Joint Preservation in Patients with Grade IV Osteoarthritis of the Knee: Use of an Acellular Collagen Scaffold (ChondroFiller® Liquid) and Blood Derived Stem Cell Rich Graft - A Prospective Controlled Trial
by Weninger Patrick, Reitböck Celina*, Steffel Caterina, Herbst Andreas, Reich Angelika, Özçelik Murat
Avancell Medical, Vienna, Vienna, Austria
*Corresponding author: Reitböck Celina, Avancell Medical, Vienna, Vienna, Austria
Received Date: 19 June 2025
Accepted Date: 25 June 2025
Published Date: 27 June 2025
Citation: Weninger P, Reitböck C, Steffel C, Herbst AJ, Reich A, Özçelik M (2025) Joint Preservation in Patients with Grade IV Osteoarthritis of the Knee: Use of an Acellular Collagen Scaffold (ChondroFiller® Liquid) and Blood Derived Stem Cell Rich Graft - A Prospective Controlled Trial. J Surg 10: 11360 https://doi.org/10.29011/2575-9760.011360
Abstract
Background: Knee Osteoarthritis (OA) is a degenerative joint disease causing pain and disability. Regenerative therapies with Mesenchymal Stem Cells (MSCs) and acellular collagen scaffolds may offer alternatives to joint replacement in end-stage OA. This prospective observational study evaluated whether combining an injectable acellular collagen scaffold (ChondroFiller® liquid) with autologous blood-derived MSCs improves outcomes in patients with Kellgren-Lawrence Grade IV knee OA compared to MSC therapy alone.
Methods: Twenty-five patients with advanced knee OA were treated with either intra-articular MSC-rich concentrate alone (n=12) or in combination with ChondroFiller® liquid (n=13). Treatment choice followed individual therapeutic decisions. Outcomes at 2 months included KOOS (Pain, Symptoms, ADL, Sport/Rec, QoL), VAS for pain, and Range of Motion (ROM). MRI evaluated structural joint changes, and MMP-13 expression served as a biomarker for cartilage degradation.
Results: Both groups improved significantly from baseline across all KOOS subscales (p < 0.01), with Group 2 achieving higher mean scores (e.g., KOOS Pain: 72.38 vs. 65.58). VAS pain scores declined substantially (Group 1: 8.33→2.42; Group 2: 8.54→2.08), and ROM improved in both groups. MRI showed greater reductions in bone marrow edema, effusion, and synovitis in Group 2. MMP-13 expression declined in both groups, with a more pronounced reduction in Group 2 (p = 0.001 vs. p < 0.001). No serious adverse events occurred.
Conclusion: Adding ChondroFiller® liquid to MSC therapy resulted in greater clinical and molecular improvements than MSCs alone, with no safety concerns. This combination may enhance joint preservation in advanced OA. Further studies are needed.
Keywords: ChondroFiller® liquid; Mesenchymal Stem Cells; MSC; Orthopedics; Regenerative Medicine
Introduction
Osteoarthritis (OA) is a prevalent and debilitating joint disease, affecting over 500 million people worldwide [1]. It is one of the leading causes of chronic pain and disability, particularly in aging populations [2]. Despite its high burden, current OA treatmentsincluding exercise, weight management, analgesics, antiinflammatory drugs, and intra-articular injections-are primarily symptomatic and do not halt progressive cartilage degeneration [3]. No disease-modifying OA therapies are currently available, and as a result, joint damage continues to worsen in many cases. Endstage disease often necessitates Total Knee Arthroplasty (TKA), a procedure that, while effective, is associated with high costs, surgical risks, and limited long-term implant survival, particularly in younger patients [4]. These limitations highlight the urgent need for regenerative approaches that can restore articular cartilage and alter the disease course of OA. Over the past two decades, stem cell–based therapies have emerged as promising strategies for cartilage repair in OA [5]. Mesenchymal Stem Cells (MSCs) have been widely explored in regenerative medicine and emerging evidence continues to highlight their critical role in cartilage regeneration and joint preservation [6,7]. MSCs are multipotent stromal cells capable of differentiating into chondrocytes, osteoblasts, and other cell types relevant to musculoskeletal repair [8]. Beyond their differentiation potential, MSCs exert significant immunomodulatory effects, releasing bioactive factors that can reduce inflammation and promote tissue repair by influencing key regenerative pathways [9,10]. Our approach focuses on advancing tissue engineering strategies by combining MSC-based therapies with biomaterial scaffolds. This method aims to improve cell retention and enhance the effectiveness of cartilage repair. Scaffolds provide a supportive three-dimensional structure that facilitates MSC engraftment and protects transplanted cells from rapid clearance [11].
