Journey to Integration of Injectable Antigens in Mass Vaccination Campaign; Benefits and Challenges using 2019 Meningitis A (Men A) and Measles Integrated Campaign in Nigeria
by Avuwa Joseph Oteri1*, Adejoke Oladele Kolawole2*, Fredrick Mogekwu9, Chidinma Blessing Nwachukwu5, Samuel Ibizugbe6, Samuel Bawa4, Chinedu Okoronkwo10, Boubacar Dieng8, Anne Eudes Jean Baptiste7, Nadia Lasri3, Nneka Onwu2
1Nigeria Governors Forum, Nigeria
2National Primary Health Care Development Agency, Nigeria
3Gavi, The Vaccine Alliance, Switzerland
4World Health Organization Country Office Abuja, Nigeria
5World Health Organization, Niger state field office
6Clinton Health Access Initiative (CHAI)
7Pan America Health Organization
8Technical Consultant, Gavi, The Vaccine Alliance
9Careland Health care Services UK
10UK Health Security Agency
*These authors contributed equally to this work.
*Corresponding author: Avuwa Joseph Oteri, Nigeria Governors Forum, Abuja, Nigeria
Received Date: 03 December, 2023
Accepted Date: 13 December, 2023
Published Date: 18 December, 2023
Citation: Oteri AJ, Kolawole AO, Mogekwu F, Nwachukwu CB, Ibizugbe S, et al. (2023) Journey to Integration of Injectable Antigens in Mass Vaccination Campaign; Benefits and Challenges using 2019 Meningitis A (Men A) and Measles Integrated Campaign in Nigeria. J Community Med Public Health 7: 392. https://doi.org/10.29011/2577-2228.100392
Abstract
Background: Campaign integration, which refers to the co-delivery of all or some of the campaign components has been one of the guiding principles of immunization strategies. Mass vaccination campaigns serve as critical interventions to address deficiencies in routine vaccination coverage, particularly within health systems facing challenges. Nigeria has been involved in many mass vaccination campaigns but mostly standalone though attempts have been made to integrate some with other health interventions. In 2019, Nigeria conducted an integrated measles and Meningitis A vaccination campaign in sixteen states in Northern Nigeria. Notably, this marked Nigeria’s most extensive integrated campaign, involving the administration of two injectable antigens. This paper aims to chronicle the nation’s progress towards the integration of injectable antigens within its immunization framework, drawing insights from the unique challenges and successes encountered during the implementation of this integrated campaign.
Methods: We documented the integration of the measles and Men A campaign from earlier planned standalone campaigns to reviewing decision making steps taken during the planned integration. We reviewed the coordination mechanism involved in the integration as well as the campaign budgets and post-campaign coverage survey report to document best practices, challenges, and recommendations for future integrations. Results: The findings reveal significant time savings, with the integrated approach. Decision-making involved extensive negotiation and collaboration at all levels, with stakeholder engagement crucial for successful integration. Cost-effectiveness analysis indicated remarkable savings in operational costs, attributed to the efficient resource utilization and prudent planning.
Conclusion: Through a detailed examination of this historic initiative, the paper contributes to the understanding of the complexities surrounding the integration of injectable antigens. It addresses the challenges associated with a crowded immunization calendar and resource limitations in developing countries. The lessons learned contributed to the understanding of campaign effectiveness and offered valuable considerations for future integrated health interventions.
Keywords: Integrated Campaign; Measles and Meningitis A Vaccination Campaign; Campaign Effectiveness; Nigeria
Introduction and Background
Mass vaccination campaigns are usually instituted to bridge the gaps of poor routine vaccination coverage usually associated with a weak health system. They are also an avenue to expediently fill delivery gaps, provide extra or booster doses of a vaccine to eligible persons, reach previously unreached with the particular antigen, and help attain program targets such as elimination or eradication goals of vaccine-preventable diseases [1-3].
Mass vaccination campaigns usually extend the coverage of the antigens to age groups outside those for Routine Immunization (RI) and as such can cover up to 10 times the number scheduled for RI within a period. Campaigns are also used to prevent or respond to disease outbreaks, and therefore require intensive planning, coordination, training of staff, mobilization of vaccines, documentation, safety surveillance and good execution to achieve the aims of the campaign [4,5].
Nigeria is a signatory to the many disease elimination and eradication goals such as measles elimination, polio eradication, eliminating yellow fever etc. and uses mass vaccination campaigns as one of its methods to achieve these goals. The country has yet to attain high routine immunization coverage to sustain eradication and elimination goals and as such relies on mass vaccination campaigns to boost coverage and achieve herd immunity [6].
An integrated campaign refers to the co-delivery of all or most of the campaign components for two or more health interventions. It could be partial or full integration depending on which areas of the campaign are being integrated [7].
Integration has also been one of the guiding principles of Global Immunization strategies, from Global Immunization Vision and Strategies 2006-2015 (GIVS) to the Global Vaccine Action Plan 2011- 2020 (GVAP) and the recent Immunization Agenda 2030 (IA 2030), as a way to deliver multiple services especially at primary healthcare level in order to improve access through immunization [8-11].
Integration of health services is not new, especially in maternal and child health; advocacy has always been to provide as many interventions as possible when a child or mother presents to the health center, resulting in programs such as Integrated Management of Childhood Illness (IMCI). Kamatsuchi M, et al. found that, on average, 100 integrated child health events take place in sub-Saharan Africa annually between 2005 and 2010 with about 154 events alone in 2010 as child health days or immunization campaigns [12]. Mass measles vaccination campaigns are commonly used as a platform for delivering other interventions, especially Vitamin A and polio vaccine [13,14].
Nigeria context
Nigeria is a signatory to disease elimination and eradication goals, such as measles elimination, polio eradication, and eliminating yellow fever and uses mass vaccination campaigns as one of its methods to achieve these goals. The country has yet to attain high routine immunization coverage to sustain eradication and elimination goals and as such relies on mass vaccination campaigns to boost coverage and achieve herd immunity [6].
The Maternal Neonatal Child Health Week (MNCHW) which has been in practice in Nigeria since 2010 as a biannual event following the recommendation of the National Council of Health delivers integrated maternal and child health services to all pregnant women and children less than five years of age [15,16]. Similarly, Periodic Intensification of Routine Immunization (PIRI) enhances routine immunization in areas with extremely weak infrastructure, security problems, or major geographic and resource challenges and uses an integrated campaign mode to deliver services [17].
In terms of antigen integration as well as integration across interventions, Nigeria has vast experience in conducting partially integrated vaccination campaigns. Immunization Plus Days (IPDs) replaced the National Immunization Days (NIDs) in 2006 as a response to the community needs. Instead of administering the oral polio vaccine alone as was done in NIDs, a range of antigens (measles and DPT vaccines) have been administered with the oral polio vaccines plus other child survival interventions such as anti-helminthics, Vitamin A, and distribution of long-lasting insecticide-treated nets (LLIN) [18].
The integration of most vaccination campaigns in the country involved maternal health and child survival interventions with immunization services. Integration of injectable and noninjectable vaccinations (i.e. measles and polio) was conducted prior to 2019. In the 2013, integrated measles and polio campaign (IMC) in a few Local Government Areas (LGA) attempts were made to include distribution of LLIN. In 2014, two injectable vaccines (tetanus and meningitis A) were combined in some Local government Areas in three states [19,20].
