Lidocaine Application for Cannulation in Endoscopic Retrograde Cholangiopancreatography
by Noriyuki Arakawa1,2*, Atsushi Irisawa3, Hiroto Wakabayashi2
1Arakawa Gastroenterology Clinic, Aizu, Japan
2Department of Gastroenterology, Takeda General Hospital, Aizu, Japan
3Department of Gastroenterology, Dokkyo Medical University, Mibu, Japan
*Corresponding author: Noriyuki Arakawa, Arakawa Gastroenterology Clinic, 2-1-29 Chuo, Aizu Wakamatsu-shi, Fukushima 965-0037, Japan.
Received Date: 08 September 2025
Accepted Date: 16 September 2025
Published Date: 19 September 2025
Citation: Arakawa N, Irisawa A, Wakabayashi H (2025) Lidocaine Application for Cannulation in Endoscopic Retrograde Cholangiopancreatography. J Dig Dis Hepatol 10: 231. https://doi.org/10.29011/2574-3511.100231
Abstract
The primary challenge in endoscopic retrograde cholangiopancreatography (ERCP) is accessing the papilla. In this study, we aimed to assess the usefulness of applying 100 mg of 1% lidocaine to the major duodenal papilla during cannulation. A retrospective observational study was conducted with control and lidocaine-treated groups. To reduce bias, 48 cases from each group were matched using propensity scores. Papilla and oral protuberance morphologies were classified based on previous reports. The primary endpoint was deep bile duct intubation time. Secondary endpoints included bile duct intubation success rate, degree of papilla opening after lidocaine application, use of the pancreatic guidewire method, ERCP-related adverse events, and blood lidocaine levels. Factors influencing intubation time were analyzed using univariate and multivariate regression. The median intubation times were 52 and 240 seconds in the lidocaine and control groups, respectively (p<0.01). No significant differences were found between groups for disease, intubation success rate, use of the pancreatic guidewire technique, or post-procedural adverse events. Moderate papilla opening was observed in most patients (n=26) after lidocaine application. Three factors influencing intubation time were identified: pancreatic duct guidewire use, papilla pattern I, and oral protrusion pattern R. The study demonstrates that applying 100 mg of 1% lidocaine to the major papilla significantly reduced the time required for deep bile duct intubation, suggesting a potential new pre-treatment strategy for ERCP.
Keywords: Cannulation; Endoscopic Retrograde Cholangiopancreatography; Lidocaine; Bile Duct Induction; Papilla; Endoscopic Procedure
Introduction
Prompt and safe deep bile duct intubation is essential for endoscopic retrograde cholangiopancreatography (ERCP). Patients with difficult bile duct intubation cannot transition easily to treatment, and prolonged procedural time increases the risk of post-ERCP pancreatitis (PEP) [1]. Ensuring prompt and safe intubation can reduce adverse events, including PEP. The primary obstacle to ERCP is accessing the major duodenal papilla.
There are few reports on pre-treatment for ERCP. One study demonstrated that nitroglycerin administration relaxes the Oddi sphincter during the removal of common bile duct stones [2]. Maintaining the sphincter of Oddi surrounding the papilla in a relaxed state is critical. A recent meta-analysis highlighted the limited effectiveness of current pharmacological pre-treatments for ERCP, emphasizing the need for alternative strategies.
A study by Nemoto et al. showed that spraying 400 mg of 2% lidocaine on the colonic mucosa suppressed colonic peristalsis, demonstrating its safety and effectiveness during endoscopy [3]. Lidocaine, a local anesthetic, blocks sodium channels in nerve tissue and voluntary and involuntary muscles. By acting on the gastrointestinal mucosa, lidocaine spray reportedly relaxes nerves and suppresses peristalsis. While lidocaine has been shown to suppress peristalsis and relax sphincter tone in other contexts, its direct effects on the major duodenal papilla during ERCP remain unexplored.
Lidocaine perfusion around the Oddi sphincter likely reduces its tone around the bile and pancreatic ducts. Consequently, the major papilla opens, potentially easing deep bile duct intubation. Additionally, spraying lidocaine may prevent intrapancreatic duct pressure increases from papilla edema and Oddi sphincter spasms post-ERCP, reducing the likelihood of PEP. However, no studies to date have examined the effects of lidocaine spraying on the major papilla during ERCP.
