Male Nipple-Sparing Mastectomy and Areolar-Sparing Mastectomy: A Multi-Institutional Retrospective Review of Indications and Outcomes
by Georgia Syrnioti1*, Leah Candell2, Taylor Anderson2, Antonia Syrnioti3, Josh Johnson4, Claire M Eden5, Jean Bao2, Irene Wapnir2, Shawna Willey6, Jay K. Harness7, Lisa A Newman8, Mardi R. Karin2.
1Department of Surgery, One Brooklyn Health-Brookdale University, Brooklyn, NY, USA
2Department of Surgery, Stanford University, Stanford, California, USA
3Department of Pathology, Aristotle University of Thessaloniki, Greece
4Department of Surgery, Columbia University Medical Center/New York-Presbyterian Hospital, New York, USA
5Department of Surgery, Memorial Sloan Kettering, NY, USA
6Department of Surgery, Inova Schar Cancer Institute, Fairfax, VA, USA
7Department of Surgery, Providence Saint Joseph Hospital, Orange, CA, USA
8Department of Surgery, New York Presbyterian, Weill Cornell Medicine, NY, USA
*Corresponding Author: Georgia Syrnioti, Department of Surgery, One Brooklyn Health-Brookdale University, Hospital Medical Center, Brooklyn, NY, USA
Received Date: 12 September 2025
Accepted Date: 19 September 2025
Published Date: 20 November 2025
Citation: Syrnioti G, Candell L, Anderson T, Syrnioti A, Karin M, et al. (2025) Male Nipple-Sparing Mastectomy and AreolarSparing Mastectomy: A Multi-Institutional Retrospective Review of Indications and Outcomes. J Surg 10: 11447 https://doi.org/10.29011/25759760.011447
Abstract
Introduction: Most male breast cancer is treated with Total Mastectomy (TM), despite Nipple-Sparing Mastectomy (NSM) and Areolar-Sparing Mastectomy (ASM) being common in women for improved aesthetic outcomes. This study evaluates the indications for male NSM and ASM, and oncologic outcomes.
Methods: A muti-institution retrospective review of male NSM and ASM during 2008-2023 at 5 institutions was performed. Indications, tumor characteristics, treatment and outcomes were analyzed.
Results: 15 males, ages 36-77, underwent 11 NSM and 3 ASM for pTis, pT1-2, pN0-N2, ER+/PR+ invasive ductal carcinoma, 5/14(36%) HER2 positive of which 3 received neoadjuvant chemotherapy. For BRCA1 mutations, a prophylactic bilateral NSM and 1 contralateral NSM resulted in 14 NSM total. Indications for NSM were no clinical nipple involvement (imaging and physical exam), and the ability to obtain clear margins. ASM indications were cancer close to the nipple (n=2) and removal for margins, or positive sub-nipple biopsy (n=1), allowing for areola preservation away from cancer and closure of areola to create appearance of a nipple. All ASM were satisfied with appearance, declining reconstruction. Following NSM, delayed fat grafting in 2/13(15%) resulted in excellent appearance. Pathology showed one pCR, pTis, pT1-T2, pN0-N2, largest tumor size 3.2 cm, and clear margins in all. Two patients (14%) with pN1-pN2 received postoperative radiotherapy. No recurrences or contralateral cancers at 5.7 years mean follow-up.
Conclusion: NSM and ASM are alternatives to TM for males. In this first reported multi-institution series, the oncologic outcomes are excellent, aesthetics appears improved compared to TM, without any local recurrences to date.
Keywords: Male breast cancer surgery, male nipple-sparing mastectomy, areola-sparing mastectomy, oncologic outcomes.
Introduction
Male breast cancer is rare, making up roughly 1% of all breast cancers diagnosed worldwide, though the United States and global incidence appears to be increasing [1-6]. Despite significant advances in the medical and surgical treatment of breast cancer in the last several years, data regarding the diagnosis and treatment of breast cancer in men is mainly extrapolated from studies involving women [7,8]. This is due to both the low incidence of breast cancer in men, and the historical exclusion of male participation in breast cancer clinical trials [9,10].
Principles regarding surgical management are generally similar between male and female breast cancer patients. However, while Breast Conserving Therapy (BCT) has become common for women, male breast cancer patients continue to undergo mastectomy at a much higher rate when compared to BCT [1117]. This is due to the relatively small amount of glandular tissue in men compared to women.
The common retro-areolar location of breast cancer in men [18], and perhaps due to an assumption that men care less about the cosmetic appearance of the breasts compared to women. In a 50 year single institution review of male breast cancer treatment from 1960-2011 by Bratman et al, surgical treatment consisted of mastectomy with or without lymph node surgery in 82% (22 patients), of which all 3 men treated between 1965-1973 underwent radical mastectomy, and following that time period through 2011 the remainder had either modified radical mastectomy or simple mastectomy with or without Sentinel Lymph Node Biopsy (SLN); four patients (18%) underwent breast conserving surgery with lumpectomy beginning in 1986 [7]. Thus, most men with breast cancer, due to the common subareolar location, and combined with a small breast size, have traditionally been previously treated usually with TM or historically more extensive surgery as above [7,19]. Since commonly most of the male breast tissue will be removed with removal of the cancer and surrounding margin due to the small amount of breast tissue present if BCT is performed, many males choose mastectomy instead of breast conservation treatment with Radiation Therapy (RT).
Nipple-Sparing Mastectomy (NSM) in women with breast cancer is common and considered oncologically safe with achieving clear margins and is usually performed with breast reconstruction [20-24] due to the excellent cosmetic outcomes and much higher patient satisfaction compared to Total Mastectomy (TM) [25-27]. However, data regarding the feasibility, approach, and outcomes of NSM and Areola-Sparing Mastectomy (ASM) in men is scarce. A recent national survey in 2022, by Chichura et al, of the male breast cancer patient experience reported many were dissatisfied with the post-surgical appearance after TM specifically due to the loss of their nipple and scar appearance [19]. This underscores the importance of nipple preservation and post-surgical appearance to men, and relevant question of whether male breast cancer can similarly be treated with NSM or Areolar-Sparing Mastectomy (ASM), provided that clear margins are obtained and achieve excellent outcomes.