Historically, Nigeria has been involved in stand-alone campaigns for meningitis and measles prior to the 2019 integrated campaign of these antigens. For example, in 2005, the first catchup measles campaign targeting children 9 months to under 15 years of age was conducted in Nigeria and thereafter, stand-alone measles vaccination campaigns have been conducted every two or three years, with that of 2013 along with oral polio vaccine [21].
Between 2011 and 2015, following Gavi’s approval of Nigeria’s proposal to conduct a preventive mass vaccination campaign using the meningitis A conjugate (men. A) vaccine, in 26 high-risk states including the Federal Capital Territory, preventive mass vaccination campaigns were conducted in these states targeting the age group one year to 29 years in phased manner due to the high target population (81.5 million) and availability of vaccine [22].
Nigeria, in keeping with global efforts to reduce the burden of vaccine-preventable diseases conducted mass vaccination campaigns involving measles, meningitis A, tetanus, yellow fever and polio antigens in 2019. The delivery of the meningitis vaccine and measles vaccine second dose were introduced into the routine immunization schedule. In the North (16 out of 20) states including the Federal Capital Territory (FCT) implemented the integrated measles and meningitis A vaccination campaign (IVC). The integration was an innovation as both campaigns were planned earlier as stand-alone campaigns, and for the first time, conducted on a very large scale as integrated.
This paper showcases the actions and steps taken to plan and coordinate the implementation of the largest integrated injectable antigens mass vaccination campaign in 16 northern states of Nigeria and the Federal Capital Territory, mentioning some best practices. The paper also examines some of the benefits of integration and provides recommendations on how to plan and implement integrated vaccination campaigns.
Methodology
Study setting
Sixteen of the 19 states in northern Nigeria and the Federal Capital Territory conducted an integrated vaccination campaign in 2019 except for Kano, Yobe who did stand-alone campaigns and Niger and Kogi states whose campaigns could not take place with the rest because of logistical issues affecting on the availability of the meningitis vaccines.
The planning and implementation of the 2019 integrated measles and meningitis A vaccination campaign was coordinated by the Non-Polio Supplemental Immunization Activities (NPSIA) unit of the Disease Control and Immunization Department of the National Primary Health Care Development Agency (NPHCDA). The coordination platform of the 2019 integrated measles men A campaign was the same as used in the 2017/2018 measles vaccination campaign and operated from the National Polio Emergency Operation Center and was led by Government [23]. Oversight for the team was provided by a steering committee. A Core Group made up of NPHCDA management and heads of both local and international partner agencies working in immunization within Nigeria and external support came from the Country Working Group (CWG). Dedicated staff of World Health Organization (WHO), United Nation Children’s Fund (UNICEF), United State Centers for Disease Control (CDC), the African
Field Epidemiology Network (AFENET), Clinton Health Access Initiative (CHAI), Bill & Melinda Gates Foundation (BMGF), European Union (EU) through her EU Support to Immunization Governance in Nigeria (EU-SIGN) project and the Nigeria Center for Disease Control (NCDC) were nominated from their country offices to support non-polio supplemental immunization activities.
Proposals for 2019 Campaigns
In the 2018 Gavi proposal application window, Nigeria applied for support from Gavi to carry out a multi-year proposal (2019-2021):
- A follow-up measles campaign in 2019,
- A measles second dose introduction into the routine immunization schedule in 2019,
- A yellow fever preventive mass vaccination campaign in four states of Katsina, Ekiti, Edo and Rivers states in 2019. A multi-year proposal (2019-2021)
These proposals were approved by the Gavi Independent Review Committee (IRC) in January 2019 and were proposed as stand-alone. The IRC recommended complementarity in some of the activities because there were other previously approved interventions earmarked for 2019. These interventions are the introduction of the meningitis vaccine into the routine immunization schedule earlier approved for 2016 after the completion of the phased preventive catch-up meningitis A campaign in 2015. This introduction is associated with a “mini catch-up meningitis A campaign” targeting the birth cohorts born after the mass catch-up campaign of 2011 to 2015 and prior to the introduction of meningitis into the routine schedule. Thus, the meningitis A target age group varied across states based on the date of the last meningitis A vaccination campaign in the state. Bauchi, Borno, Gombe, Jigawa, Kano Katsina and Sokoto States targeted children aged 1 to 7 years while the other states targeted children aged 1 to 6 years.
The decision to change from stand-alone to integrated campaigns
Nigeria’s immunization calendar for 2019 was busy as it had multiple antigen campaigns and two introductions of vaccines into the routine immunization schedule: the measles second dose starting with the seventeen southern states and meningitis A vaccine introduction starting in the nineteen northern states as well as the FCT. The supplemental immunization activities were measles follow-up campaign in the 19 northern states and the FCT, yellow fever mass preventive campaign in Edo, Katsina, Ekiti and Rivers states as well as meningitis A follow up campaign in 25 states with variation in the target age group per state. The polio immunization programs (outbreak response and planned national and subnational polio campaigns) were also being carried out side by side with the busy programs in line with the country’s polio eradication efforts. In the same vein, Maternal and Neonatal Tetanus Elimination (MNTE) campaigns were planned for implementation across 108 Local Government Areas in the Northern and Southern states.
The Leadership of the National Primary Health Care Development Agency, the CORE Group made up of team leaders of immunization Partners in the country (WHO, UNICEF, CDC, AFENET, EU, BMGF, World Bank etc.), the Country Working Group (external support to immunization campaigns with Gavi and US CDC as alternate chair) all had various meetings to review the way forward to address the busy immunization Agenda for 2019. Coincidentally, at the same time, the government of Nigeria was unable to provide her counterpart funds for the planned measles campaign due to the economic crunch in the nation following falling crude oil prices globally. This led to the government’s decision to integrate men A, which had operational funding, with the measles campaign. The decision was backed by Gavi which pooled Gavi’s in-country resources domiciled with WHO and UNICEF to mitigate the financial shortfall and alleviate the unusually busy immunization calendar. This led to the development of a revised chronogram.
Reviews of reports
We reviewed the minutes of meetings and activities of all the technical working groups, NPSIA unit, Steering committee, Core group and Country Working Group. These meetings were held at various levels:
- High-level meetings/engagement with global partners - Country Working Group (CWG), Gavi and United States Centers for Disease Control made up the secretariat for the group.
- Meetings with the Steering committee and the Core group that was chaired by the Executive Director of the NPHCDA.
- Technical working committee meetings (Training, Logistics, Advocacy, Communication, and social mobilization etc.)
- Campaign coordinating committee activities and weekly meetings.
The minutes gave the authors insight to the various steps taken in arriving at a decision to integrate the campaign and actions taken to mitigate likely challenges that could emanate from the decision.
Similarly, we reviewed the Gavi IRC approved budgets for the stand-alone campaigns – for men A, the measles vaccination campaign and the yellow fever campaigns approved by Gavi and also the later approved integrated campaign budgets for the northern states that integrated their men A with measles antigens. The review of the budget is to see areas where savings were generated due to the integration. The operational cost of the campaign is arrived at by summing up all the cost related to activities such as implementation trainings at the states, LGAs and ward levels, campaign waste management, ACSM activities including ACSM tracking and job aids. Others are personnel allowances national, states and LGA supervisors, stipends for transportation of vaccination team, LGA team, independent monitors as well as transportation and logistics for the campaign.
