do occur, they typically include hematuria, flank pain, and a palpable abdominal mass [2]. Recognized risk factors for RCC include cigarette smoking, obesity, hypertension, and hereditary syndromes such as von Hippel–Lindau disease [1]. Diagnostic evaluation is primarily based on Computed Tomography (CT) and Magnetic Resonance Imaging (MRI), followed by histopathological confirmation. Treatment options for localized disease include partial or radical nephrectomy, depending on tumor size, location, and patient comorbidity. In advanced or metastatic stages, targeted therapies and immunotherapy have significantly improved clinical outcomes. Liposarcoma is a malignant mesenchymal neoplasm derived from adipose tissue and accounts for approximately 20% of all adult soft-tissue sarcomas [2,3]. Its occurrence within the kidney is exceedingly rare. The peak incidence is observed in the fifth and sixth decades of life, with a male predominance. Liposarcoma is classified into four subtypes: well-differentiated, dedifferentiated, myxoid, and pleomorphic. The Well-Differentiated Subtype (WDLPS) represents a low-grade malignancy characterized by mature adipocytes with minimal atypia and a relatively indolent course, although it carries a significant risk of local recurrence (20–85%) following surgical excision [3]. It may arise primarily from the renal capsule or renal sinus. The most important prognostic factor for survival is histological grade, which reflects the degree of differentiation and completeness of resection. The principal differential diagnosis includes Angiomyolipoma (AML), as both tumors may appear as large, fat-containing lesions radiographically. Immunohistochemically, WDLPS often expresses S100 and calretinin. The cornerstone of therapy is complete surgical resection with negative margins. Although the prognosis is generally favorable, long-term follow-up is required due to the risk of recurrence [3]. The coexistence of RCC and WDLPS within a single kidney is exceptionally uncommon. The underlying mechanisms remain speculative, with hypotheses including independent tumorigenesis or a shared progenitor cell line. Management is primarily surgical, typically involving radical nephrectomy, as standardized treatment guidelines are lacking due to the rarity of this clinical scenario.
Case Report
A 78-year-old woman presented to a urology clinic with intermittent left flank discomfort persisting for approximately six months. She denied hematuria, dysuria, or a history of urinary tract infection and renal inflammation. Laboratory findings revealed mild anemia (hemoglobin 109 g/L, hematocrit 0.323 L/L) and normal renal function (creatinine 90 µmol/L, urea 7.7 mmol/L). Her medical history was notable for hypertension and well-controlled type 2 diabetes mellitus managed with oral hypoglycemics. On physical examination, a palpable mass was detected in the left flank and hemiabdomen. Abdominal ultrasonography revealed a poorly defined lesion within the left kidney, approximately 90 × 60 mm in size. Contrast-enhanced CT of the thorax, abdomen, and pelvis demonstrated multiple small cystic lesions up to 25 mm in the left kidney, along with two major solid masses of clinical significance. A lobulated mass at the upper pole (67 × 60 mm) exhibited central calcifications and contrast enhancement consistent with RCC (Figure 1). A second, well-circumscribed lipomatous lesion (110 × 100 mm) was identified at the lower pole and renal hilum (Figure 2), causing compression and deformation of the collecting system without invasion, initially interpreted as an angiomyolipoma. The renal vein demonstrated a retroaortic course, a relevant anatomic variation for surgical planning. After multidisciplinary evaluation including urologists, oncologists, abdominal surgeons, and interventional radiologists, preoperative embolization of the left renal artery was recommended, followed by radical nephrectomy.
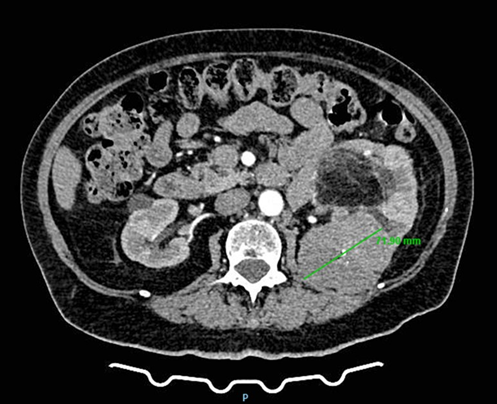
Figure 1: CT scan demonstrating a left renal mass with radiologic features of clear cell renal carcinoma.
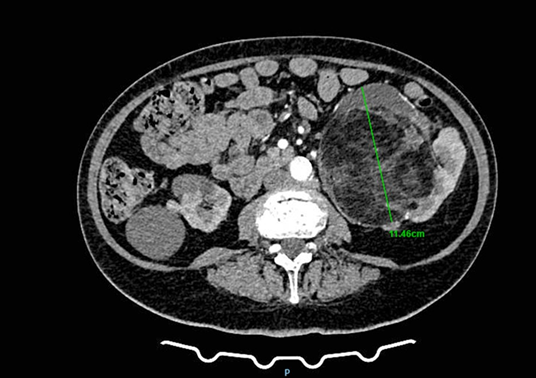
Figure 2: CT image showing a giant liposarcoma occupying the lower pole of the left kidney.