One such biomaterial-based approach is ChondroFiller® liquid, an injectable, resorbable type I collagen scaffold designed to fill focal cartilage defects. The scaffold polymerizes in situ, forming a protective matrix that enhances cell attachment and promotes tissue regeneration [12]. Research suggests that ChondroFiller® liquid can support the homing and retention of circulating progenitor cells, including MSCs, thereby improving cartilage repair outcomes [13]. In a prospective cohort study, patients receiving ChondroFiller® liquid for hip cartilage defects demonstrated significant defect filling on MRI and sustained clinical improvements over a 1-5 year follow-up period [14]. Another pre-clinical study showed that low-dose MSCs in combination with a scaffold yielded the same regenerative effect as the single high dose MSC-injection [15]. Given these advantages, the combination of MSC therapy with ChondroFiller® liquid represents an innovative strategy for cartilage repair, potentially offering a more effective and durable regenerative solution for OA. The present study aims to evaluate the therapeutic potential of combining intra-articular MSC therapy with ChondroFiller® liquid in patients with grade IV knee OA. We hypothesize that the addition of ChondroFiller® liquid will enhance pain relief and functional improvement compared to MSC therapy alone. By leveraging the synergistic effects of an acellular collagen scaffold and MSCs, this study aims to advance the field of regenerative medicine and provide a novel, joint-preserving alternative to surgical intervention in OA.
Material and Methods
Study Design and Patients
The underlying study was conducted as a prospective, noninterventional observational trial comparing Mesenchymal Stem Cell (MSC) injection alone with the combination of ChondroFiller® liquid and MSC injection in patients with advanced knee osteoarthritis. A total of 25 patients with end-stage knee OA were assessed. Eligible patients were adults aged 50–99 years with exclusively Kellgren–Lawrence grade IV OA of the knee affecting at least two compartments of the joint. Both patients with and without prior arthroscopic procedures on the knee were allowed. Major exclusion criteria included active smoking, any history of rheumatoid or inflammatory joint disease, prior knee arthroplasty on the affected knee and pregnancy. All patients provided informed consent to participate.
Sample Size Justification
A total of 25 patients were included in this study. The sample size was determined based on feasibility considerations, given the exploratory nature of the investigation and the strict inclusion criteria related to advanced (Kellgren & Lawrence grade IV) knee osteoarthritis. Previous pilot studies in the field of regenerative medicine, particularly those involving cell-based and scaffoldassisted interventions, have employed similar or even smaller sample sizes to assess short-term clinical outcomes and biomarker changes [16,17]. In line with these precedents, the current sample allows for a first clinical and mechanistic evaluation of treatment effects, with sufficient statistical power for within-group comparisons and descriptive analyses of clinically meaningful trends. Moreover, as this study involves complex biologic preparation and arthroscopic procedures, the number of participants was calibrated to ensure consistent treatment application, quality control and close clinical follow-up. The results are intended to inform the design of larger, controlled trials and to support hypothesis generation for future confirmatory studies.