There is very little literature on NSM in men with breast cancer [19,28]. The first case report of a male NSM was published in Italy in 2007 [29]. The first case series of male NSM and ASM to our knowledge, published in 2024 was a small single institution retrospective review of males treated at Stanford from 2015-2021, demonstrating the feasibility and excellent clinical outcomes associated with NSM and ASM in men, even for subareolar breast cancer, with no cancer recurrences at median follow up of 46 months [28]. That case series contained the first description in the literature to our knowledge of a novel technique for male ASM described by Karin et al. for subareolar cancer close to the nipple or positive sub-nipple biopsy with nipple removal and partial areolar sparing; then closure to create an outpouching or the areola resembling a nipple [28]. In that study, following NSM or ASM all patients reported satisfaction with appearance and being comfortable without a shirt in public, without any additional breast reconstruction surgery, indicating a substantial improvement compared to prior reports of patient dissatisfaction with appearance after TM [19,28].
The purpose of this first multi-institutional study of male NSM and ASM is to evaluate a larger cohort of male breast cancer patients from multiple institutions, to assess if similar excellent oncologic outcomes and results are confirmed and present an algorithm for offering male NSM and ASM as current alternatives to TM.
Methods
We performed a retrospective study based on database and chart review of males who underwent NSM or ASM at 4 institutions in the United States: New York Presbyterian-Weill Cornell Medicine, Stanford University, Georgetown University, and Providence St. Joseph’s Hospital. Institutional Review Board (IRB) approval was obtained to collect data and evaluate outcomes. All performed procedures and data gathering were conducted according to the ethical standards of the institutional research committee and the Helsinki declaration. Patients gave consent for photography when applicable.
Patient information was collected from the Electronic Medical Record (EMR) at each study site, and additional details were added by the attending surgeon when available. Clinical and histopathological characteristics were collected including age at diagnosis, race/ethnicity, Body Mass Index (BMI) at time of diagnosis, presenting symptom, laterality, known germline mutations, clinical and pathologic staging, nodal status, grade of tumor, Estrogen Receptor (ER) status, Progesterone Receptor (PR) status, Human Epidermal Growth Factor 2 (HER2) status, location of tumor within the breast, and margin status. Disease-free survival was defined as no locoregional or distant recurrences from the time of surgery to most recent known follow-up. Negative margins were defined as no ink on tumor. Data regarding neoadjuvant and adjuvant treatment including chemotherapy, radiation, and endocrine therapy was collected and data on cosmetic appearance and patient satisfaction data was reported if available.
Indications for NSM were no clinical nipple involvement (imaging and physical exam), the ability to obtain clear margins and a negative sub-nipple biopsy which was performed in all men undergoing NSM. ASM indications were cancer close to or involving the nipple, or sub-nipple biopsy positive for cancer necessitating nipple removal, and adequate residual areolar tissue away from the cancer to facilitate closure and creation of a pseudonipple (Figures 1,2) [28].
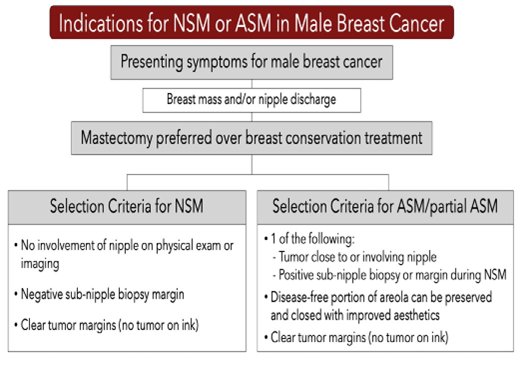
Figure 1: Selection criteria for NSM and/or ASM for male breast cancer.
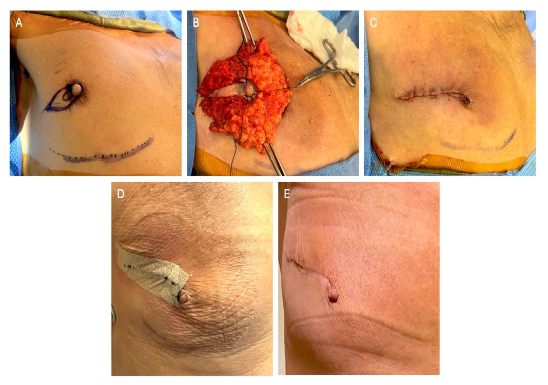
Figure 2: Preoperative, intraoperative, and postoperative photos of male ASM with nipple reconstruction.
A: Preoperative marking of palpable cancer adjacent to nipple, and outer elliptical ASM incision and around the base of the nipple medially, since palpable cancer extended to medial nipple. Avoidance of incision medial to areola for lateral subareolar cancers.
B: Right ASM surgical specimen with complete removal of nipple.
C: Closure by approximating edges of preserved areolar skin first, to create nipple appearance with outpouching of areolar skin with interrupted 4-0 vicryl deep dermal sutures, and 4-0 Prolene interrupted skin suture.
D: ASM initial postoperative photo, showing areolar closure to reconstruct appearance of nipple, with prolene suture on reconstructed nipple Final pathology clear margins.
E: ASM post-operative photo of same patient at 1 month, showing healed appearance of reconstructed nipple from residual areolar skin, and avoidance of scar medial to areola creates much better appearance than TM.
Results
Between 2008 and 2023, 15 men underwent 18 mastectomies: 14 NSM and 4 ASM. The mean age was 56.4 (range 36-77) years. Mean BMI was 29.0 (range 22.1-37.5) kg/m2 (Table 1). Most patients (12/15, 80%) presented with palpable mass. All patients were recommended to have genetic testing and all but one agreed to testing. Three patients (21.4%, 3/14,) tested positive for BRCA 1 or 2 mutations, one patient (7.1%, 1/14,) tested positive for an ATM mutation and genetic testing was negative in 71.4% (10/14 patients). Race/ethnicity was reported in 10 patients demonstrating 70% (7/10) Caucasian, and 10% (1/10) for each of the following: Asian, Hispanic, and African American, and was unknown in the remaining 5 patients. Neoadjuvant chemotherapy was given to 3 patients who all had HER2 positive cancer, and pathology showed one Pathologic Complete Response (pCR). Otherwise, pathology showed pTis, pT1-T2, pN0-N2, with largest tumor size 3.2 cm, and clear margins in all. Two patients (2/15, 13.3%) with pN1-pN2 lymph node metastasis received postoperative radiotherapy.
