Livedo Reticularis of the Nose Following HA Filler Injection
by Zoe Sinkins1, Kathleen Collis2, Daniyal Elahi2, Chiara Penafiel RN3, Mohammed Elahi4*
1Medical Student, University of Limerick, Ireland
2Research Student, Toronto Institute of Plastic Surgery, Toronto, Canada
3Advanced Nurse Injector, Toronto Institute of Plastic Surgery, Toronto, Canada
4Department of Surgery, Clinical Faculty, University of Toronto and Medical Director, Toronto Institute of Plastic Surgery, Toronto, Canada
*Corresponding author: Mohammed Elahi, Department of Surgery, Clinical Faculty, University of Toronto and Medical Director, Toronto Institute of Plastic Surgery, Toronto, Canada
Received Date: 29 September 2025
Accepted Date: 06 October 2025
Published Date: 08 October 2025
Citation: Sinkins Z, Collis K, Elahi D, Penafiel RNC, Elahi M (2025) Livedo Reticularis of the Nose Following HA Filler Injection. J Surg 10: 11462 https://doi.org/10.29011/2575-9760.011462
Abstract
Hyaluronic Acid (HA) fillers have gained significant popularity as a non surgical technique to augment soft tissues. HA injections to the nose have become increasingly common to reshape the nose, address minor imperfections and correct asymmetries. While generally considered safe, HA injection to the nasal region can be associated with potentially severe complications. A case of a 37-year-old woman who had undergone two prior open rhinoplasty procedures received intranasal HA filler. Within 12 hours, she developed a mottled net-like pattern on the nasal tip and dorsal skin recognized immediately as Livedo Reticularis (LR). Prompt treatment with pulsed high dose hyaluronidase, low dose salicylic acid, transdermal nitropatch and warm compresses were able to achieve complete resolution of this complication. LR results from the disruption of local cutaneous circulation. In the case of HA fillers, it is thought to occur when injected filler obstructs the local vasculature. If left untreated, LR can progress to soft tissue ischemia and ultimately necrosis. We present this report to highlight the importance of prompt recognition and treatment of this vascular insult, particularly in the post rhinoplasty patient, in order to avoid potentially significant skin necrosis, scarring, wound care and surgical reconstruction.
Keywords: Ha Fillers; Hyaluronidase; Livedo Reticularis; Prevention; Rhinoplasty; Vascular Compromise
Introduction
In recent years, there has been a significant increase in the demand for minimally invasive cosmetic surgery interventions. Traditional areas of soft tissue augmentation include the nasolabial folds, lips, labiomental, temporal and malar regions of the face and more recently, the nose. Dermal filler injections to the nose have become a popular alternative to surgical rhinoplasty and have been contemporarily coined as “liquid rhinoplasty” [1]. One of the most common substrates used for dermal nasal filling is Hyaluronic Acid (HA) [2]. Hyaluronic Acid (HA) is a naturally occurring glycosaminoglycan polysaccharide chain present in the extracellular matrix [3]. As a dermal filler, HA attracts and retains water molecules to restore volume and provide structural support to the treated area [3]. HA injection is generally considered a safe option owing to its low allergic potential and reversibility with hyaluronidase [2-4]. Despite its relative safety, HA injections can be associated with complications, including damage to the nerves, soft tissues, and vasculature. The nasal region has a complex vascular network which can be at risk of occlusion during HA injections. This risk is particularly pertinent for patients who have undergone previous rhinoplasty where the blood supply is often damaged or compromised. If nasal surgery, especially open rhinoplasty, was done prior to HA injections, there is a risk of skin discolouration, ischemia, and, in severe cases, tissue necrosis [4]. In order to avoid such complications, it is imperative that HA injectors be intimately familiar with the risks and potential complications associated with HA nasal fillers to this region. In this report, we present the case of a 37-year-old woman who, after undergoing two previous open rhinoplasty procedures, received intranasal HA filler from an experienced nurse injector. The patient immediately developed a skin complication known as Livedo Reticularis (LR), which presented as a blue-purple discolouration in a net-like pattern over the treatment area and surrounding region. We administered pulsed hyaluronidase, 81 mg of salicylic acid, warm compress, and transdermal nitroglycerin and were able to achieve complete resolution within 7 days.
Case Report
A 37 year old female non smoker who had undergone 2 previous rhinoplasty procedures requested HA filler injection to the nasal tip to enhance rotation. Her first rhinoplasty operation was performed in Columbia in 2011. This procedure was an open rhinoplasty operation that involved placement of an alloplastic medpor nasal graft. She subsequently underwent a second open rhinoplasty procedure in 2023. The medpor graft which had migrated was removed and was replaced with a septal cartilaginous graft. The patient had no wound healing problems from this most recent operation. She has no significant past medical history, has no drug allergies and does not take any medications. In 2025, due to her perception of a lack of tip definition, she requested a non surgical approach to this aesthetic goal. Hyaluronic acid filler was offered as a way to enhance nasal tip rotation and definition. After discussing the risks and complications as well as alternatives to this treatment, 0.2 cc of Hyaluronic Acid (HA) filler was injected using a 31 g needle. She was satisfied with the initial results and was discharged from the clinic without issue. 12 hours later, she noticed a reddish-purple discoloration of the nasal tip and dorsum (Figure 1).