Interventions
Group 1 (Stem Cell Only) received an intra-articular injection of an autologous blood-derived, MSC-rich concentrate. To prepare this graft, 30 mL of the patient’s venous blood was drawn from the arm and processed using a specialized closed centrifugation system (SMART Cell device, Miracell Co., South Korea). Centrifugation (2,500×g for 17 minutes) separated the blood components. This process yielded approximately 5 mL of plasma rich in peripheral Mesenchymal Stem Cells (MSCs). The concentrate (total ~9 mL) was injected into the affected knee joint during an arthroscopy under sterile conditions. After the injection, the knee was gently cycled through its range of motion to distribute the fluid. Patients were advised to use crutches for 3 days post-procedure, maintaining partial weight-bearing limited to approximately half of their body weight. They were also instructed to avoid high-impact or stop-and-go sports for 4 weeks to protect the knee during early healing. Group 2 (Stem Cell + ChondroFiller® liquid) received the same stem cell concentrate injection as Group 1, followed immediately by an injection of 1 mL of ChondroFiller® liquid acellular collagen scaffold. The scaffold was prepared according to the manufacturer’s instructions and injected intra-articular into the knee joint during an arthroscopy to ensure accurate delivery to the cartilage lesion area. Within minutes of injection, the liquid collagen gel polymerizes in the joint, conforming to the defect morphology and forming a stable scaffold in the area of cartilage damage, enabling matrix-supported self-assembly with the MSCs. The post-procedure rehabilitation recommendations for Group 2 were the same as for Group 1. All patients in both groups received standard supportive care (analgesics as needed and physical therapy exercises) during the recovery period.
Outcomes and Evaluations
Outcome assessments were performed at baseline (pre-intervention) and at 2 months after the treatment. The primary outcome measure was the Knee Injury and Osteoarthritis Outcome Score (KOOS). The KOOS is a validated knee-specific instrument that includes five subscales: Pain, Symptoms, Activities of Daily Living (ADL), Sport and Recreation (Sport/Rec) and knee-related Quality of Life (QOL), each scored 0–100 (with higher scores indicating better). Secondary outcomes included the knee pain severity, evaluated by the Visual Analog Scale (VAS) scored from 0 (no pain) to 10 (worst pain). Objective functional assessment included measurement of knee range of motion (ROM), specifically the maximum passive extension and flexion angles, using a goniometer. These outcome parameters were collected for each patient at the pre-treatment visit and at the two-month follow-up visit; two-month results are reported here as the primary endpoint. MRI assessments were conducted to evaluate structural changes in the knee joint following treatment. Both groups demonstrated significant improvements in key MRI parameters, including bone marrow edema, effusion, and synovitis. Since the data for all three MRI parameters were not normally distributed, a non-parametric Wilcoxon signed-rank test was applied to assess these changes. To assess potential molecular effects of the interventions, MMP-13 (matrix metalloproteinase-13) levels were quantified from peripheral blood samples obtained at baseline and at the two-month follow-up. MMP-13 is a key enzymatic marker associated with cartilage degradation and structural progression in osteoarthritis. Changes in serum MMP-13 concentrations were analyzed to explore the biological response to treatment. These outcome measures were selected to capture both patient-reported experiences and objective functional as well as biochemical changes, providing a multidimensional evaluation of treatment efficacy in advanced knee osteoarthritis.
Statistical Analysis
All analyses were performed on an intention-to-treat basis including all 25 patients (12 in Group 1, 13 in Group 2). Continuous outcome data (KOOS, VAS, ROM) were summarized as means ± standard deviation. Within each treatment group, pre- vs post-intervention scores were compared using paired two-tailed t-tests for normally distributed data or Wilcoxon signed-rank tests for non-parametric analysis. Normality of distributions was checked with Shapiro– Wilk tests. Between-group comparisons of outcomes at 2 months (e.g. mean change from baseline and absolute scores) were conducted using independent-samples t-tests. Categorical data were described in proportions and compared using Fisher’s exact test. A significance level of α = 0.05 was used for all hypothesis tests. Prior to the study, a power analysis determined that a sample size of 25 patients would provide 80% power to detect an effect size of 0.45 between groups at α=0.05. This sample size was chosen for this proof-of-concept trial given practical recruitment limitations. Data were analyzed using SPSS software (v27, IBM Corp).