This study aimed to evaluate whether the application of 100 mg of 1% lidocaine to the major duodenal papilla in patients undergoing ERCP reduces bile duct intubation time and adverse events compared to standard practice.
Materials and methods
Patients
This study was conducted and reported in accordance with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines. As context for this study, we note that our hospital has used lidocaine since February 2021. We conducted a retrospective comparative study of cases before and after the introduction of lidocaine during ERCP at our institution. A total of 432 patients who underwent ERCP between June 2020 and September 2021 were included. Of these, 226 cases from June 2020 to January 2021 were assigned to the control group, and 206 cases from February to September 2021 were assigned to the lidocaine-treated group. To minimize patient bias, 48 cases were selected from each group for comparisons using a propensity score matching method. The statistical analysis was performed with the assistance of a biostatistician.
The indication criteria are as follows.
- Patients scheduled for ERCP for examination of bile duct or pancreatic disease
- Target disease: obstructive jaundice due to benign or malignant disease
- Untreated papilla
- Age ≥ 20 years old
- Written informed consent has been obtained
Patients who met any of the following conditions were excluded: those who did not provide consent, those undergoing pancreatography, those with a history of papilla incision, those with pre-ERCP pancreatitis, those allergic to xylocaine, those with postoperative bowel reconstruction, those with major papilla tumors, and those with arrhythmias or heart disease.
As a result, in the control group, 146 of the 226 patients were excluded, leaving 80 patients. Among the 206 patients who underwent ERCP during the lidocaine use period, 158 were excluded, and 48 were included in the lidocaine spray group. Using propensity score matching, 48 cases were selected from each group (Figure 1).
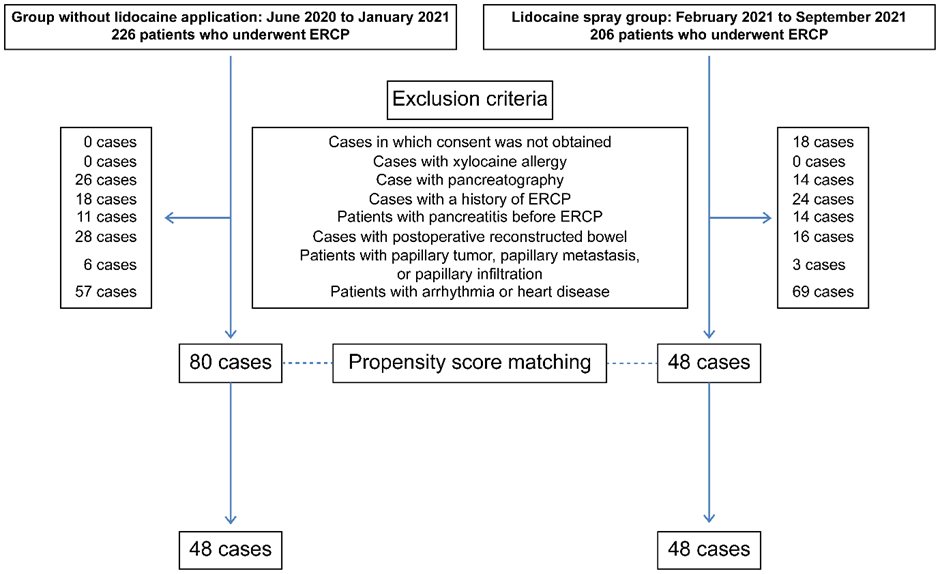
Figure 1: Patient study group allocation.
In a retrospective observational study, 48 patients were extracted from each group (a control group and a lidocaine-treated group) using a propensity score matching method. Intergroup comparisons were conducted. ERCP, endoscopic retrograde cholangiopancreatography.
Study Design
The primary endpoint was deep bile duct intubation time in both groups. Secondary endpoints included an analysis of factors related to bile duct intubation success rate, degree of papilla opening after lidocaine application (four-grade qualitative evaluation), use of the pancreatic duct guidewire method, adverse events following ERCP (pancreatitis, cholangitis, bleeding), blood lidocaine concentrations in the lidocaine spray group, and time for deep bile duct intubation in the lidocaine group. This study was not registered as it is a retrospective observational study.