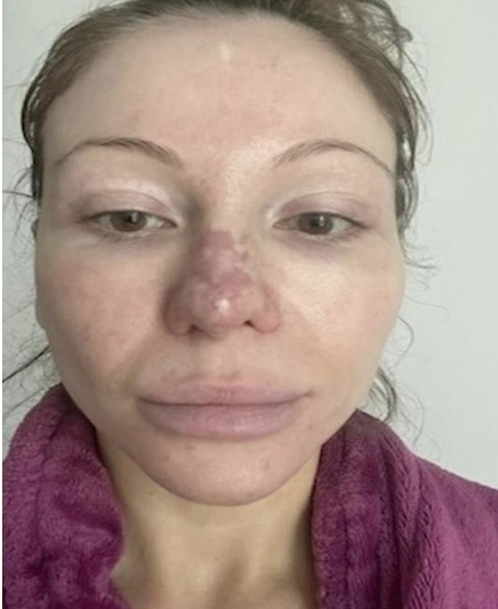
Figure 1: 12 hours post treatment with Hyaluronic Acid Filler to the nasal tip.
Prompt diagnosis of LR was made and 280iU of hyaluronidase was injected within 24 hours to dissolve the HA filler. She was also started on 81 mg of salicylic acid and told to keep warm compresses on the nose. A prescription was also given for nitropaste to apply to the nose. A further 100iU injection of hyaluronidase was given 2 days later followed by another 90iU of hyaluronidase was injected in similar fashion on day 6. The mottled appearance of the nose continuously improved in this period of time such that on post treatment day 10, there was complete resolution of this condition (Figure 2).
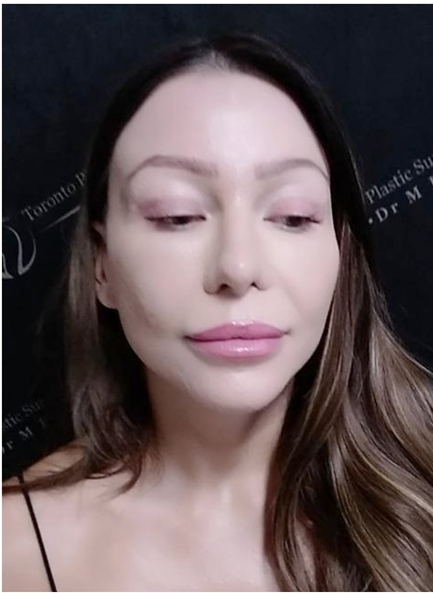
Figure 2: Appearance of nose 10 days after initial onset of LR.
Discussion
Livedo Reticularis (LR) is a dermatologic condition characterized by blue-purple discolouration that forms in a netlike pattern1. It derives its name from the Latin, livedo (“blueness”) and reticulum (“fishing net”) [2-4]. LR was first described in 1860 by Austrian dermatologist Ferdinand von Hebra who coined the term to refer to violet discolouration of the skin resulting from disturbance to cutaneous circulation [4,5]. The blood vessels of the skin supply a particular area of tissue or angiosome and when blood supply is compromised for whatever reason, deoxygenation leads to the mottled appearance that can progress to ischemia and necrosis [4,6]. LR is typically categorized as either primary or secondary. Primary Livedo Reticularis, also known as Cutis Marmorata, Idiopathic LR, or Physiologic LR, is caused by a benign dysregulation of autonomic skin perfusion. It most commonly results from cold exposure of the lower extremities and classically presents as symmetric skin mottling that spontaneously resolves when the skin is warmed [1]. It is worth noting that a subtype of primary LR is drug-induced LR. This reaction occurs as a result of certain medications resulting in the disruption of local cutaneous circulation, most commonly amantadine [6]. Secondary Livedo Reticularis, also known as Livedo Racemosa or Pathological LR, is caused by local obstruction of blood flow [7]. In contrast to primary LR, secondary LR is typically more widespread and irregular. It does not resolve with warmth alone [1,4]. Secondary LR is a documented symptom of several factors including autoimmune conditions, connective tissue disorders, hematological disorders and of course, vascular insult from either surgery or non-invasive techniques that compromise soft tissue vasculature [1,8]. In the setting of injectable Hyaluronic Acid (HA) fillers, LR is a rare but well-documented manifestation of either direct vascular occlusion or indirect vascular compression from the resultant edema leading to ischemia of local tissues [9,10]. In these cases, LR is often accompanied by pallor, coolness, and delayed capillary refill. If left untreated, filler-associated vascular occlusion can progress to skin necrosis [11]. The case presented involved LR limited to the nasal tip, ala and dorsum, however, occlusion of other areas of the face can lead to severe complications like stroke and blindness [12-14]. Current estimates place the incidence of hyaluronic acid-associated vascular occlusion between 0.01 - 0.05% [11]. While this number is low, the increasing popularity of filler augmentation may lead to a greater number of patients facing potentially severe complications [11,15]. It is imperative for clinicians to be able to make a prompt diagnosis and initiate immediate treatment to mitigate potential complications of this condition. In the case of our 37 year old patient, the development of LR may have been a sequela of her previous rhinoplasties. During a rhinoplasty