Results
Patient Characteristics
A total of 25 patients were enrolled (14 male, 11 female), with 12 assigned to Group 1 (MSC) and 13 to Group 2 (MSC + ChondroFiller® liquid). Seven male patients were included in both groups, whereas Group 1 comprised five female patients and Group 2 comprised six female patients. The groups were comparable in age, with a mean of 72.25 ± 6.67 years in Group 1 and 72.08 ± 5.79 years in Group 2. All patients presented with radiographic grade IV osteoarthritis (OA) involving at least two knee compartments, including multicompartmental cases. Baseline clinical measures indicated advanced symptomatic disease in both groups. KOOS subscale scores were uniformly low at baseline: KOOS Pain (41.33 ± 10.62 in Group 1 vs. 38.15 ± 7.36 in Group 2), Symptoms (41.25 ± 7.91 in Group 1 vs. 39.46 ± 3.87 in Group 2), ADL (37.25 ± 4.62 vs. 38.69 ± 6.78), Sport/Rec (37.50 ± 4.50 vs. 34.08 ± 3.12), and QOL (34.42 ± 2.88 vs. 35.15 ± 3.13) for Groups 1 and 2, respectively. Mean VAS pain scores were 8.33 ± 1.07 in Group 1 and 8.54 ± 0.97 in Group 2 (p = 0.64), reflecting similarly high pain levels prior to treatment. None of the between-group differences reached statistical significance. These data confirm that both groups had comparable levels of pain, functional impairment, and quality of life at baseline (Table 1).
Characteristic | Group 1 (MSC only) | Group 2 (MSC plus CF) |
Number of patients | n = 12† | n = 13† |
Age (years), mean ± SD | 72.25 ± 6.67* | 72.08 ± 5.79* |
Sex (male/female) | 7/5† | 7/6† |
VAS pain (0-10) | 8.34 ± 1.07* | 8.54 ± 0.97* |
KOOS pain (0-100) | 41.3 ± 10.62* | 38.2 ± 7.36* |
KOOS symptoms (0-100) | 41.3 ± 7.91* | 39.5 ± 3.89* |
KOOS ADL (0-100) | 37.3 ± 4.61* | 38.7 ± 6.80* |
KOOS Sport/Rec (0-100) | 37.5 ± 4.50* | 34.1 ± 3.12* |
KOOS QOL (0-100) | 34.4 ± 2.87* | 35.2 ± 3.13* |
Baseline characteristics were similar between groups. Values are † = number or mean ± SD*
Table 1: Patient Demographics and Baseline Characteristics.
KOOS Subscales
All five KOOS subscales improved significantly from baseline in both groups (p < 0.01 for all within-group comparisons). Improvements were consistently greater in Group 2 (MSC + ChondroFiller® liquid) compared to Group 1 (MSC only) (Figures 1,2). Due to non-normal distribution of KOOS Sport/Rec values in Group 1, the Wilcoxon signed-rank test was applied; all other comparisons were performed using paired t-tests. KOOS Pain increased from 41.33 ± 10.62 to 65.58 ± 7.86 in Group 1 and from 38.15 ± 7.36 to 72.38 ± 8.12 in Group 2 (p < 0.01 for both), representing improvements of +24 and +34 points, respectively. KOOS Symptoms improved from 41.25 ± 7.91 to 60.33 ± 4.46 in Group 1 and from 39.46 ± 3.87 to 73.08 ± 9.93 in Group 2 (p < 0.01), with a between-group difference of +13 points at follow-up. KOOS ADL increased from 37.25 ± 4.62 to 63.50 ± 4.01 in Group 1 and from 38.69 ± 4.80 to 75.77 ± 6.73 in Group 2 (p < 0.01), reflecting a 12-point higher score in Group 2. KOOS Sport/Rec rose from 37.50 ± 4.50 to 63.50 ± 5.00 in Group 1 (p = 0.02, Wilcoxon test) and from 34.10 ± 3.12 to 68.15 ± 4.71 in Group 2 (p < 0.01), corresponding to improvements of +25 and +34 points, respectively. KOOS QOL improved from 34.40 ± 2.87 to 63.42 ± 4.32 in Group 1 and from 35.20 ± 3.13 to 67.62 ± 5.20 in Group 2 (p < 0.01 for both). Although the between-group difference in QOL at follow-up (+4.2 points) did not reach statistical significance, a consistent trend in favor of the combined treatment was observed across all subscales (Table 2).