Renal artery embolization was performed under local anesthesia using the Seldinger technique, with complete occlusion confirmed angiographically (Figures 3-4). Six days later, a transabdominal left radical nephrectomy was performed. Intraoperatively, the kidney appeared markedly enlarged and deformed, containing cystic and soft-tissue components but without invasion of adjacent structures (Figures 5-6). The operation was uneventful, with minimal blood loss (approximately 300 mL) and no transfusion requirement. The postoperative course was smooth, and the patient was discharged on postoperative day five in good condition. Histopathological examination revealed that the upper pole lesion corresponded to a low-grade clear cell RCC with focal necrosis (Figures 7-8). The larger lesion was identified as a well-differentiated liposarcoma (Figures 9-10), correcting the initial radiologic misdiagnosis of angiomyolipoma. At the three-month follow-up, the patient remained asymptomatic. Laboratory results indicated mild renal impairment (creatinine 169 µmol/L, urea 9.2 mmol/L) but normal inflammatory markers. Ultrasonography revealed no recurrence or lymphadenopathy. Nephrology follow-up was continued due to reduced renal function. Six months postoperatively, CT imaging of the thorax, abdomen, and pelvis demonstrated no evidence of local recurrence or distant metastasis. Given the absence of standardized surveillance protocols for synchronous RCC and WDLPS, followup was conducted in accordance with conventional RCC and retroperitoneal sarcoma guidelines.
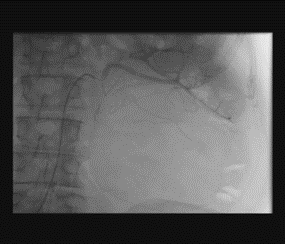
Figure 3: Angiographic visualization of arterial vascularization of the left kidney before embolization.

Figure 4: Post-embolization angiography showing successful

Figure 5:Anterior view of the resected left kidney specimen with tumor dimensions indicated.
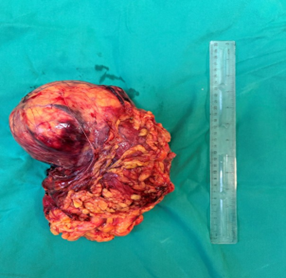
Figure 6: Posterior view of the resected kidney specimen
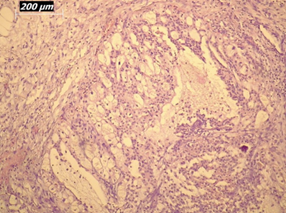
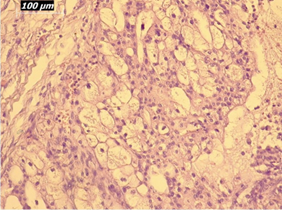
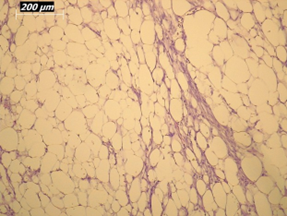
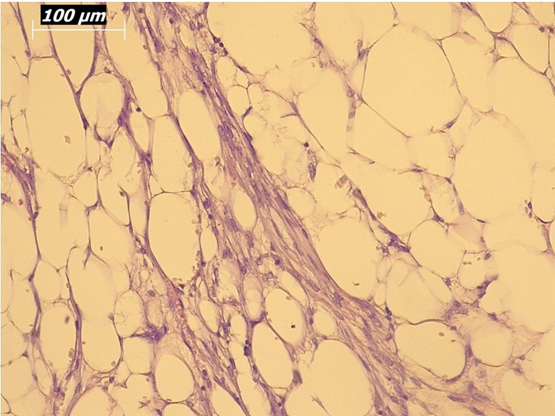
Figure 10: Higher-magnification view (20×) of liposarcoma showing mature adipocytes and minimal atypia.