This study was conducted at Takeda General Hospital (Aizuwakamatsu, Japan). Approval was obtained from Clinical Research Ethics Committee of Takeda General Hospital. (No. 2020-059D), and the study adhered to the principles of the Declaration of Helsinki. Informed consent was not obtained for this retrospective study; instead, a means to opt-out was provided, allowing research subjects to be notified and enabling the publication of research information on our website.
Endoscopic Procedure
The operator in this study was a single endoscopist with experience performing over 1000 successful ERCP procedures. ERCP was conducted using a duodenoscope (TJF-260V; Olympus Corp., Tokyo, Japan) under conscious sedation with intravenous midazolam and/or pentazocine hydrochloride.
During ERCP, the major papilla was observed frontally, and its shape and oral protrusion were morphologically classified according to a previous report (Figure 2) [4]. Papilla shape was categorized into five patterns. Pattern A was annular, characterized by a ring-shaped papilla with a nodular center. Pattern U was unstructured, representing papillae lacking a distinct opening. Pattern LO referred to papillae with an opening elongated in the longitudinal direction of the duodenum. Pattern I was characterized by two openings, where the bile duct opening is on the mouth or left side, and the pancreatic duct opening is on the anus or right side. Pattern G referred to papillae with a gyrate structure.
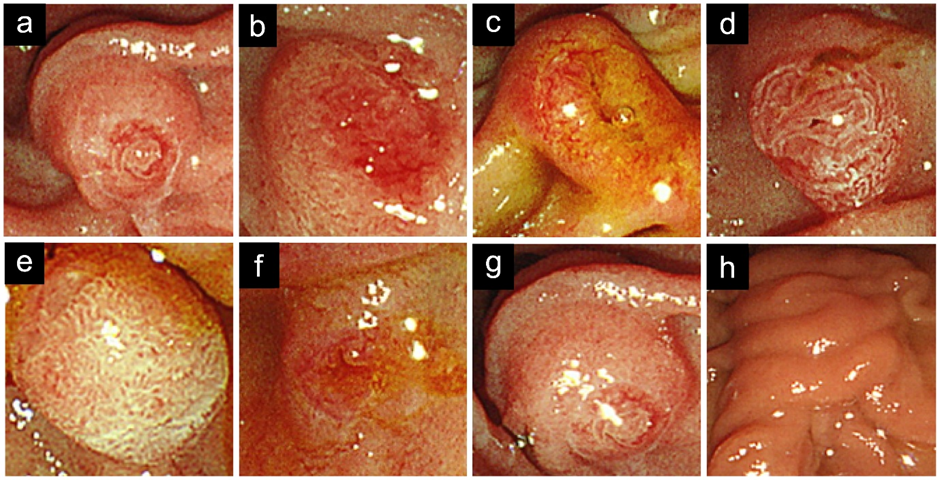
Figure 2: Morphological classification of papilla patterns (a-e); and oral protrusion patterns (f-h). Patterns include annular (A), unstructured (U), longitudinal opening (LO), two openings (I), and gyrate structure (G). Oral protrusion ratios include S (<0.5), R (0.5 ≤ L/D < 2), and L (≥2).
Oral protrusion patterns were classified into three types. The ratio of the oral protrusion length to the transverse diameter of the papilla was calculated, and pattern designations were assigned as follows: Pattern S was assigned when the ratio was <0.5, Pattern R when 0.5 ≤ L/D < 2, and Pattern L when the ratio was ≥2.
Using an ERCP catheter (MTW ERCP catheter; ABIS, Hyogo, Japan), 1% lidocaine (100 mg/10 mL) was sprayed into the major papilla over 10 seconds. After observing for 1 minute, the degree of papilla opening was qualitatively evaluated on a four-point scale (Figure 3). A fully open papilla was defined as an opening in which the papilla was visible and the ERCP catheter (tip thickness: 2 mm) did not hit the surroundings. A mildly open papilla was defined as a very slight opening visible to the naked eye. A moderately open papilla was defined as an intermediate state between a fully open and mildly open papilla. No change was defined as no observable change in the opening after spraying.