We also reviewed the activities carried out during the integration to document what the authors considered as best practices and challenges encountered during the integration. The integrated post-campaign coverage survey results from the National Bureau of statistics (NBS) for the measles and meningitis A in the study states (integrated and stand-alone campaigns) were also reviewed. Post campaign coverage surveys are conducted after a vaccination campaign by an independent body to estimate the coverage of the antigen at state level. The review was to ascertain if there were any significant differences between the stand-alone and integrated states in the number of children reached during the campaign.
Results
Planning and Coordination
Minutes of Meetings of the Steering Committee, CORE Group and Country Working Group - Following the approval of the measles vaccination campaigns and the measles second dose introduction proposal, the campaign coordinating committee made up of Partners and the government made regular presentations on preparations for the campaigns as well as the measles second dose introduction to the above committees on a regular basis as detailed in Table 1.
|
Meetings |
CORE Group |
Steering Committee |
Country Working Group |
NPSIA Meetings |
Teleconference with States |
Stakeholder meetings |
|
Monthly/weekly Frequency |
As needed |
Monthly then weekly |
Weekly except public holidays and days of states engagement |
Twice weekly from January to Nov 2019 |
Weekly (Wed, Thurs, Fridays 10 am - 3 pm (states were batched |
Once |
|
Total No of meetings held |
6 |
24 |
29 |
60 |
12 meetings per batch |
5 (integration meeting with Polio and the routine immunization committee) |
Table 1: Frequency and number of coordinating meetings held during the 2019 integrated measles Men A campaign.
Presentations at these meetings were centered around the development of a chronogram of activities to cover the four (4) antigens, funding and financing updates for the campaigns, readiness, coordination at national and state levels, logistics, advocacy and social mobilization, and human resources.
Development of One Microplan and Tool - Microplan, which is a detailed bottom-up planning process conducted at the ward level and aggregated from the state to the national level quantifies the human, material and financial resources required to reach all the targeted persons in a campaign. Microplanning processes, which included training and the two-stage verification processes of the states’ microplan for measles and the Men. A vaccination campaign was harmonized from the planning stage since it involved the same persons at the lowest (ward) levels. It was cost and timesaving to have one microplan activity covering all the SIAs. The electronic copy of the tool was also integrated with routine immunization features, as the country was planning to introduce the second dose of measles onto the routine immunization schedule. (Link below shows a sample template of one of the states (Plateau).
https://docs.google.com/spreadsheets/d/1_3ji6RZoScXNX7kVH Qz6zsLqpJ6DxHB/edit?usp=sharing&ouid=109599797999890257930&rtpof=true&sd=true)
Micro plan verification, a part of the microplanning process, stands out as one of the core components of pre-implementation activity and is the final step before the plans are used. It is a 2-step process conducted at the state level. Microplanning, entails physically confirming the detailed inputs, such as population size by wards, LGAs, terrain of targeted places, names of religious places, schools, influential persons in the community, required commodities (vaccines and devices) for implementation etc. This variety of data is entered into the microplanning tools at the state level. The verification is conducted after a desk review and field visit to selected Local Government Areas to ensure all the aspects of the microplanning tools are correctly filled. The Daily Implementation Plans (DIP) drawn from the microplan are used to inform and train state and LGA teams on the approach for integration [24].
One Advocacy, Communication, and Social Mobilization (ACSM)/Stakeholders Plan – Advocacy, communication and social mobilization activities are key to generating demand, increasing uptake, and improving knowledge about vaccines whether during SIAs (Polio and Non-Polio) or RI implementation. A harmonized meeting was held with various stakeholders such as professional medical bodies, other Government Ministries, Department and Agency (e.g., education, National Youth Service Corps, Information, etc.,) Trade Unions, Development partners etc. informing the stakeholders of the integrated SIA and their roles to ensure a successful implementation.
Immunization Chronogram for 2019
Following the approval of the various immunization interventions for 2019, a chronogram of activities, especially the second half of the year when the majority of the planned interventions were to be implemented was developed as shown in Figure 1.
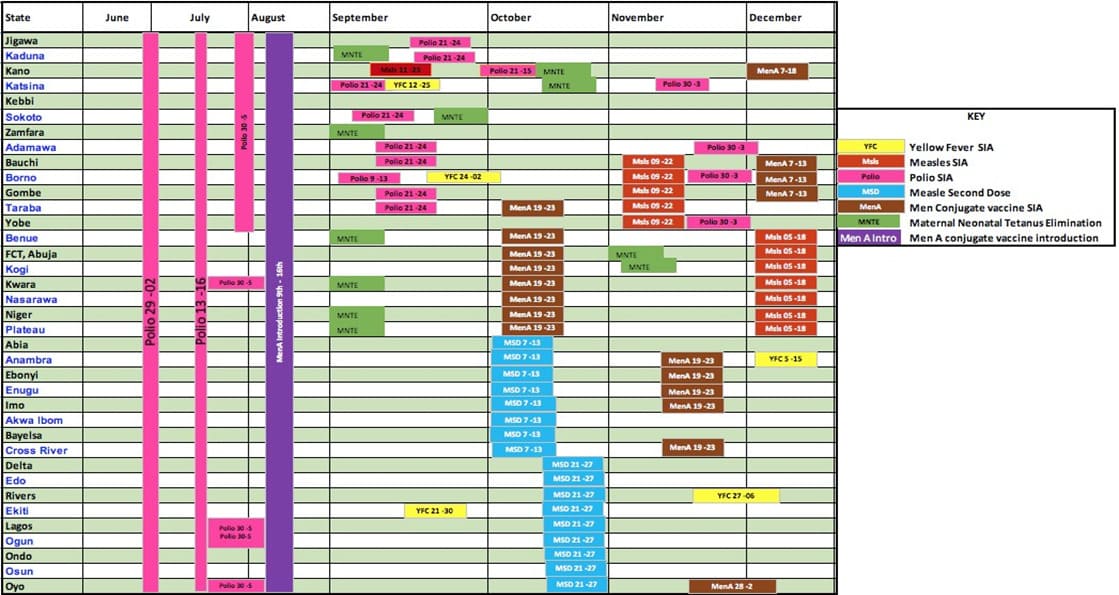
Figure 1: Immunization chronogram for 2019 based on the approvals.
With the decision to integrate the measles and men A vaccination campaigns in some states a new chronogram was developed as shown in Figure 2.