NSM was performed in 78% (14/18 breasts); ASM in 22% (4/18 breasts) with partial areolar sparing, either due to proximity of tumor to the nipple for margins (3 breasts) or for positive intraoperative sub-nipple biopsy (1 breast). One cancer case undergoing planned NSM required conversion to ASM because of a positive sub-nipple biopsy (6.7% of planned NSM), however all the other planned NSM were completed in 93% (14/15) which included a negative sub-nipple biopsy for cancer cases. Notably, no patients required conversion to TM. All final pathologic margins were negative. Three patients developed seromas, treated with aspiration in 2 patients. There were no serious surgical complications including no ischemic complications of the skin or nipple. All ASM reported being satisfied with appearance, declining any additional nipple reconstruction, fat grafting or nipple-areolar tattoo. Following NSM, 14% (2/14) underwent delayed fat grafting resulting in improved chest wall contour and appearance.
At mean follow up of 68.1 months (range 18-156 months) there were no locoregional or distant recurrences, though one patient lost to follow up 3 months after surgery for pT1N0 cancer was excluded from follow up data. However, one male, (1/15, 6.6%) developed contralateral breast cancer as described below, treated with ASM (Figure 2). Treatment, complications, pathologic characteristics and recurrence data can be found in Table 2.
Three men were BRCA gene mutation carriers for whom bilateral NSM or ASM, were performed. One man with BRCA 2 mutation and initial left breast pT2N0 invasive ductal cancer planned for NSM had a positive sub-nipple biopsy during surgery and was converted to left ASM (patient 2); then 7 years later, had a contralateral screening mammogram detected right subareolar breast cancer with positive sub-nipple biopsy treated with ASM, representing the only contralateral breast cancer in this series (contralateral cancer not included in follow up data since under 3 months) Figure 2. A second man with BRCA 1 gene mutation and right breast cancer underwent right NSM with simultaneous contralateral prophylactic left NSM (patient 11). A third man underwent bilateral prophylactic NSM for his BRCA 1 mutation carrier state, bilateral gynecomastia and significant family history of male breast cancer in in his father, paternal grandfather and paternal uncle (patient 7).
|
Patient |
Age at dx |
BMI |
Presentation |
Laterality |
Genetic mutation |
Pathologic type |
ER |
PR |
HER2 |
Pathologic stage |
|
1 |
52 |
27 |
Palpable mass |
Left |
Declined testing |
IDC, DCIS |
+ |
+ |
+ |
T2N0 (IIA) |
|
2a |
66 |
28 |
Palpable mass |
Left |
BRCA 2 |
IDC, DCIS |
+ |
- |
- |
T2N0 (IIA) |
|
2b |
73 |
28 |
Screening mammogram (for BRCA2 + previous hx L IDC) |
Right |
BRCA 2 |
IDC |
+ |
- |
- |
T1N0 (IA) |
|
3 |
64 |
32 |
Palpable mass + nipple discharge |
Right |
Negative |
IDC, DCIS |
+ |
+ |
- |
T1aN0 (IA) |
|
4 |
47 |
28 |
Palpable mass |
Left |
ATM |
IDC |
+ |
+ |
+ |
ypT0N0 |
|
5 |
43 |
33 |
Palpable mass |
Left |
Negative |
DCIS |
+ |
- |
N/A |
TisN0 (0) |
|
6 |
49 |
25 |
Palpable mass |
Left |
Negative |
IDC |
+ |
+ |
- |
T1N0 (IA) |
|
7 |
53 |
32 |
Gynecomastia + known BRCA mutation |
Bilateral prophylactic |
BRCA 1 |
Benign |
N/A |
N/A |
N/A |
N/A |
|
8 |
70 |
35 |
Palpable mass |
Left |
Negative |
IDC, DCIS |
+ |
+ |
+ |
T2N0 (IA) |
|
9 |
62 |
27 |
Palpable mass |
Left |
Negative |
IDC (papillary features) |
+ |
+ |
- |
T1N0 (IA) |
|
10 |
51 |
26 |
Nipple discharge |
Left |
Negative |
IDC |
+ |
+ |
- |
T1miN0 (IA) |
|
11 |
36 |
22 |
Palpable mass |
Right |
BRCA 1 |
IDC |
+ |
+ |
- |
T1bN0 (IA) |
|
12 |
63 |
28 |
Palpable mass |
Left |
Negative |
IDC |
+ |
+ |
- |
T1cN2 (IIIA) |
|
13 |
64 |
25 |
Palpable mass, breast pain |
Left |
Negative |
IDC (papillary features) |
+ |
+ |
- |
T1cN0 (IA) |
|
14 |
77 |
38 |
Unknown |
Left |
Negative |
IDC |
+ |
+ |
- |
T1cN0 (IA) |
|
15 |
49 |
31 |
Palpable mass |
Right |
Negative |
IDC |
+ |
+ |
+ |
T2N1 (2B) |
|
Mean |
57 |
29.33 |
Table 1: Clinical and pathologic characteristics of male breast cancer patients undergoing NSM or ASM.