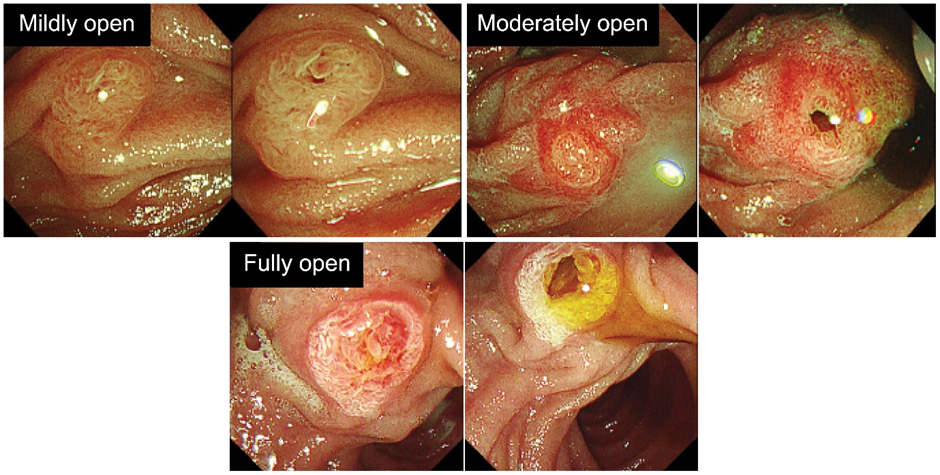
Figure 3: Evaluation of papilla opening before and after lidocaine application.
Mildly open indicates a slightly visible opening, moderately open is an intermediate state, and fully open is visible without the catheter hitting the surroundings.
ERCP was performed using a wire-guided cannulation method in all cases. Specifically, a 0.025-inch guidewire (VisiGlide2; Olympus Medical Systems, Tokyo, Japan) was used. The time to deep bile duct intubation was measured; the start time was when the catheter was applied to the papilla, and the end time was when the catheter was inserted into the bile duct and confirmed to be placed within the bile duct by imaging. After successful intubation, the necessary endoscopic procedures were performed for each case (for stones, papilla incision, and lithotripsy were performed; for bile duct strictures caused by cancer or benign disease, stent placement was performed). Serum amylase and blood lidocaine levels were measured two hours after ERCP and at 6:00 the next morning in all cases.
Acute pancreatitis, cholangitis, and bleeding were evaluated as adverse events related to ERCP. Based on a report by Cotton et al., PEP was defined as pancreatic amylase ≥3 times the normal value accompanied by abdominal pain. Cholangitis was defined as a fever of 38°C or higher for ≥24 hours and elevated biliary enzymes associated with cholestasis. Bleeding was defined as hematemesis, rectal bleeding, or a hemoglobin (Hb) drop of ≥2 g/dL [5].
Adverse events associated with lidocaine spraying were defined as confirmation of any of the following [6]: local anesthesia poisoning (cardiopulmonary events: suppression of the conduction system, such as PQ interval prolongation or QRS width increase on electrocardiography, blood pressure drop, shock, pulse abnormalities, or respiratory depression; central nervous system events: tremors, convulsions, drowsiness, anxiety, agitation, nausea, vomiting; malignant hyperthermia: sudden rise in body temperature, unexplained tachycardia/arrhythmia/blood pressure fluctuation, hyperventilation, sweating, muscle rigidity, etc.; other: hives, edema, etc.) or elevated blood lidocaine concentrations (≥5 μg/mL).
Statistical Analysis
For propensity score matching, both groups were matched one-to-one based on propensity scores calculated using sex, age, and the presence or absence of parapapillary diverticula. For statistical analysis, Mann–Whitney U tests were applied for comparisons of continuous variables. For nominal variables, chi-square tests, Fisher's exact tests, and Yates' correction were used.
To clarify the factors associated with the time of deep bile duct intubation in the lidocaine group, the following items were examined: time to deep intubation, sex, age, diagnosis, presence or absence of parapapillary diverticula, papilla pattern, oral protrusion pattern, and presence or absence of concomitant use of a pancreatic duct guide. For the analysis of categorical variables, Mann–Whitney U tests were implemented after testing the normality of the data. For continuous variables, Spearman's rank correlation coefficients were used after testing the normality of the data.