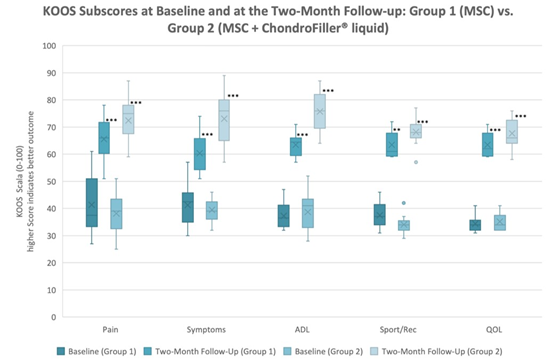
Figure 1: KOOS subscores at baseline and at the two-month follow-up in Group 1 (MSC) and Group 2 (MSC + ChondroFiller® liquid). Higher scores reflect better function. Highly significant improvements were observed in both groups (p < 0.001) for all subscores except Sport/Rec in Group 1 (p = 0.002).
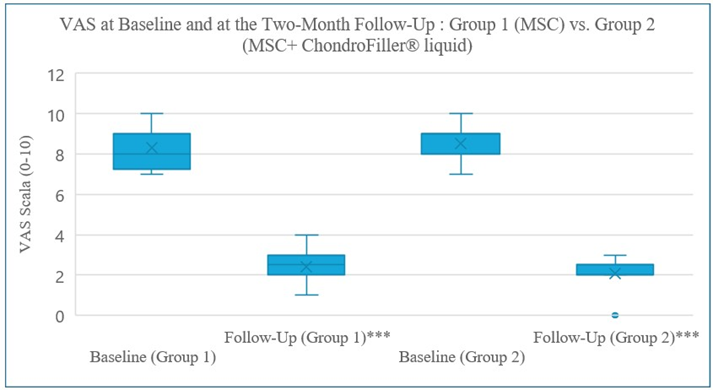
Figure 2: VAS pain levels at baseline and at the two-month follow-up. VAS pain levels decreased significantly in both groups. A paired t-test confirmed a highly significant reduction in Group 1 (p < 0.001, normal distribution), while a Wilcoxon signed-rank test confirmed a highly significant reduction in Group 2 (p < 0.001, non-normal distribution). No significant difference between both groups at the twomonth follow up (p < 0.321), very highly significant ***.
|
KOOS Subscore |
Group 1 Baseline → Two-Month Follow-Up |
p-value |
Group 2 Baseline → Two- Month Follow-Up |
p-value |
|
Pain |
41.3 → 65.6† |
p < 0.001*** |
38.2 → 72.4† |
p < 0.001*** |
|
Symptoms |
41.3 → 60.3† |
p < 0.001*** |
39.5 → 73.1† |
p < 0.001*** |
|
Activities of Daily Living (ADL) |
37.3 → 63.5† |
p < 0.001*** |
38.7 → 75.8† |
p < 0.001*** |
|
Sport and Recreation (Sport/Rec) |
37.5 → 63.5† |
p < 0.002** |
34.1 → 68.2† |
p < 0.001*** |
|
Quality of Life (QOL) |
34.4 → 63.42† |
p < 0.001*** |
35.2 → 67.6† |
p < 0.001*** |
Higher KOOS scores indicate better clinical status (normalized 0-100 scale) *** Very highly significant, ** highly significant, Values are † = mean
Table 2: KOOS Subscores Difference between baseline and the two-month follow-up including significance.