Mean age and BMI excludes patient 13 due to loss to follow-up.
|
Patient |
Neoadjuvant chemotherapy |
Surgery |
Complication |
Adjuvant therapy |
Ipsilateral recurrence |
Contra-lateral breast cancer |
Disease-free survival to date (months) |
|
1 |
No |
ASM + SLNB |
None |
Chemotherapy + endocrine therapy |
No |
No |
41 |
|
2a |
No |
ASM + SLNB |
None |
Chemotherapy + endocrine therapy |
No |
Yes |
103 |
|
2b |
No |
NSM converted to ASM + SLNB |
Seroma |
Pending possible endocrine therapy |
No |
N/A |
18 |
|
3 |
No |
NSM |
None |
Endocrine therapy |
No |
No |
49 |
|
4 |
Yes |
NSM |
None |
Endocrine therapy |
No |
No |
116 |
|
5 |
No |
NSM + SLNB |
None |
None |
No |
No |
94 |
|
6 |
No |
NSM + SLNB |
None |
Endocrine therapy |
No |
No |
106 |
|
7 |
No |
Prophylactic B/L NSM |
None |
N/A |
N/A |
N/A |
156 |
|
8 |
Yes |
ASM + SLNB |
Seroma |
Chemotherapy; pending possible endocrine therapy |
No |
No |
25 |
|
9 |
No |
NSM + SLNB |
None |
None |
No |
No |
32 |
|
10 |
No |
NSM + SLNB |
Seroma |
None |
No |
No |
119 |
|
11 |
No |
NSM + SLNB; contralateral prophylactic NSM |
None |
Endocrine therapy |
No |
No |
91 |
|
12 |
No |
NSM + axillary dissection |
Hypertrophic scar |
Radiation + endocrine therapy |
No |
No |
16 |
|
13 |
No |
NSM + SLNB |
None |
Endocrine therapy |
No |
No |
Lost to follow up 3 months after surgery * |
|
14 |
No |
NSM + SLNB |
None |
Endocrine therapy |
No |
No |
29 |
|
15 |
Yes |
NSM + SLNB |
None |
Radiation + endocrine therapy |
No |
No |
27 |
|
Mean |
68.1 * |
Table 2: Treatment, complications, and recurrence data of male breast cancer patients undergoing NSM or ASM.
Mean follow-up disease-free survival excludes patient 13 due patient lost to follow-up.
Discussion
This study presents the first multi-institutional case series of NSM and ASM in men, with description of clinical and pathologic features and outcome data. There were no locoregional recurrences or distant metastasis at a mean follow up of 68 (range 18-156) months and one contralateral breast cancer. This demonstrates the oncologic safety of NSM and ASM for male breast cancer. For men with breast cancer who are not candidates for NSM due to proximity or involvement of the nipple, ASM was performed with clear margins and no evidence of recurrence at mean follow up of 41.6 (range 7-83) months.
We have formulated a current algorithm of indications for male NSM and ASM (Figure 1) with our proposed surgical management of male breast cancer patients which was previously published in a smaller single-institution case series [28]. Similar to women with breast cancer, men with breast cancer may be candidates for BCT if tumor to breast size ratio can accommodate a lumpectomy usually combined with RT. As in women, breast conserving surgery in men is associated with similar oncologic outcomes compared to mastectomy, with reported locoregional recurrence rates of 0-17.4% [7,14-17,30,31] though there are no randomized clinical trials directly comparing BCT and mastectomy for men. This study, with no locoregional recurrences, demonstrates that NSM and ASM for male breast cancer are oncologically safe alternatives, and comparing favorably to TM and BCT.
Men with a relatively large cancer to breast size ratio, or those who are interested in potentially avoiding adjuvant Radiation Therapy (RT), may be better candidates for mastectomy than BCT, and now can be offered NSM or ASM for improved appearance compared to TM. Moreover, some have questioned the benefit/cost ratio of BCT in men, given minimal glandular tissue to preserve, added resources required for RT, and difficulty of obtaining necessary future mammograms to screen for recurrence [32]. BCT may also be a less desirable option for some men due to RT-associated alopecia, which can be managed with laser hair removal on the contralateral side, however in men with significant chest hair that that might not be desirable. Furthermore, male chest hair can conceal the scar well from NSM and result in a better appearance, a factor that is not a consideration in women selecting for BCT or NSM (Figure 3). Due to these factors, and the typically small contralateral breast in men, the authors have observed that NSM or ASM, provide reasonable symmetry and significantly improved appearance and patient satisfaction compared to TM. Based on these multiple considerations and excellent oncologic outcomes in this study, NSM or ASM are demonstrated to be good options for surgical treatment of males with breast cancer, provided patients meet selection criteria as outlined in Figure 1.
Extrapolating from studies of female breast cancer patients [3337] we would expect a higher rate of locoregional recurrence with lumpectomy in the absence of RT, although ongoing clinical trials such as NRG-BR007 [38] may show that omission of RT is safe in certain low-risk patients; this trial is open to both women and men with breast cancer. These results may impact the surgical decision making for men with breast cancer in the future. Nevertheless, at present it appears that most male breast cancer patients, for various reasons, undergo mastectomy rather than BCT [17,19]. Thus, it is important to offer men the modern mastectomy options of NSM or ASM for improved appearance, either without reconstruction or with fat grafting for improved chest contour.
The concept of NSM in women was first introduced in 1962 [39] and its practice has gained popularity over the subsequent decades. Main concerns for the preservation of the Nipple-Areola Complex (NAC) include increased risk of local recurrence and risk of ischemia/necrosis of the NAC [20,40-42]. In the last 2 decades several large meta-analyses [20-22,24] have reported on the oncologic safety of NSM and ASM in women with breast cancer and the inclusion criteria for women who are candidates for NSM has widely expanded [43]. Currently, NSM is considered a safe option for most women whose tumors do not directly involve the NAC [23,44]. The absolute contraindication for NSM is direct tumor invasion of the nipple, or a positive sub-nipple biopsy margin [45-47]. We propose that these same criteria be applied to men, so that men would similarly be candidates for possible NSM or ASM.
Data for male breast cancer patients are currently lacking due to both rarity of the disease and the fact that breast cancer in men has traditionally been treated with TM without significant consideration of alternatives [19]. Like their female counterparts, selection criteria for NSM in male breast cancer patients should include no involvement of the nipple clinically on physical examination or imaging, negative sub-nipple biopsy, and clear microscopic tumor margins (no tumor on ink) [23,47]. NSM in males is performed in the same manner as females, with removal of all of the breast tissue from under the dermis of the nipple and removing the tissue directly under the nipple for a sub-nipple biopsy [41,42,47], in contrast to subcutaneous mastectomy for men with gynecomastia which leaves breast tissue under nipple. Male breast cancer patients can be candidates for ASM if the tumor is close to or involving the nipple, provided at least a portion of the areola is disease free and can be preserved for improved cosmesis and symmetry, and the surgical technique in males developed by the senior author (MK) was previously described in detail [28]. Figure 2 shows preoperative, intraoperative, and postoperative photographs of male ASM with partial areolar sparing, which is an option if unable to undergo NSM for breast cancer due to proximity to nipple or involvement of the nipple. This ASM technique with partial areolar sparing and areola closure to reconstruct the appearance of a nipple avoids a scar on the medial breast, and usually provides improved aesthetics compared to TM (Figure 1, Figure 3).