Factors related to the time to deep intubation were extracted from multiple factors. The time to deep intubation was taken as the dependent variable, and variables that were statistically significantly associated with the time to deep intubation on univariate analysis (using Mann–Whitney U tests and Spearman's rank correlation coefficients) were used as the independent variables. Multiple regression analysis was then performed using a stepwise method. Analysis of variance was used to test the statistical significance of the multiple regression model. We ensured that the variance inflation factor (VIF) of all input independent variables was <10 to address issues of multicollinearity. Residuals and outliers were examined using the Durbin–Watson ratio.
SPSS (v.25; Chicago, IL, USA) was used for statistical analysis. A two-sided p-value of <0.05 was considered statistically significant.
Results
Patient characteristics
Patient demographic and medical characteristics are shown in (Table 1). There were 48 subjects in the control group (29 males, 19 females) and 48 subjects in the lidocaine-treated group (23 males, 25 females). The median age was 73.5 years in the control group and 77 years in the lidocaine-treated group.
|
Control |
Lidocaine |
P |
|
|
Patients |
48 |
48 |
|
|
Sex (M / F) |
29 / 19 |
23 / 25 |
NS |
|
Age (range) |
73.5 (48–101) |
77.0 (48–101) |
NS |
|
Disease |
|||
|
CBD stone |
35 |
33 |
NS |
|
Bile duct cancer |
6 |
3 |
|
|
Gallbladder cancer |
1 |
1 |
|
|
Pancreatic cancer |
3 |
6 |
|
|
AIP |
1 |
0 |
|
|
SOD |
1 |
3 |
|
|
Malignant biliary obstruction by LNM |
1 |
0 |
|
|
Chronic pancreatitis |
0 |
2 |
|
|
Periampullary diverticulum |
13 |
12 |
NS |
|
Papilla pattern |
|||
|
Pattern A |
28 |
30 |
NS |
|
Pattern U |
6 |
4 |
|
|
Pattern LO |
3 |
3 |
|
|
Pattern I |
7 |
8 |
|
|
Pattern G |
4 |
3 |
|
|
Oral protrusion |
NS |
||
|
S |
5 |
4 |
|
|
R |
37 |
40 |
|
|
L |
6 |
4 |
Table 1: Demographic characteristics in the lidocaine and control groups; AIP: autoimmune pancreatitis; SOD: sphincter of Oddi Dysfunction; LNM: lymph node metastasis; NS: Not Significant.
In the control group, there were 35 patients with common bile duct stones, six with cholangiocarcinoma, one with gallbladder cancer, three with pancreatic cancer, one with autoimmune pancreatitis, one with papilla sphincter dysfunction, and one with a malignant biliary stricture caused by hilar lymph node metastasis. In the lidocaine treatment group, there were 33 patients with common bile duct stones, three with cholangiocarcinoma, one with gallbladder cancer, six with pancreatic cancer, three with papilla sphincter dysfunction, and two with chronic pancreatitis.
Parapapillary diverticula were present in 13 patients in the control group and 12 patients in the lidocaine-treated group. Regarding papilla patterns, the breakdown in the control group was Pattern A in 28 patients, Pattern U in six, Pattern LO in three, Pattern I in seven, and Pattern G in four. In the lidocaine spray group, the papilla patterns were Pattern A in 30 patients, Pattern U in four, Pattern LO in three, Pattern I in eight, and Pattern G in three. In the control group, oral protrusions were classified as S in five patients, R in 37, and L in six. There were four S cases, 40 R cases, and four L cases in the lidocaine group. No statistical differences were observed for any of the above items.