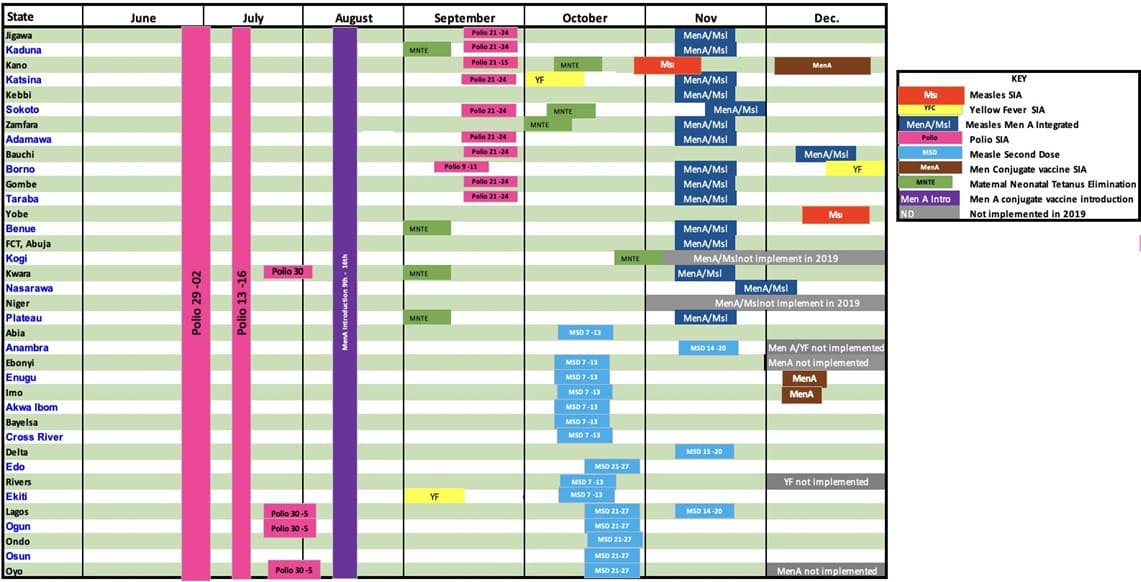
Figure 2: Revised chronogram of immunization activities for 2019.
Training plans
Training, is a major component of vaccination campaigns as health workers’ knowledge is critical for the effective delivery of immunization programs [25]. In the planning for a vaccination campaign, various trainings are carried out to ensure adequate knowledge of the health workers. For the 2019 integrated Men A and measles vaccination campaign, PowerPoint presentations for use at the subnational levels were developed by the National implementing team i.e. government and partners and these presentations covered all the thematic areas of the vaccination campaign; Coordination, planning and financing, cold chain and logistics, ACSM activities, monitoring, supervision and evaluation, data and management of AEFI and surveillance used in monitoring the preparation and readiness of the states using the campaign readiness dashboard [5].
With the integration of the antigens, revisions to the earlier plans ensured a seamless implementation of the integrated campaign. Major additional topics introduced at the implementation training were: https://docs.google.com/spreadsheets/d/1_I3ji6R-ZoScXNX7kVHQz6zsLqpJ6DxHB/edit?usp=drive_link&ouid=109599797999890257930&rtpof=true&sd=true
1. Vaccination Post Layout: Since the integration involved a huge number of clients of different ages, there was a special need to guard against wrong administration of vaccines for age, for example, children 9 months to 11 months were to receive only measles vaccination and could easily be mistakenly given meningitis A vaccine that was not intended for them at that age. Similarly, a child could mistakenly receive two shots of same antigen in place of the two different antigens if the layout was wrong and the team members were not specially trained. This led to the development of a special training on vaccination post layout (Figure 3) and flow of clients including specified roles and responsibilities in the team composition (Figure 4). An animated video was developed which team members downloaded and shared in their mobile phones for ease of understanding the layout, team composition and client flow.https://drive.google.com/drive/folders/1uOPVY47aO7zNs6YbEZ hw8BtKGxlqE0E2?usp=sharing 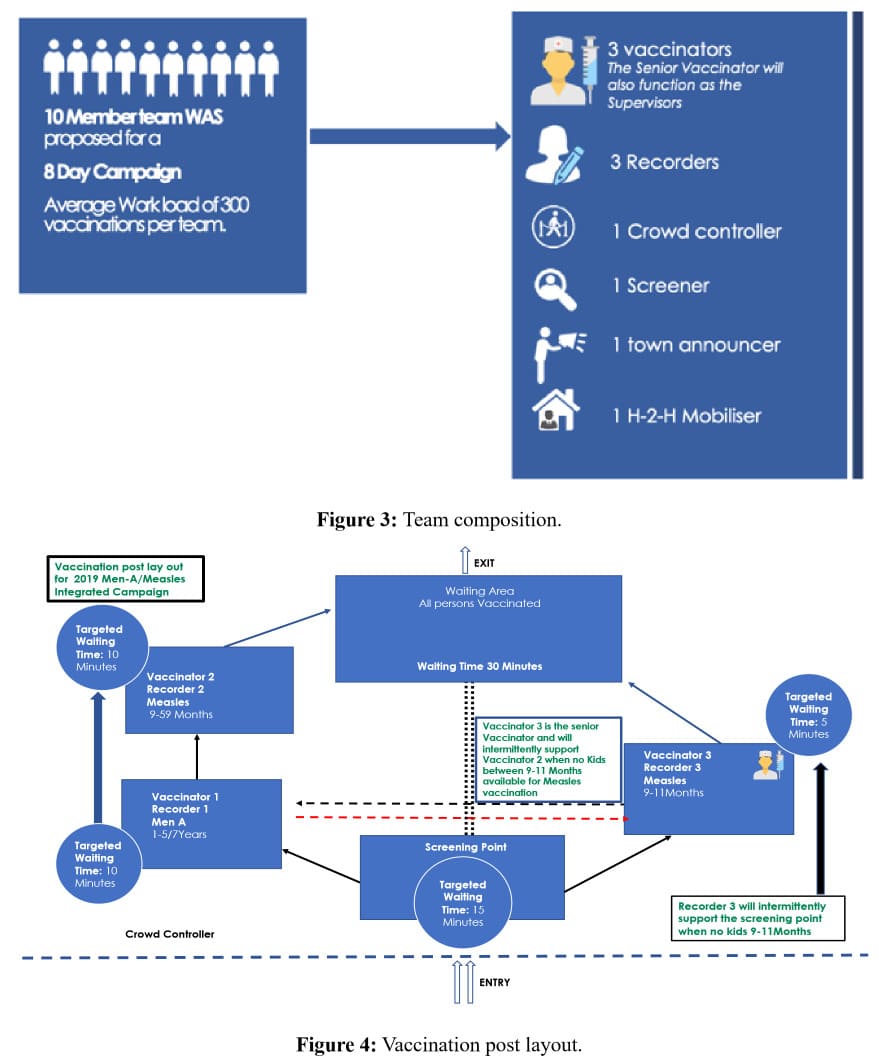

Figure 5: Orderly arrangement of vaccination post with children and caregivers queuing for their turn.

Figure 6: Sitting arrangement in a temporary fixed post showing the two different antigens with different vaccine storages and the two different recorders for each antigen.