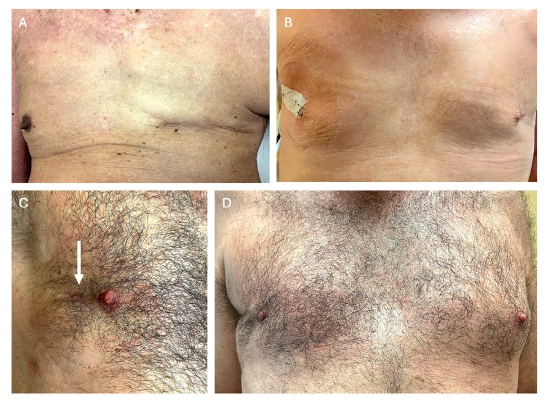
Figure 3: Appearance of TM, ASM, or NSM surgical treatment for male breast cancer.
A: Typical appearance following left total mastectomy.
B: Bilateral ASM, for initial left breast cancer, then subsequent right breast cancer (following technique of ASM with partial areolar sparing and closure described in Figure 2).
C: Right NSM frontal view of preserved nipple with arm raised, and radial scar barely visible with chest hair (white arrow).
D: Right NSM bilateral frontal view with comparison to normal left breast (same patient as c).
A sub-nipple biopsy is recommended in men undergoing planned NSM, due to the common subareolar location and relative smaller breast size compared to women. Similarly, sub-nipple biopsy is commonly performed to determine eligibility for NSM in women [23]. For NSM in men, sending the sub-nipple biopsy to pathology for evaluation during surgery is recommended to provide important information intraoperatively to assess for NSM, which resulted in one conversion to ASM in this series. Patients should be counseled for the possibility of conversion to ASM or TM in the case of positive sub-nipple biopsy. This series contains the first report in the literature to our knowledge of bilateral ASM for bilateral male breast cancer involving the nipple (Figure 3).
There is a significant body of literature regarding the psychologic impact of a breast cancer diagnosis and particularly of mastectomy in women. While there is much less written about male psychologic stress related to breast cancer diagnosis and treatment, certain studies have demonstrated high levels of cancer-specific distress in men with breast cancer [48], and when compared to agematched controls, poorer life satisfaction [49] and major deficits in emotional functioning [50]. The emotional impact of being diagnosed with cancer and with being diagnosed with a disease traditionally associated with women may be compounded by the physical and psychological changes associated with treatment of the disease, leading to altered body image and feelings of isolation and stigma [51-53]. Men who experience shame associated with post-surgical appearance may seek to conceal their scars or be hesitant to engage in activities such as swimming where scars may be conspicuous [48,54,55]. Concerns about masculinity may further hinder emotional expression and discourage patients from seeking support [53]. A study of 161 male breast cancer patients showed that 23% of participants reported cancer-related distress with depressive symptoms being associated with altered body image [48]. Similarly, in 2022 the Male WhySurg national survey of patient reported outcomes in 63 men undergoing breast cancer surgery, reported 98.6% had their nipple removed during surgery and 33% reported feeling uncomfortable with their postoperative appearance related to feelings of imbalance or asymmetry, scar, lack of nipple, or lack of hair on the surgical side [19].
Despite these psychological and aesthetic concerns, postmastectomy reconstruction is rarely considered or discussed with male breast cancer patients [19,56]. Unlike women with breast cancer, who are routinely offered plastic surgery referrals, men with breast cancer are seldom provided the same opportunity. This practice likely stems from the misconception that men with breast cancer are less likely to be impacted by the cosmetic outcome of breast surgery [57]. In the Male Breast Cancer WhySurg study with 485 surgeons responding to the survey about surgical options offered to men with breast cancer, only 34% would offer NSM regardless of reconstruction and 20.8% routinely offered reconstruction, most commonly fat grafting, while 36.6% do not even consider it in their surgical planning [19]. Consequently, men with breast cancer often endure distorted chest appearance without being offered the option of reconstruction, possibly further exacerbating the psychologic burden already being suffered.
A systematic review of breast reconstruction following male mastectomies in 2022 by Deldar, et al, identified five studies and 29 males undergoing breast reconstruction [56]. Mastectomy type reported was radical mastectomy in 34.5%, modified radical mastectomy in 17.2% and not reported in the remainder, however, no NSM or ASM were reported. Breast reconstruction consisted of flap reconstruction in 89% (n=26) including latissimus flap, Transverse Rectus Abdominus Muscle (TRAM), or local flap, and the remainder had fat grafting (n=1), implant (n=1), or wound closure with subsequent Nipple Areolar Complex (NAC) reconstruction (n=1); patient satisfaction was recorded and although numbers are small, all patients who underwent reconstruction reported satisfaction with postoperative appearance [57-61]. Interestingly, the patient who underwent fat grafting had it performed at the time of mastectomy under the pectoralis fascia, then repeat delayed fat grafting and NAC reconstruction from groin skin [58]. Also described following mastectomy in men, is contralateral liposuction, for symmetry, instead of filling in the mastectomy site, combined with NAC reconstruction with a skin graft from the groin or scrotum after TM [56,60]. The magnitude of the reconstruction performed, and nipple-areolar reconstruction described with skin graft, was likely reflective of men previously commonly undergoing much more extensive surgery without any nipple or areolar preservation.