procedure, whether open or closed, it is commonly accepted that the vascular supply to the nose will be compromised to varying degrees. The most at risk vessels include the lateral nasal and columellar arteries. The result of having undergone rhinoplasty can be damage to the circulation of the nasal tip, rendering it vulnerable to ischemia in the event of vascular occlusion. To our knowledge, this paper presents the first case report of LR developing after intranasal HA filler in a patient with previous rhinoplasty. Other reports have acknowledged the occurrence of LR after HA filler, however, they do not acknowledge its potential appearance post rhinoplasty. This case highlights the importance of prompt recognition and treatment of HA-associated vascular occlusion. By being aware of the early signs of ischemia, such as LR, clinicians are better able to prevent potentially irreversible complications. While HA filler injection is generally considered a low-risk procedure, it is important to remain aware of the potential complications, particularly in the post rhinoplasty patient. Vascular occlusion by HA filler can result in Livedo Reticularis, a net-like blue-purple discolouration of the skin. If not appropriately addressed, LR can progress to tissue ischemia and permanent necrosis. This report highlights the importance of prompt recognition and treatment of this vascular insult in order to avoid potentially significant skin necrosis, scarring, wound care and surgical reconstruction. With prompt diagnosis and treatment, using a combination of pulsed hyaluronidase injections, baby aspirin, transdermal nitroglycerin, and warm compress, total resolution was achieved within 10 days.
References
- Turk CB, Dagtas BB, Izci NF, Gokdemir G (2024) Microinjection Technique: A Novel and Comprehensive Approach for Nose Reshaping with Hyaluronic Acid Fillers. J Clin Aesthet Dermatol 17: 43-47.
- Baser B, Singh P, Shubha P, Roy PK, Chaubey P (2021) Non-surgical Rhinoplasty and Use of Hyaluronic Acid Based Dermal Filler-User Experience in Few Subjects. Indian J Otolaryngol Head Neck Surg 73: 52-58.
- Walker K, Basehore BM, Goyal A, Zito PM (2023) Hyaluronic acid. National Library of Medicine.
- Ling LI (2019) Successful management of nose arterial occlusion and impending skin necrosis after filler injection. Journal of Cosmetic Medicine 3: 108-113.
- Sajjan VV, Lunge S, Swamy MB, Pandit AM (2015) Livedo reticularis: A review of the literature. Indian Dermatol Online J 6: 315-321.
- Lewis C, Short C (2025) Perseus Digital Library.
- Parsi K, Partsch H, Rabe E, Ramelet AA (2011) Reticulate eruptions: Part 2. Historical perspectives, morphology, terminology and classification. Australas J Dermatol 52: 237-244.
- Champion RH (1965) Livedo Reticularis. A Review. Br J Dermatol 77: 167-179.
- Kraemer M, Linden D, Berlit P (2005) The spectrum of differential diagnosis in neurological patients with livedoreticularis and livedo racemosa. J Neurol 252: 1155-1166.
- Sneddon IB (1965) Cerebro-Vascular Lesions and Livedo Reticularis*. Br J Dermatol 77:180-185.
- (1907) An Epitome of Current Medical Literature. Br Med J 2: E73–76.
- Gibbs MB, English JC, Zirwas MJ (2005) Livedo reticularis: An update. J Am Acad Dermatol 52: 1009-1019.
- Beleznay K, Humphrey S, Carruthers JDA, Carruthers A (2014) Vascular Compromise from Soft Tissue Augmentation. J Clin Aesthetic Dermatol 7: 37-43.
- Murray G, Convery C, Walker L, Davies E (2021) Guideline for the Management of Hyaluronic Acid Filler-induced Vascular Occlusion. J Clin Aesthetic Dermatol 14: E61-69.
- Soares DJ, Hynes SD, Yi CH, Shah-Desai S, Irving SC (2024) Cosmetic Filler–Induced Vascular Occlusion: A Rising Threat Presenting to Emergency Departments. Ann Emerg Med 83: 59-67.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.