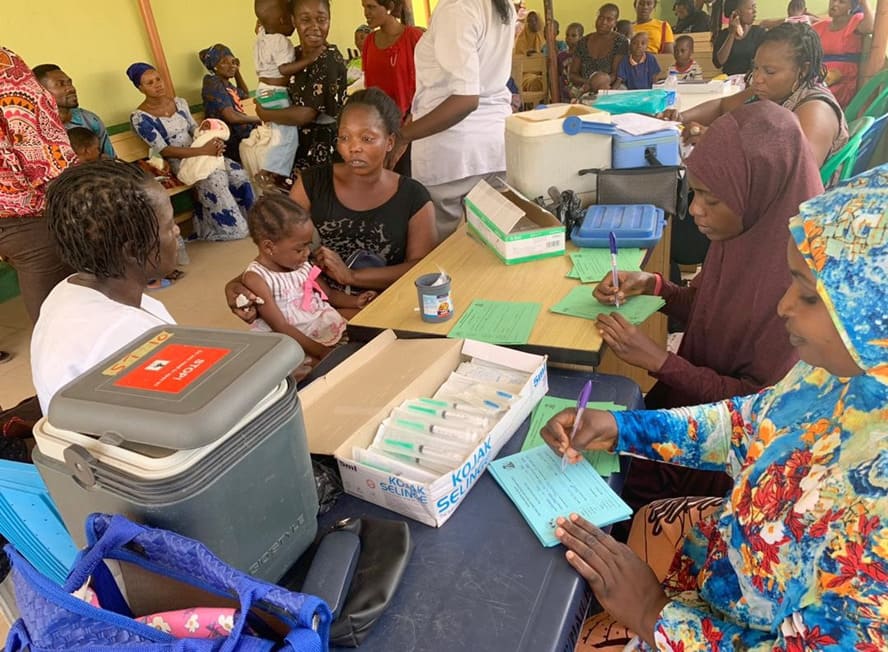
Figure 7: Sitting arrangement of vaccinators and recorders made to prevent a child receiving one antigen twice in error. The cold boxes only store a particular antigen and diluents to avoid mix-up.
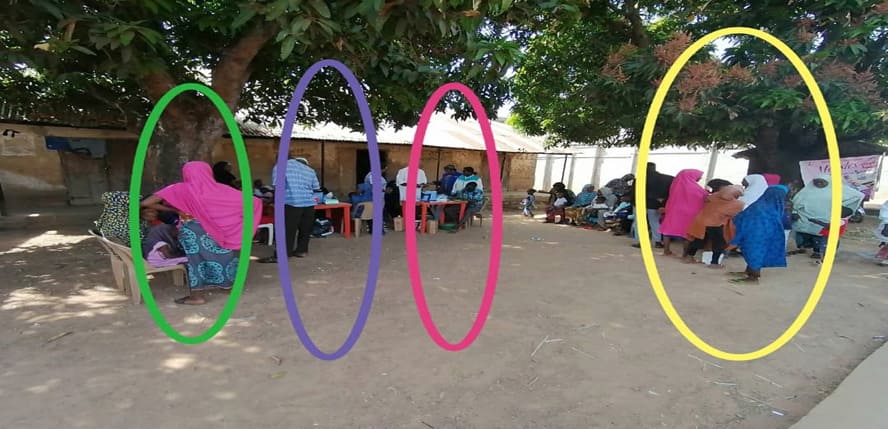
Figure 8: Showing orderly arrangement at a temporary vaccination post to avoid errors. Yellow circle - screening area, Pink and purple - vaccination points and Green - post vaccination waiting area.

Figure 9: Sitting arrangement in a vaccination post between care givers, vaccinator and recorders. Note caregiver sits across from 2 vaccinators. First one administers menA, then mother turns child and 2nd vaccinator injects MCV. Recorders sitting next to them hand cards to caregiver after both injections complete.
The training modules were developed and vetted for use at the implementing sub-national level by the Steering Committee.
Budgets
Table 2 shows the summary of the operational cost of the campaign by reviewing the budget for the measles and the meningitis A stand-alone and that of the integrated campaign in the implementing states. The operational cost for the earlier planned measles stand-alone and the meningitis A vaccination campaign for the study states was 2,666,198,363.66 Naira and 1,839,553,529.36 Naira respectively, totaling 4,505,751,893.02 Naira while the integrated measles and meningitis A operational cost was 2,112,533,935.92 Naira, a difference of 2,393,217,957.10 Naira. The average cost saving with the integration of the two vaccination campaigns was 45.83% with FCT savings being the lowest at 39.66% while savings from Borno was 53.56%.
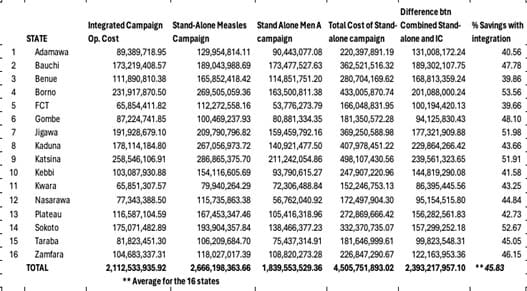
Table 2: Summary budget for standalone and integrated campaign for the states.
Post Campaign Coverage Survey
Table 3 shows the post-campaign coverage survey results for both antigens including the coverage for Kano state that conducted two stand-alone campaigns. For meningitis A, the state with the least coverage at 78.5% was Kebbi while the highest was Kwara with 95.4% coverage. For the measles vaccine, Kebbi had the lowest coverage of 80.6% while Kwara state also had the highest coverage of 95.9%. Comparing coverage between the two antigens, in the PCCS, Kano state which conducted two stand-alone campaigns had a range of 6.8% while for the states that targeted children 12-83 months for meningitis A, Zamfara state had a range of -4.8%, while Gombe and Sokoto states was -1.8%. For states that targeted children 12 months to 59 months, Taraba state had a range of -2.4% while Benue was 0.1%.
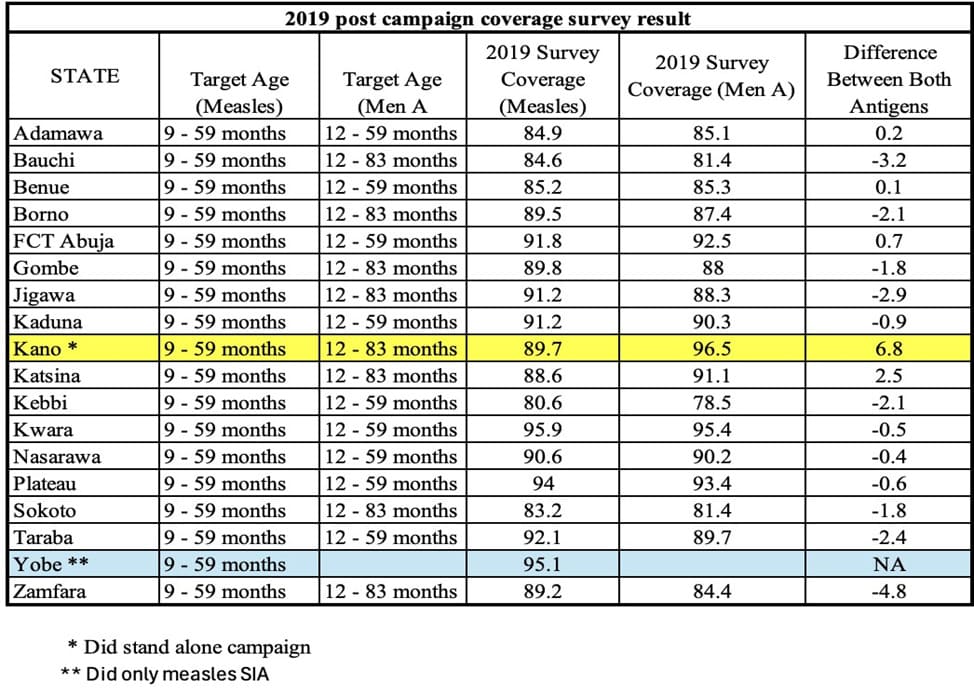
Table 3: 2019 Post campaign coverage survey result for Measles and Men A vaccines.