Integrating plastic and reconstructive surgery consultations is an option for men with breast cancer and could potentially offer a more comprehensive and supportive care model. Notably, however, insurance companies in the United States are mandated to cover reconstructive procedures for women undergoing breast cancer surgery, yet breast reconstruction is not regularly provided to men with breast cancer [19]. In this series, for the men that had satisfaction recorded, they reported following NSM or ASM being satisfied with their appearance and comfortable without a shirt in appropriate sports, swimming, and other settings. Furthermore, all ASM patients were offered additional nipple reconstruction or breast reconstruction such as fat grafting, for symmetry and all declined reconstruction being satisfied with appearance. Delayed fat-grafting after male NSM was done in 2 men (14%) following NSM, and the surgeon (S.W.) noted excellent appearance after. Therefore, it appears that if men desire further reconstruction after NSM or ASM, much less extensive procedures such as fat-grafting, or contralateral liposuction for symmetry, can successfully provide excellent aesthetic appearance, compared to the type of reconstruction previously described following total mastectomies.
The authors have observed significantly improved appearance with male NSM or ASM compared to TM in men (Figure 3).
Post-mastectomy complication rates are rare in the male population. A retrospective cohort study of the American College of Surgeons National Surgical Quality Improvement Program Database (NSQIP) that examined treatment and outcomes of male breast cancer patients between 2008 and 2016 showed overall morbidity of 4.6% of which most were wound healing complications (overall rate of 3.2%) [62]. In the current series we show that NSM and ASM are both oncologically safe options with few minor surgical complications of seromas, without any major complications and no locoregional recurrence was noted, even in the presence of node-positive disease. In contrast to reported rates of 4.1-35.9% for Nipple Areolar Complex (NAC) or mastectomy flap ischemic complications in female NSM [63,64], none of our male NSM patients experienced either NAC or mastectomy flap ischemia.
Regarding guidelines for screening of men at increased risk for breast cancer, the National Comprehensive Cancer Network (NCCN) recommends annual clinical breast exam as well as monthly self-examination in men with pathologic or likelypathologic BRCA 1 or 2 mutations starting at age 35 [65]. However, even in men with BRCA mutations, routine imaging screening is not recommended by NCCN 2024 guidelines. In men with a history of breast cancer treated with lumpectomy, the American Society of Clinical Oncology (ASCO) recommends offering annual ipsilateral mammogram; in men with a history of breast cancer AND a high-risk genetic mutation, ASCO recommends offering annual contralateral mammogram [66]. Screening breast Magnetic Resonance Imaging (MRI) is not routinely recommended in men with a history of breast cancer [67]. Men with known BRCA gene mutations, particularly BRCA2 mutations, have a lifetime risk of breast cancer as high as 8%, approaching the lifetime average risk for women of 12%, thus some have proposed these men undergo screening mammograms to detect breast cancer at earlier stages [68]. We support incorporating the ASCO guidelines for contralateral screening mammogram in men with BRCA mutations following unilateral mastectomy for breast cancer. Perhaps screening mammography should be considered in asymptomatic male BRCA genetic mutation carriers, to identify cancer at an earlier stage and more likely allow for treatment with NSM or ASM.
In contrast to female BRCA mutation carriers, prophylactic bilateral mastectomies in male BRCA mutation carriers are not routinely recommended [69], due to the lifetime risk of breast cancer as stated above of approximately 8%, which is similar to average risk women [68]. In addition, while Contralateral Prophylactic Mastectomy (CPM) is often done for women with breast cancer, with or without a high-risk genetic mutation, the same is not true of men with breast cancer due to this low risk of future contralateral breast cancer. Thus, for men with a high-risk genetic mutation, mammogram screening is reasonable to assess the contralateral breast, but CPM is not usually recommended.
The strengths of this study are the multi-institutional dataset assessing long term outcomes for male NSM or ASM, a topic rarely discussed in the literature, and presenting selection criteria that can be applied to men with breast cancer. The weaknesses of this study are that it is retrospective, and did not include formal patient reported outcomes of aesthetic appearances with NSM or ASM compared to TM. Future studies of male breast cancer surgical options, including NSM, ASM, TM, and BCT, with patient reported outcomes to evaluate surgical appearance, and RT side effects with BCT such as alopecia or muscle tightness, in addition to oncologic outcomes, would provide further information for surgical decision making for male breast cancer patients
Conclusions
This is the first multi-institutional case series of NSM and ASM in men with breast cancer, demonstrating the oncologic safety and improved appearance compared to TM. Similar surgical oncological principals for female breast cancer should be applied to men, and NSM and ASM should be considered acceptable surgical options in select male breast cancer patients based on the surgical treatment algorithm presented. Nipple and/or areolar preservation not only improve the aesthetic appearance compared to TM but may also decrease psychologic distress.
Conflict of Interest: The authors do not have any conflict of interest.
References
- Surveillance, Epidemiology, and End Results (SEER) Program (http:// www.seer.cancer.gov) SEER*Stat Database (2007): Incidence- SEER 9 Regs Limited-Use, Nov 2006 Sub (1973–2004), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, based on the November 2006 submission
- Stang A, Thomssen C (2008) Decline in breast cancer incidence in the United States: what about male breast cancer? Breast Cancer Res Treat 112: 595-596.
- Speirs V, Shaaban AM (2009) The rising incidence of male breast cancer. Breast Cancer Res Treat 115: 429-30.
- Noone AM, Howlader N, Krapcho M, et al. SEER cancer statistics review. In: SEER web site. Bethesda, MD: National Cancer Institute; April 2018. p. 1975e2015. Based on November 2017 SEER data submission, https:// seercancergov/csr/1975_2015/.
- Chen Z, Xu L, Shi W, Zeng F, Zhuo R et al. (2020) Trends of female and male breast cancer incidence at the global, regional, and national levels, 1990-2017. Breast Cancer Res Treat 180: 481-490.
- Konduri S, Singh M, Bobustuc G, Rovin R, Kassam A (2020) Epidemiology of male breast cancer. Breast 54: 8-14.
- Bratman SV, Kapp DS, Horst KC (2012) Evolving trends in the initial locoregional management of male breast cancer. Breast 21: 296-302.
- Fentiman IS (2018) Surgical options for male breast cancer. Breast Cancer Res Treat 172: 539-544.
- Corrigan KL, Mainwaring W, Miller AB, Lin TA, Jethanandani A, et al. (2020) Exclusion of Men from Randomized Phase III Breast Cancer Clinical Trials. Oncologist 25: e990-e992.