Discussion
The simultaneous occurrence of RCC and WDLPS within the same kidney raises several clinical considerations, including diagnostic challenges, potential pathogenetic links, and therapeutic strategies for optimal oncologic control. Our case underscores the importance of multidisciplinary collaboration in achieving favorable outcomes. Surgical management remains the cornerstone of therapy. Although the current follow-up period is limited to six months, no recurrence has been observed, and the patient remains in good clinical condition. Longer follow-up is warranted to assess long-term survival. Williamson et al. [4] reported the first case of concomitant RCC and retroperitoneal liposarcoma, emphasizing the aggressive nature of liposarcoma, its recurrence potential, and the necessity of complete resection with negative margins. Kinebushi et al. [5] highlighted the diagnostic difficulties associated with atypical imaging findings but achieved a favorable 24-month disease-free outcome following surgical resection. Triphaty et al. [6] emphasized meticulous preoperative planning and surgical precision to minimize recurrence, noting that retroperitoneal sarcomas pose a major therapeutic challenge due to complex anatomy and limited responsiveness to chemotherapy or radiation. Similarly, Hoshi et al. [2] described a young male with concurrent RCC and retroperitoneal liposarcoma, suggesting a possible genetic predisposition. Complete resection resulted in long-term disease-free survival. Chemotherapy may be considered for unresectable, recurrent, or metastatic liposarcoma; however, its benefit remains limited. Pérez et al. [7] reported modest responses to doxorubicin and ifosfamide, without significant survival improvement (median 69 months for truncal versus 78 months for retroperitoneal liposarcomas, p = 0.668). Rioja et al. [8] reinforced the importance of radical nephrectomy even in dedifferentiated subtypes, reporting no recurrence after 15 months. Beltran et al. [9] described a case of dedifferentiated liposarcoma synchronous with metastatic clear cell RCC, in which disease progression occurred despite multiple therapeutic interventions, underscoring the prognostic significance of early-stage detection. Crago et al. [10] demonstrated that survival correlates strongly with surgical margin status, tumor grade, and histologic subtype, noting higher recurrence rates in dedifferentiated forms. Novel agents targeting MDM2 and CDK4 amplification show promise in preclinical trials. Knebel et al. [11] further identified tumor grade, liposarcoma subtype, resection margins, metastatic status, and tumor size as independent prognostic factors for disease-specific survival.
Patients with local recurrence had significantly poorer outcomes.
Conclusion
Synchronous ipsilateral renal cell carcinoma and well-differentiated liposarcoma within the same kidney represent an exceedingly rare and diagnostically challenging clinical entity. Accurate diagnosis requires advanced imaging and histopathological evaluation, while surgical resection remains the cornerstone of management. A multidisciplinary approach is essential to optimize outcomes. Further research is warranted to elucidate the underlying mechanisms and to establish standardized diagnostic and therapeutic guidelines for this rare coexistence.
References
- Liu Z, Xu Y, Long J, Guo K, Du R (2014) A case report of huge perirenal liposarcoma associated with renal cell carcinoma and reviews of three previous cases. Int J Clin Exp Med 7: 4526-4529.
- Hoshi S, Hayashi N, Yagi M, Ookubo T, Muto A, et al. (2014) Long term survival in a case of concurrent retroperitoneal liposarcoma and renal cell carcinoma: a case report. BMC Res Notes 7: 538.
- Parizi MK, Ohadian Moghadam S, Momeni SA (2019) Primary upper pole liposarcoma of the kidney with invasion to inferior vena cava: A case report. Urol Case Rep 27: 101002.
- Williamson JML, König TC, Canelo R (2008) Incidental finding of renal cell carcinoma in recurrent retroperitoneal liposarcoma. Ann R Coll Surg Engl 90: W4-W5.
- Kinebuchi Y, Ishizuka O, Minagawa T, Nisizawa O, Shimojo H (2009) Concurrent perirenal liposarcoma associated with renal cell carcinoma. Hinyokika Kiyo 55: 571-574.
- Tripathi M, Pavithira G, Dubey S, Verma R, Garg V (2023) Surgical excision of a giant retroperitoneal liposarcoma with renal cell carcinoma: A case report of the largest retroperitoneal sarcoma. International Journal of Surgery Case Reports 109: 108515.
- Perez EA, Gutierrez JC, Moffat FL (2007) Retroperitoneal and truncal sarcomas: prognosis depends upon type not location. Ann Surg Oncol 14: 1114-1122.
- Rioja P, Valencia G, Centurión-Rodriguez C, Morante Z, Bravo M, et al. (2021) Renal liposarcoma: case report and review of systemic treatment. Ecancermedicalscience 15: 1173.
- Beltran E, Garcia-Robledo JE, Rodríguez-Rojas LX, Rengifo M, Perez B, et al. (2020) Clear cell renal carcinoma synchronous with dedifferentiated liposarcoma: a case report and review of the literature. J Med Case Rep 14: 4.
- Crago AM, Singer S (2011) Clinical and molecular approaches to well differentiated and dedifferentiated liposarcoma. Curr Opin Oncol 23: 373-378.
- Knebel C, Lenze U, Pohlig F, Lenze F, Harrasser N, et al. (2017) Prognostic factors and outcome of liposarcoma patients: a retrospective evaluation over 15 years. BMC Cancer 17: 410.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.