Discussion
The ever-increasing burden of limited resources to carry out health campaigns was a driver for integrating the many mass vaccination campaigns that were slated for 2019 in Nigeria. Personnel, funds, time etc. were in short supply in an unusually busy immunization year. From our results, we presented findings in integrating the measles and meningitis A vaccination campaign.
Time saved
From the chronograms in Figures 1 and 2, all the twelve months had an immunization activity across all the states, and it was obvious that without the decision to integrate the measles and the meningitis A vaccination, it could have been impossible to effectively carry out the 2019 Gavi approved interventions. This became more pertinent as, at least twenty-eight days could have been needed to administer two live vaccines if not administered at the same time [26,27], that time could have further stretched the immunization calendar further into the following year. Anambra state was scheduled to carry out integrated meningitis and yellow fever campaign, but this could not happen because when the mandatory twenty-eight days interval needed for injecting another live vaccine after the measles second dose introduction in the state was projected, their campaign fell into the Christmas season, and this was not advisable. Based on lessons learnt, December being holiday season is not the best of time to schedule any major health intervention as the quality will not only be poor, some, of the targeted clients would have been missed because of travels as found in other studies on holiday migration [28].
Decision, Coordination and Collaboration
Integration of the meningitis A and measles campaign did not just fall from the rooftop. It involved a lot of thorough thinking and negotiation before arriving at the decision to integrate. Negotiation to integrate was done at the highest level as proposed in the Health Campaign Effectiveness Coalition Decision guidelines [29]. From our study, the importance of good coordination and collaboration among stakeholders at both National and subnational levels cannot be over emphasized. All the relevant stakeholders (partners, donors, implementers, religious and Traditional leaders, national and subnational government) were engaged early enough as soon as the decision to integrate was taken to get their buy-in. The government played a leading role in the decisions to integrate amongst the stakeholders. The coordination and collaboration seen in this study are similar to the findings of Chehab, et al. in their review of best practices from the integration of Vitamin A and Polio campaign in five African countries of Angola, Chad, Cote d’Ivoire, Tanzania and Togo, and Bazant et al. in their promising practices for the collaborative planning of integrated health campaigns from a synthesis of case studies [30,31]. The team appreciated the complexity of integrating both antigens since there were children under one year who were taking only the measles vaccine and some children above 59 months that were taking only the meningitis A vaccine. This complexity resulting from age and antigen-specific differences amongst the target population as well as the dynamic iterative process involving the numerous relevant stakeholders was noted, respected and considered in the planning and coordination of the integrative campaign as recommended by Cathain, et al., in their guidance on how to develop complex interventions to improve health and healthcare [32].
While the decision to co-administer immunization antigens was left with the policymakers and program managers, decision for vaccine co-administration was provided by the National Immunization Technical Advisory Groups (NITAG), who used well reviewed and tested evidence to recommend to the policymakers and program managers. Nigeria’s equivalent - the Nigeria Immunization Technical Advisory Group (NGI- TAG) had severally assisted the immunization program with decisions on vaccine types, formulations but for the 2019 integration of measles and men A antigens, NGI-TAG applied the principle of use of previous studies to approve the integration [33].
Cost-effectiveness and efficient use of resources
From the budget Table 2, there was remarkable savings in the operational cost of delivering the integrated vaccines when compared to the earlier stand-alone budgets. The average 45.83% saved from the sixteen states based on integration could have been more as the additional birth cohort due to the one-year delay of the earlier approved men. A mini catch-up campaign added to the target population. The mini catch-up targeted children that were born after the major menafrivac campaigns between 2012-2015 that happened in phases. The savings from the integration was not unconnected to the efficient use of resources and more prudent planning. For example, in the integrated budget House-to-house mobilisers were reduced from 10/ward to 1/ward. The number of teams was reduced based on the increased number of days for the campaign (integrated campaign – 8 days and standalone – 5 days). The measles campaign projected a vaccination rate of 35 persons/ hour compared with the 60 persons/hour projection of both the Standalone Men-A and integrated campaign budgets. Still on prudent planning, one major challenge that campaigns in Nigeria has had is the issue of target population which is derived from the population of the states. Prior to the 2017/2018 measles vaccination campaign, proposals were developed using projections from the 2006 national census and that had contributed to over budgeting as some of the population figures were not accurate due to the differences in growth rate since the census in 2006. The 2017/2018 measles campaign afforded the immunization team the opportunity of getting a more accurate target population as Geographic Information System (GIS) was used in arriving at the population used for the Northern states while the southern states did a household enumeration for the target population [34]. The exercise gave the team a near accurate population for subsequent immunization interventions, this was applied in reworking the earlier approved men A catch up campaign for these 16 states leading to more savings. These and other detailed savings have been documented by Anne Eudes Jean Baptiste et al, who documented the cost savings between the stand-alone and the integrated strategy [35].
Our findings are not different from other studies that showed that integration of interventions reduces cost when compared to individual interventions. In Bosselli, et al., a study in Lao PDR, a ten times reduction in the cost of deworming individuals was observed when the deworming program was integrated with an existing immunization program [36]. Savings from appropriate implementation of health interventions have been linked with efficiency and have been recommended in Baraimi, et al. study as a way of expanding the fiscal space for the health care system [37]. In the same vein, Lankester, et al. in their study on integrated health delivery platforms, targeting Soil-Transmitted Helminths (STH) and canine-mediated human rabies, demonstrated that integrated delivery resulted in a lower cost per dose delivered than if each intervention had been delivered independently [38]. However, in a study by Immunization Costing Action Network in Nigeria, there was no significant difference in financial cost per targeted individual when they compared the two states that did stand-alone yellow fever campaign with a state that integrated yellow fever with meningitis vaccine [39]. This observation may be due to the differences in the delivery strategies and the timing of the campaign. The integrated campaign came up post-COVID-19 pandemic while the stand-alone campaigns were pre-COVID19. Vaccination campaigns in Nigeria and other low- and mediumincome countries have been associated with disruption of routine services as health facilities are almost shut down to other services because the few available health workers are deployed for the campaigns [40,41].
Therefore, integration which reduces the number of campaigns could be seen as a means of reducing the disruption of services associated with multiple campaigns. The efficient use of resources, reduction in fragmentation of programs, improvement in collaboration amongst stakeholders and programs associated with integration have been demonstrated by Wirtz, et al., Engel et al. and Morgan et al. studies that looked at integrating the Human Papillomavirus vaccination program with enhanced cervical cancer screening and treatment, promoting adolescent health and other life course services [42-44].