Endoscopic procedure type and deep bile duct intubation time
(Table 2) shows a comparative study of endoscopic procedures and deep bile duct intubation time. The success rate of bile duct intubation was 100% in both groups. The median deep bile duct intubation time was 240 seconds (402–1162 seconds) in the control group and 52 seconds (5–540 seconds) in the lidocaine-treated group (p<0.01). The pancreatic guidewire method was used in eight cases in the control group and four cases in the lidocaine-treated group. Median glucagon consumption was 0.5 mg in the control group and 0 mg in the lidocaine group (p<0.01). Adverse events following ERCP were limited to pancreatitis, observed in three patients (0.0625%) in the control group, with no cases of cholangitis or bleeding. No adverse events were observed in the lidocaine-treated group.
|
Control |
Lidocaine |
P |
|
|
Patients |
48 |
48 |
|
|
Cannulation success rate (%) |
100 |
100 |
NS |
|
Selective bile duct cannulation time (sec, range) |
240 (40–1162) |
52 (5–540) |
< 0.01 |
|
Pancreatic guidewire cannulation |
8 |
4 |
NS |
|
Usage of glucagon (mg) |
0.5 (0–1) |
0 (0–2) |
< 0.01 |
|
Postoperative complications |
|||
|
Pancreatitis |
3 |
0 |
NS |
|
Cholangitis |
0 |
0 |
NS |
|
Hemorrhage |
0 |
0 |
NS |
Table 2: Endoscopic procedural characteristics in the lidocaine and control groups.
Blood lidocaine concentrations
In all 48 patients in the lidocaine-treated group, blood lidocaine concentrations were below the detection sensitivity at two hours following ERCP and at blood sampling the next day.
Papilla opening
An evaluation of the papilla opening is shown in Figure 3. There were five cases of fully open papilla, 26 cases of moderately open papilla, nine cases of mildly open papilla, and eight cases with no change.
Factors involved in deep bile duct intubation in the lidocaine group
As a result of univariate analysis, the following five variables were statistically significantly associated with the time to deep intubation: the presence or absence of parapapillary diverticula (p=0.047), papilla Pattern I (p=0.001), oral protrusion Pattern R (p<0.001), oral protrusion Pattern S (p<0.001), and use of a concomitant pancreatic duct guide (p<0.001). The time to deep intubation was statistically significantly longer in those with parapapillary diverticula, whereas it was statistically significantly shorter in the papilla Pattern I group than in comparative groups. Moreover, the time to deep intubation was statistically significantly shorter in the oral protrusion Pattern R group than in comparative groups. In contrast, the time to deep intubation was statistically significantly longer in the oral protrusion Pattern S group than in the other groups. The time to deep intubation was also statistically significantly longer in the pancreatic duct guide combined group than in the non-combined group.
As a result of multiple regression analysis, three variables were extracted as factors affecting the time to deep intubation: use of a pancreatic duct guide (p<0.001), papilla Pattern I (p=0.019), and oral protrusion Pattern R (p=0.032) (Table 3). The generated multiple regression model was statistically significant (p<0.001), with an R² of 0.639 and an adjusted R² of 0.615. VIF values ranged from 1.042 to 1.875, with no multicollinearity. The Durbin-Watson ratio was 1.846, and there were no outliers where predicted values exceeded ±3 standard deviations relative to the observed values.
|
Variables |
Unstandardized factor |
Standardized factor |
Significance probability |
95.0% confidence intervals for partial regression coefficients |
Collinearity statistic |
|
|
Partial regression coefficients |
β |
Lower bound |
Upper bound |
VIF |
||
|
(Constant) |
196.25 |
0 |
111.474 |
281.026 |
||
|
Concomitant pancreatic duct guide |
250.5 |
0.516 |
0 |
130.608 |
370.392 |
1.833 |
|
Papilla pattern I |
-81.094 |
-0.225 |
0.019 |
-148.115 |
-14.072 |
1.042 |
|
Oral protrusion pattern R |
-98.656 |
-0.274 |
0.032 |
-188.575 |
-8.737 |
1.875 |
Table 3: Three variables extracted from the results of multiple regression analysis.
Discussion
We investigated deep bile duct intubation time as the primary endpoint in a comparison between control and lidocaine-treated groups. In comparing the deep bile duct intubation time, we found that the median time in the control group was 240 seconds, while that in the lidocaine group was 52 seconds (p<0.01). Additionally, 54% (26 of 48) of the lidocaine-treated group achieved successful deep bile duct intubation within one minute. We believe that deep bile duct intubation time was shortened to such a degree in the lidocaine-treated group because lidocaine treatment enabled visual observation of the papilla opening, thereby facilitating the cannulation procedure. The findings suggest that lidocaine pre-treatment could reduce procedural times and potentially improve patient outcomes by minimizing the risk of papillar trauma during ERCP. Implementing this approach in routine practice could enhance procedural efficiency, particularly in high-risk patients.