- Scheike O (1973) Male breast cancer 5. Clinical manifestations in 257 cases in Denmark. Br J Cancer 28: 552-561.
- Guinee VF, Olsson H, Moller T, Shallenberger RC, van den Blink JW, et al. (1993) The prognosis of breast cancer in males. A report of 335 cases. Cancer 71: 154-161
- Goss PE, Reid C, Pintilie M, Lim R, Miller N (1999) Male breast carcinoma: a review of 229 patients who presented to the Princess Margaret Hospital during 40 years: 1955–1996. Cancer 85: 629-639.
- Cutuli B, Le-Nir CC, Serin D, Kirova Y, Gaci Z, et al. (2010) Male breast cancer. Evolution of treatment and prognostic factors. Analysis of 489 cases. Crit Rev Oncol Hematol 73: 246-254
- Cloyd JM, Hernandez-Boussard T, Wapnir IL (2013) Outcomes of partial mastectomy in male breast cancer patients: analysis of SEER, 1983–2009. Ann Surg Oncol 20: 1545-1550
- Fields EC, DeWitt P, Fisher CM, Rabinovitch R (2013) Management of male breast cancer in the United States: a surveillance, epidemiology and end results analysis. Int J Radiat Oncol Biol Phys 87: 747-752
- Fogh S, Kachnic LA, Goldberg SI, Taghian AG, Powell SN, et al. (2013) Localized therapy for male breast cancer: functional advantages with comparable outcomes using breast conservation. Clin Breast Cancer 13: 344-349
- Zaenger D, Rabatic BM, Dasher B, Mourad WF (2016) Is breast conserving therapy a safe modality for early-stage male breast cancer? Clin Breast Cancer 16: 101-104
- Yap HY, Tashima CK, Blumenschein GR, Eckles NE (1979) Male breast cancer. A natural history study. Cancer 44: 748-754.
- Chichura A, Attai DJ, Kuchta K, Nicholson K, Kopkash K, et al. (2022) Male Breast Cancer Patient and Surgeon Experience: The Male WhySurg Study. Ann Surg Oncol 29: 6115-6131.
- Piper M, Peled AW, Foster RD, Moore DH, Esserman LJ (2013) Total skin-sparing mastectomy: a systematic review of oncologic outcomes and postoperative complications. Ann Plast Surg 70: 435-437.
- De La Cruz L, Moody AM, Tappy EE, Blankenship SA, Hecht EM (2015) Overall Survival, Disease-Free Survival, Local Recurrence, and Nipple-Areolar Recurrence in the Setting of Nipple-Sparing Mastectomy: A Meta-Analysis and Systematic Review. Ann Surg Oncol 22: 3241-3249.
- Youn S, Lee E, Peiris L, Olson D, Lesniak D, et al. (2023) Spare the Nipple: A Systematic Review of Tumor Nipple-Distance and Oncologic Outcomes in Nipple-Sparing Mastectomy. Ann Surg Oncol Dec;30: 8381-8388.
- Shanno JN, Daly AE, Anderman KA, Cruz HSS, Webster AZ, et al. (2024) Positive Nipple Margins in Nipple-Sparing Mastectomy: Management of Nipples Containing Cancer or Atypia. Annals of surgical oncology 31: 5148-5156.
- Spillane S, Baker C, Lippey J (2025) Therapeutic nipple-sparing mastectomy: a scoping review of oncologic safety and predictive factors for in-breast recurrence. ANZ J Surg 95: 34-40.
- Moyer HR, Ghazi B, Daniel JR, Gasgarth R, Carlson GW (2012) Nipplesparing mastectomy: technical aspects and aesthetic outcomes. Ann Plast Surg 68: 446-450.
- Djohan R, Gage E, Gatherwright J, Pavri S, Firouz J, et al. (2010) Patient satisfaction following nipple-sparing mastectomy and immediate breast reconstruction: an 8-year outcome study. Plast Reconstr Surg 125: 818-829.
- Yueh JH, Houlihan MJ, Slavin SA, Lee BT, Pories SE, et al. (2009) Nipple-sparing mastectomy: evaluation of patient satisfaction, aesthetic results, and sensation. Ann Plast Surg 62: 586-590.
- Anderson TN, Bao J, Ayala C, Wapnir I, Karin MR (2024) Contemporary mastectomy options for male breast cancer: nipple-sparing and areolar-sparing mastectomy—a case series. Annals of Breast Surgery 8: 27-37.
- Luini A, Gatti G, Brenelli F, Silva LS, Ivaldi G, (2007) Male breast cancer in a young patient treated with nipple-sparing mastectomy: case report and review of the literature. Tumori 93: 118-120.
- Leone JP, Leone J, Zwenger AO (2017) Locoregional treatment and overall survival of men with T1a,b,cN0M0 breast cancer: a populationbased study. Eur J Cancer 71: 7-14.
- Selcukbiricik F, Tural D, Aydoğan F, Beşe N, Büyükünal E, et al. (2013) Male breast cancer: 37-year data study at a single experience center in Turkey. J Breast Cancer 16: 60-65.
- Takabe K (2016) Breast-conserving surgery should not be recommended to men with early-stage breast cancer simply because we can perform these operations. HemOnc Today 17: 13.
- Fisher B, Anderson S, Redmond CK, Wolmark N, Wickerham DL, et al. (1995) Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med 333: 1456-1461.
- Forrest AP, Stewart HJ, Everington D (1996) Randomised controlled trial of conservation therapy for breast cancer: 6-year analysis of the Scottish trial. Lancet 348: 708-713.
- Veronesi U, Luini A, Del Vecchio M (1993) Radiotherapy after breastpreserving surgery in women with localized cancer of the breast. N Engl J Med 328: 1587-1591.
- Uppsala-Orebro Breast Cancer Study Group (1990) Sector resection with or without postoperative radiotherapy for stage I breast cancer: a randomized trial. J Natl Cancer Inst 82: 277-282.
- Clark RM, Whelan T, Levine M (1996) Randomized clinical trial of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer: an update. J Natl Cancer Inst 88: 16591664.