Reaching More Targets
From our study, looking at the difference in the percentage coverage of the antigens in the post-campaign study, the difference in coverage between the states that integrated was small compared to Kano state that conducted two stand-alone campaigns. This could mean the integration probably led to more reach of clients when compared to when they were given separately. Studies on antigens preference by caregivers in Nigeria are not available but from the most recent National Immunization Cluster Survey report, antigens given at the same time showed no significant difference in coverage, for example the third dose of Oral Polio Vaccine, Pentavalent vaccine and the Pneumococcal conjugate vaccine taken same time were 56%, 57% and 55% respectively [45], meaning clients don’t select what antigen they are willing to take. In other integrated interventions, especially between different but related interventions, the extent to which the intervention reaches the target population (REACH) is usually on the positive. HIV services integrated with family planning, antenatal care, and tuberculosis screening have all shown positive reach in both integrated services [46,47]. For integrated antigens, more research may be needed to ascertain if integration has any effect on the number of clients reached per antigen based on the integration. However, vaccine coadministration practices which have been shown to be cost-effective in facilitating the introduction of new vaccines into the immunization programs has also been shown to improve coverage rates [48].
In summary, this article has thrown some historical perspective on the integration of health campaigns in Nigeria and the integration of the measles and Men A vaccines in the 2019 supplementary immunization activity has demonstrated some of the benefits of integrating antigens amid a crowded immunization activities calendar as usually witnessed in most developing countries where routine immunization alone does not provide the needed herd immunity for addressing vaccine-preventable diseases. However, it is pertinent to note that dedication to details in the planning of a huge integrated campaign is necessary as some Nuts-and-Bolts issues such as differences in target age group, number of vaccine doses, immune response, and safety of coadministration as well as unintended consequences are worth paying attention to for successful integration.
Strength and Limitations
The utilization of meticulously documented meeting minutes stands out as a significant strength in this study, reducing reliance on informant interviews and thereby minimizing the potential for recall bias. This methodological choice significantly bolsters the credibility and reliability of the study’s findings.
However, the study is not without its limitations. The expansive scope covering 16 northern states and the Federal Capital Territory (FCT) presented a challenge in delving into specific details at the state level where interventions were implemented. This limitation may impact the depth of insights and impede the ability to capture nuanced variations in implementation practices across different states. Another noteworthy limitation is the absence of guidelines, including definitions, from the Health Campaign Effectiveness Coalition for integrating campaigns at the time of the 2019 integrated campaign. These guidelines and definitions were not used to guide the integration of the antigens. However, some members of the study team have been contributors to the development of these global guidelines and experiences from the Nigeria’ 2019 integrated campaign were used in drafting the guidelines.
Recommendations
From our study, integration of antigens in a vaccination campaign is possible and it should be encouraged for resources (human and finance) limited settings. Factors that determine the success of such integration include good coordination and planning with a single planning, coordination, and funding source paramount with all the relevant stakeholders carried along in the decision making, that is from conception through to post implementation. It is recommended that the funders of some of these campaigns at the Global level such as Global Fund and Gavi need to collaborate in ensuring integration is considered while approving country proposals for support. Enablers and facilitators of integration within and across health interventions are well elaborated in the campaign integration decision toolkit of the Health Campaign Effectiveness Coalition [49].
Credit Authorship Contribution Statement
Adejoke Oladele Kolawole: Conceptualization, Methodology, Formal analysis, Writing – Original draft, Writing – Review and editing, Visualization – Tables and figures. Avuwa Joseph Oteri: Conceptualization, Methodology, Formal analysis, Writing – Original draft, Writing – Review and editing, Visualization – Tables and figures. Samuel Ibizugbe: Review and editing, Visualization – Tables and figures, Methodology, Writing – Review and editing. Samuel Bawa: Conceptualization, Methodology, Writing – Review and editing, Visualization – Tables and figures. Fredrick Mogekwu: Conceptualization, Methodology, Writing – Review and editing, Visualization – Tables and figures. Chidinma Amara Nwachukwu: Writing – Review and editing, Visualization. Chinedu Okoronkwo: Review and editing. Boubacar Dieng: Review and editing, Validation. Anne Eudes Jean Baptiste: Conceptualization, Writing – Review and editing, Visualization – Tables and figures. Nadia Lasri: Review and editing, Validation. Nneka Onwu: Review and editing, Validation.
Acknowledgments
The authors would like to gratefully acknowledge the committed and passionate work of members of the National Measles Technical Coordinating Committee (NMTCC) that later became the National Measles/Yellow Fever Technical Coordinating Committee and the members of the various Working Groups at National and State levels. We also appreciate the invaluable contribution of Gavi, The Vaccine Alliance for making the integration decision possible with funding support. Bill & Melinda Gate Foundation and the rest membership of the Country Working Group led by Mark Papania of the US CDC are also acknowledged for their international support. In the same vein, we appreciate and acknowledge Dr Lydia Taiwo of AFENET and Cheryl William of the US CDC for the pictures used in this article. We also acknowledge Eva Bazant of the Health Campaign Effectiveness Coalition and Maryam Musa Yahaya of the Nigeria Governors Forum for reviewing and proofreading the article before submission.
Authors’ Contribution
AOK, AJO, FM, and SB conceived of the study idea, contributed to the study design and literature search and analysis of findings. CBN, SI, CO, BD, AEJB, NL and NO contributed to data collection, preparation of figures and tables, and performed the analysis. All contributed to interpretation and writing.
Research Data for this Article
Some of the data sets used in this paper are publicly available via the sources referenced in the manuscript. Other data was generated as part of the activities supporting measles elimination strategy and Supplemental Immunization Activities. Campaign budget data are available on request from the corresponding author.
References
- Haenssgen MJ, Closser S, Alonge O (2021) Impact and effect mechanisms of mass campaigns in resource-constrained health systems: quasi-experimental evidence from polio eradication in Nigeria. BMJ Glob Health 6: e004248.
- Task for Global Health (2020) Why are Health Campaigns Important? - Health Campaign Effectiveness Coalition.
- Bright T, Felix L, Kuper H, Polack S (2018) A systematic review of strategies to increase access to health services among children in low and middle income countries. BMC Health Serv Res 17: 252.
- Grabenstein JD, Nevin RL (2006) Mass immunization programs:principles and standards. Curr Top Microbiol Immunol 304: 31-51.
- Richard MT, Taiwo L, Baptiste AEJ, Bawa S, Dieng B, et al. (2021) Planning for supplemental immunization activities using the readiness assessment dashboard: Experience from 2017/2018 Measles vaccination campaign, Nigeria. Vaccine 39: C21-C28.
- Fed Ministry of Health (2018) Nigeria Strategy for Immunization and PHC System Strengthening [NSIPSS].
- Health Campaign Effectiveness Coalition. Task Force for Global Health (2020) Integration between Health Campaigns: Intervention Codelivery and Collaboration - Health Campaign Effectiveness Coalition.
- World Health Organization (2005) GIVS: Global Immunization Vision and Strategy: 2006-2015.
- Hwang A, Veira C, Malvolti S, Cherian T, MacDonald N, et al. (2020) Global Vaccine Action Plan Lessons Learned II: Stakeholder Perspectives. Vaccine 38: 5372-5378.
- MacDonald N, Mohsni E, Al-Mazrou Y, Andrus JK, Arora N, et al. (2020) Global vaccine action plan lessons learned I: Recommendations for the next decade. Vaccine 38: 5364-5371.
- WHO (2019) Immunization Agenda 2030.
- Kamatsuchi M, Gheorghe A, Balabanova D (2019) The global scale and implications of delivering multiple interventions through integrated child health events. BMJ Glob Health 4: e001333.