In this study, 40 out of 48 cases showed some degree of change in the papilla, with mildly open, moderately open, and fully open papilla observed. Moreover, understanding papilla anatomy is necessary for interpreting its pathology. We investigated the relaxation mechanism of the sphincter of Oddi by spraying xylocaine. The Oddi sphincter consists of three types of sphincters: the sphincter choledochus, the sphincter pancreaticus, and the sphincter ampullae. Judging from the lidocaine distribution area, the driving factor appears to be the sphincter ampullae. Lidocaine is absorbed through the absorptive epithelium with brush borders among the epithelia that comprise the papilla and is thought to exert a muscle relaxant effect [7].
In examining the factors involved in deep bile duct intubation in the lidocaine-treated group, the results of multiple regression analysis identified the presence or absence of a concomitant pancreatic duct guide, papilla Pattern I, and oral protrusion Pattern R as important predictors. In terms of standardization factors, papilla with papilla Pattern I and oral protrusion Pattern R were found to shorten the time to deep intubation.
In papilla Pattern I, the bile and pancreatic ducts are separated. Because of this unique anatomy, not only the sphincter ampullae but also the sphincter choledochus and sphincter pancreaticus are likely to be penetrated by lidocaine. Watanabe et al. concluded that oral protrusion Pattern L is a factor that makes bile duct intubation difficult [4]. Our data showing that oral protrusion Pattern R is a factor for shortening bile duct intubation also supports these findings. Additionally, the fact that the combined use of the pancreatic duct guide lengthened the time until deep intubation suggests that cases with difficult bile duct intubation (those requiring a longer procedural time) were switched to the pancreatic duct guide.
ERCP is a crucial procedure for diagnosing and treating biliary tract and pancreatic diseases. However, the adverse events following this procedure remain a significant concern. In particular, the incidence of severe pancreatitis is reported to be 0.3%–0.6%, making prevention extremely important [8]. The pathogenesis of PEP has long been attributed to increased intrapancreatic pressure caused by papillar edema and sphincter of Oddi spasm [9]. Reducing intubation stimulation to the major duodenal papilla directly results in faster deep bile duct intubation. Several treatments have been reported to prevent PEP. Prophylaxis with rectal diclofenac and sublingual nitrate has been shown to statistically significantly reduce the incidence of pancreatitis compared with a diclofenac suppository alone [10]. The usefulness of a pancreatic stent has also been documented [11].
Risk factors for pancreatitis onset have been investigated. These risk factors include patient and procedural factors. Patient factors include age of under 50 years, a history of pancreatitis, and female sex, while procedural factors include two or more pancreatographies, difficult bile duct intubation, and precut cases [12]. However, regarding pre-treatment for the major papilla, to our knowledge, no reports exist other than those on nitroglycerin administration.
In this study, we evaluated the efficacy and safety of spray-based lidocaine administration around the sphincter of Oddi to reduce sphincter tone around the biliary and pancreatic ducts. Although no statistical difference was observed, none of the lidocaine-treated groups developed PEP. We believe this was because the time required for bile duct intubation was significantly reduced, minimizing physical irritation to the duodenal papilla, reducing papilla edema, and preventing spasms of the Oddi sphincter muscle. Evaluating additional cases in the future may demonstrate a statistically significant difference in preventing PEP. Future studies should explore the optimal lidocaine dosage for different patient subgroups and assess its efficacy in reducing other complications, such as PEP. Multi-center trials could also provide insights into its applicability across diverse patient populations.
The results of this study demonstrate the safety of lidocaine application to the major duodenal papilla. Lidocaine may cause side effects such as anaphylactic shock and lidocaine poisoning. To prevent this, the topical dose of lidocaine should not exceed 200 mg. A topical dose of 400 mg has been reported to be safe for the colonic mucosa [3]. In this study, we used 100 mg of lidocaine, which was a smaller amount than previously reported, and no lidocaine was detected in the blood following ERCP. Moreover, using a small amount of lidocaine effectively relaxed the Oddi sphincter and demonstrated its safety. Furthermore, considering the high papilla opening rate of 83% (40 out of 48 evaluated patients), we believe lidocaine application to the major duodenal papilla can be a viable option to enable rapid and safe deep bile duct intubation.