- De-Escalation of Breast Radiation Trial for Hormone Sensitive, HER2 Negative, Oncotype Recurrence Score Less Than or Equal to 18 Breast Cancer (DEBRA).
- Freeman BS (1962) Subcutaneous mastectomy for benign breast lesions with immediate or delayed prosthetic replacement. Plast Reconstr Surg Transplant Bull 30: 676-682.
- Ahn SJ, Woo TY, Lee DW (2018) Nipple-areolar complex ischemia and necrosis in nipple-sparing mastectomy. Eur J Surg Oncol 44: 11701176.
- Karin MR, Momeni A, Thompson C (2023) Internal Mammary Perforator Preserving Nipple-sparing mastectomy (IMP-NSM) to Reduce Ischemic Complications. Journal of Medical Insight.
- Karin MR, Pal S, Ikeda D, Silverstein M, Momeni A (2023) Nipple Sparing Mastectomy Technique to Reduce Ischemic Complications: Preserving Important Blood Flow Based on Breast MRI. World J Surg 47: 192-200.
- Krajewski AC, Boughey JC, Degnim AC, Jakub JW, Jacobson SR, et al. (2015) Expanded Indications and Improved Outcomes for NippleSparing Mastectomy Over Time. Ann Surg Oncol 22: 3317-3323.
- Coopey SB, Tang R, Lei L, Freer PE, Kansal K, et al. (203) Increasing eligibility for nipple-sparing mastectomy. Ann Surg Oncol 20: 32183222.
- NCCN Clinical Practice Guidelines: Breast Cancer Risk Reduction v1.2016.
- Dawood S, Merajver SD, Viens P (2011) International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol 22: 515-523.
- Smith BL, Coopey SB (2018) Nipple-Sparing Mastectomy. Adv Surg 52: 113-126.
- Brain K, Williams B, Iredale R, France L, Gray J (2006) Psychological distress in men with breast cancer. J Clin Oncol 24: 95-101.
- Andrykowski MA (2012) Physical and mental health status and health behaviors in male breast cancer survivors: a national, pop ulationbased, case-control study. Psycho Oncol 21: 927-934.
- Kowalski C, Steffen P, Ernstmann N, Wuerstlein R, Harbeck N, et al. (2012) Health-related quality of life in male breast cancer patients. Breast Cancer Res Treat 133: 753-757.
- Yaghan RJ, Bani-Hani KE (2004) Male breast disorders in Jordan. Disease patterns and management problems. Saudi Med J 25: 18771883.
- Donovan T, Flynn M (2007) What makes a man a man? The lived experience of male breast cancer. Can Nurs 30: 464-470.
- Co M, Lee A, Kwong A (2020) Delayed presentation, diagnosis, and psychosocial aspects of male breast cancer. Cancer Med 9: 33053309.
- Levin-Dagan N, Baum N (2021) Passing as normal: negotiating boundaries and coping with male breast cancer. Soc Sci Med 284: 114239.
- Quincey K, Williamson I, Winstanley S (2016) “Marginalised malignancies”: a qualitative synthesis of men’s accounts of living with breast cancer. Soc Sci Med 149: 17-25.
- Deldar R, Sayyed AA, Towfighi P, Aminpour N, Sogunro O, et al. (2022) Postmastectomy Reconstruction in Male Breast Cancer. Breast J 2022: 5482261.
- Schaverien MV, Scott JR, Doughty JC (2013) Male mastectomy: an oncoplastic solution to improve aesthetic appearance. J Plast Reconstr Aesthet Surg 66: 1777-1779.
- Al-Kalla T, Komorowska-Timek E (2014) Breast total male breast reconstruction with fat grafting. Plast Reconstr Surg Glob Open 2: e257.
- Bamba R, Krishnan NM, Youn R, Economides JM, Pittman TA (2018) The Use of Low-Profile Silicone Breast Implants in Male Breast Reconstruction. Plast Reconstr Surg 141: 324e-325e.
- Giunta G, Rossi M, Toia F, Rinaldi G, Cordova A (2017) Male breast cancer: Modified radical mastectomy or breast conservation surgery? A case report and review of the literature. Int J Surg Case Rep 30: 89-92.
- Spear SL, Bowen DG (1998) Breast reconstruction in a male with a transverse rectus abdominis flap. Plast Reconstr Surg 102: 16151657.
- Elmi M, Sequeira S, Azin A, Elnahas A, McCready DR, et al. (2018) Evolving surgical treatment decisions for male breast cancer: an analysis of the National Surgical Quality Improvement Program (NSQIP) database. Breast Cancer Res Treat 171: 427-434.
- Woodward S, Willis A, Lazar M, Berger AC, Tsangaris T (2020) Nipplesparing mastectomy: A review of outcomes at a single institution. Breast J 26: 2183-2187.
- Ju T, Chandler J, Momeni A, Gurtner G, Tsai J, et al. (2021) Two-Stage Versus One-Stage Nipple-Sparing Mastectomy: Timing of Surgery Prevents Nipple Loss. Ann Surg Oncol 28: 5707-5715.
- Daly MB, Pal T, Maxwell KN, Churpek J, Kohlmann W, et al. (2023) NCCN Guidelines® Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2024. J Natl Compr Canc Netw 21: 1000-1010.
- Hassett MJ, Somerfield MR, Baker ER, Cardoso F, Kansal KJ, et al. (2020) Management of Male Breast Cancer: ASCO Guideline. J Clin Oncol 38: 1849-1863.
- Marino MA, Gucalp A, Leithner D, Keating D, Avendano D, et al. (2019) Mammographic screening in male patients at high risk for breast cancer: is it worth it? Breast Cancer Res Treat 177: 705-711.
- Woods RW, Salkowski LR, Elezaby M, Burnside ES, Strigel RM, et al. (2020) Image-based screening for men at high risk for breast cancer: Benefits and drawbacks. Clin Imaging 60: 84-89.
- Mouelle M, Meka E, Mathelin C, Taris N (2023) Management of men with high genetic risk of breast cancer. Is there a place for screening or risk-reducing surgery? Case report and review. Curr. Probl. Cancer: Case Rep 9: 100220.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.