- Davis R, Mbabazi WB (2017) Challenges to global measles eradication: is it all in the timing? Pan Afr Med J 27: 11.
- Johri M, Verguet S, Morris SK, Sharma JK, Ram U, et al. (2016) Adding interventions to mass measles vaccinations in India. Bull World Health Organ 94: 718-727.
- CISLAC Nigeria (2018) MNCH Week: It’s time to improve maternal, child health service delivery in Nigeria.
- Executive Summary Overview of MNCHW evaluation objectives and intended audience Evaluation methodology most important findings and conclusions main recommendations.
- WHO, USAID (2009) Periodic Intensification of Routine Immunization. Lessons Learned and Implications for Action. Pre-Print Release.
- Federal Ministry of Health Nigeria (2006) Comprehensive Multi-Year Plan. The National Programme on Immunization.
- CHAI (2021) Lessons Learned from the 2019-2020 Implementation of Measles and Meningitis A in an Integrated Campaign in the Context of COVID-19: A Retrospective Case Study of Kogi, Niger, and Kwara States in Nigeria Key Messages.
- Mogekwu FI, Kolawole A, Kariya S, Baptiste AJ, Gbenewei E, et al. (2021) Integration of Multiple Antigens Nigeria experience in integrated Men A measles campaign.
- Jean Baptiste AE, Masresha B, Wagai J, Luce R, Oteri J, et al. (2021) Trends in measles incidence and measles vaccination coverage in Nigeria, 2008-2018. Vaccine 39: C89-C95.
- NPHCDA, Federal Ministry of Health (2011) Application Form for Country Proposals The Government of (NIGERIA). Gavi, The Vaccine Alliance.
- Oteri AJ, Adamu U, Dieng B, Bawa S, Terna N, et al. (2021) Nigeria experience on the use of polio assets for the 2017/18 measles vaccination campaign follow-up. Vaccine 39: C3-C11.
- Hamisu M, Dieng B, Taiwo L, Jean Baptiste AE, Bawa S, et al. (2021) Microplanning verification and 2017/2018 measles vaccination campaign in Nigeria: Lessons learnt. Vaccine 39: C46-C53.
- Okoronkwo C, Taiwo LA, Asolo JA, Jean Baptiste AE, Wagai J, et al. (2021) Leveraging on the 2017/2018 measles vaccination campaign to improve health workers knowledge and practice on injection safety: A case study of north-central states, Nigeria. Vaccine 39: C54-C59.
- CDC (2023) ACIP Vaccine Administration Guidelines for Immunization.
- CDC (2023) Multiple Vaccinations at Once |Vaccine Safety|.
- Lai S, Sorichetta A, Steele J, Ruktanonchai CW, Cunningham AD, Rogers G, et al. (2022) Global holiday datasets for understanding seasonal human mobility and population dynamics. Sci Data 9: 17.
- Health Campaign Effectiveness Coalition (2021) Decision Guidance Tool for People -Centered integration of Health Campaigns.
- Chehab ET, Anya BPM, Onyango AW, Tevi-Benissan MC, Okeibunor J, et al. (2016) Experience of integrating vitamin A supplementation into polio campaigns in the African Region. Vaccine 34: 5199-5202.
- Bazant E, McPhillips-Tangum C, Shrestha SD, Preetha GS, Khera A, et al. (2022) Promising practices for the collaborative planning of integrated health campaigns from a synthesis of case studies. BMJ Glob Health 7: e010321.
- O’Cathain A, Croot L, Duncan E, Rousseau N, Sworn K, et al. (2019) Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open 9: e029954.
- Steffen CA, Henaff L, Durupt A, El Omeiri N, Ndiaye S, et al. (2021) Evidence-informed vaccination decision-making in countries:Progress, challenges and opportunities. Vaccine 39: 2146-2152.
- Oteri J, Idi Hussaini M, Bawa S, Ibizugbe S, Lambo K, et al. (2021) Application of the Geographic Information System (GIS) in immunisation service delivery; its use in the 2017/2018 measles vaccination campaign in Nigeria. Vaccine 39: C29-C37.
- Jean Baptiste AE, Van der Schans J, Bawa S, Masresha B, Wagai J, et al. (2023) The cost of implementing measles campaign in Nigeria: comparing the stand-alone and the integrated strategy. Health Econ Rev 13: 1-13.
- Boselli G, Yajima A, Aratchige PE, Feldon KE, Xeuatvongsa A, et al. (2011) Integration of deworming into an existing immunisation and vitamin A supplementation campaign is a highly effective approach to maximise health benefits with minimal cost in Lao PDR. Int Health 3: 240-245.
- Bairami F, Takian A, Sari AA, Harirchi I, Sakha MA (2020) Expanding fiscal space for healthcare system through efficiency: A qualitative study from Iran. Iran J Public Health 49: 727-735.
- Lankester F, Davis A, Kinung’hi S, Yoder J, Bunga C, et al. (2019) An integrated health delivery platform, targeting soil-transmitted helminths (STH) and canine mediated human rabies, results in cost savings and increased breadth of treatment for STH in remote communities in Tanzania. BMC Public Health 19: 1-12.
- ICAN, Thinkwell, UNN (2022) The cost of delivering yellow fever and meningitis A vaccines through campaigns in Nigeria.
- Mounier-Jack S, Edengue JM, Lagarde M, Baonga SF, Ongolo-Zogo P (2016) One year of campaigns in Cameroon: effects on routine health services. Health Policy Plan 31: 1225-1231.
- Haenssgen MJ, Closser S, Alonge O (2021) Impact and effect mechanisms of mass campaigns in resource-constrained health systems: quasi-experimental evidence from polio eradication in Nigeria. BMJ Glob Health 6: e004248.
- Wirtz C, Mohamed Y, Engel D, Sidibe A, Holloway M, et al. (2022) Integrating HPV vaccination programs with enhanced cervical cancer screening and treatment, a systematic review. Vaccine 40: A116-A123.
- Engel D, Afeli ADJ, Morgan C, Zeck W, Ross DA, et al. (2022) Promoting adolescent health through integrated human papillomavirus vaccination programs: The experience of Togo. Vaccine 40: A100-A106.
- Morgan C, Giattas MR, Holroyd T, Pfitzer A, Engel D, et al. (2022) Integration of other services with human papillomavirus vaccination; lessons from earlier in the life course highlight the need for new policy and implementation evidence. Vaccine 40: A94-A99.
- NBS, NPHCDA, GAVI, UNICEF, BMGF (2022) Nigeria MICS-NICS 2021 report rev 2 | Enhanced Reader.
- Balasubramanian BA, Fernald D, Dickinson LM, Davis M, Gunn R, et al. (2015) REACH of Interventions Integrating Primary Care and Behavioral Health. J Am Board Fam Med 28: S73-S85.
- fhi 360 (2012) The Role of Service Integration in Strengthening Health Systems.
- Bauwens J, De Lusignan S, Weldesselassie YG, Sherlock J, Künzli N, et al. (2022) Safety of routine childhood vaccine coadministration versus separate vaccination. BMJ Glob Health 7: e008215.
- Gittleman D, Patel V, Bazant E (2021) Decision Guidance Toolkit for People-Centered Integration of Health Campaigns.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.