This study has some limitations. Specifically, it was a single-center, retrospective, observational study with a small enrolled sample size. Although propensity score matching was used, unadjusted confounding variables may remain, limiting the ability to draw causal inferences. The single-center design and relatively homogeneous patient population may limit the generalizability of these findings to other clinical settings or more diverse populations. Future multi-center studies are needed to validate these results. Further investigation is therefore necessary.
Conclusions
This study clarified that spraying 100 mg of 1% lidocaine on the major papilla reduces the time required for ERCP. This suggests that this treatment may be beneficial if implemented as a routine procedure during ERCP. These findings support the routine use of 1% lidocaine as a safe and effective pre-treatment during ERCP, with further research needed to confirm its broader applicability.
Author Contribution Statement
Collected the data and wrote the manuscript: N.A, A.I.; provided clinical advice: H.W. All authors read and agreed to the published version of the manuscript.
Ethics Approval Statement
The study has been approved by the Clinical Research Ethics Committee of Takeda General Hospital (Approval No. 2020-059D), and it adhered to the principles of the Declaration of Helsinki. Informed consent was not obtained for this retrospective study; instead, a means to opt-out was provided, allowing research participants to be notified and enabling the publication of research information on our website.
Acknowledgments
We thank the members of the Department of Endoscopy at Takeda General Hospital for their technical assistance and logistical support in conducting this research. We would like to thank Editage (www.editage.jp) for English language editing.
Funding
This research received no external funding.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author, N.A., upon reasonable request.
Conflict of Interest Statement
The authors declare no conflict of interest.
References
- Dumonceau JM, Andriulli A, Elmunzer BJ, Mariani A, Meister T, et al. (2014) Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - updated June 2014. Endoscopy 46: 799-815.
- Staritz M, Poralla T, Dormeyer HH, zum Büschenfelde KH (1985) Endoscopic removal of common bile duct stones through intact papilla after medical sphincter dilation. Gastroenterology 88: 1807-1811.
- Nemoto D, Suzuki S, Mori H, Katsuki S, Iwaki T, et al. (2019) Inhibitory effect of lidocaine on colonic spasm during colonoscopy: a multicenter double-blind, randomized controlled trial. Dig Endosc 31: 173-179.
- Watanabe M, Okuwaki K, Kida M, Imaizumi H, Yamauchi H, et al. (2019) Transpapillary biliary cannulation is difficult in cases with large oral protrusion of the duodenal papilla. Dig Dis Sci 64: 2291-2299.
- Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, et al. (2010) A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc 71: 446-454.
- Mulroy MF, Hejtmanek MR. (2010) Prevention of local anesthetic systemic toxicity. Reg Anesth Pain Med 35: 177-180.
- Yamano M. (2015) Pathology of papilla of Vater―practical pathology of papilla of Vater for clinician at diagnosis and therapy. JJBA 29: 152-162.
- Andriulli A, Clemente F, Solmi L, Terruzzi V, Suriani R, et al. (2002) Gabexate or somatostatin administration before ERCP in patients at high risk for post-ERCP pancreatitis: a multicenter, placebo-controlled, randomized clinical trial. Gastrointest Endosc 56: 488-495.
- Polack EP, Fainsinger MH, Bonnano SV (1977) A death following complications of roentgenologic nonoperative manipulation of common bile duct calculi. Radiology 123: 585-586.
- Tomoda T, Kato H, Ueki T, Akimoto Y, Hata H, et al. (2019) Combination of diclofenac and sublingual nitrates is superior to diclofenac alone in preventing pancreatitis after endoscopic retrograde cholangiopancreatography. Gastroenterology 156: 1753-1760.
- Mazaki T, Mado K, Masuda H, Shiono M. (2014) Prophylactic pancreatic stent placement and post-ERCP pancreatitis: an updated meta-analysis. J Gastroenterol 49: 343-355.
- Chen JJ, Wang XM, Liu XQ, Li W, Dong M, et al. (2014) Risk factors for post-ERCP pancreatitis: a systematic review of clinical trials with a large sample size in the past 10 years. Eur J Med Res 19: 26.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.