Visual Analog Pain Score (VAS)
All patients completed the two-month follow-up. Both groups experienced substantial reductions in VAS pain scores. In Group 1, mean pain scores decreased from 8.33 ± 1.07 at baseline to 2.42 ± 0.90 at follow-up (p < 0.01, paired t-test). In Group 2, scores declined from 8.54 ± 0.97 to 2.08 ± 0.76 (p < 0.001, Wilcoxon signed-rank test), reflecting a non-normal distribution at follow-up. A clinically important reduction in pain, defined as a ≥2-point decrease in VAS, was achieved by 100% of patients in both groups. The betweengroup difference in final mean VAS (2.08 vs. 2.42) was not statistically significant (p = 0.321). At follow-up, most patients in both groups reported minimal or no pain (VAS 0–1). Changes in pain scores are presented in Figure 2.
Range of Motion
Objective knee Range of Motion (ROM) improved modestly and comparably in both groups. Baseline extension was mildly limited in many patients due to pain and stiffness, as reflected by lowgrade flexion contracture. In Group 1, the mean extension deficit decreased from 1.42° ± 2.23° at baseline to 0.50° ± 0.9° at the twomonth follow-up (p < 0.102, not significant, Wilcoxon signed rank-test). Group 2 demonstrated a significant improvement, from 1.15° ± 1.68° at baseline to 0.15° ± 0.56° at the two-month followup (p < 0.038, Wilcoxon signed rank-test). By the end of the twomonth follow-up, nearly all patients had regained full extension (0°) or lacked only a few degrees, with no meaningful difference between groups (Figure 3).
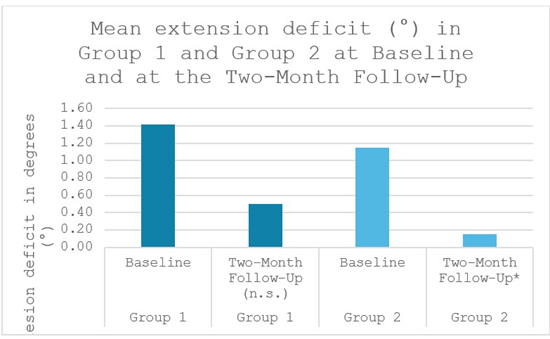
Figure 3: Mean knee-extension deficit (°) in Group 1 and Group 2 at baseline and at the two-month follow-up. Group 2 showed a significant reduction. * significant, (n.s.) not significant.
Knee flexion improved by approximately 8° in both groups. In Group 1, flexion increased significantly from 115.42° ± 7.82° at baseline to 123.75° ± 6.44° at the two-month follow-up (p < 0.01, paired t-test). Group 2 showed a similar gain, from 120.38° ± 8.28° at baseline to 128.46° ± 5.16° at the two-month follow-up (p < 0.01, paired t-test), reaching values within the normal functional range. These ROM improvements reflect reduced joint pain and stiffness following treatment. No cases of motion loss were observed; notably, two patients in Group 2 who had pre-treatment extension deficits recovered full extension after the combined therapy (Figure 4).

Figure 4: Mean knee flexion (°) in Group 1 and Group 2 at baseline and at the two-month follow-up. Both groups presented a statistically significant improvement in knee flexion. ** statistically significant.
Safety and Adverse Events
Both treatment approaches were found to be safe and well tolerated over the two-month study period. No severe adverse events were observed in either group. No infections, deep vein thromboses, or allergic reactions occurred following the injections. This is consistent with prior reports that adverse responses to ChondroFiller® liquid are very rare, with no serious complications attributable to the scaffold documented to date.
MMP-13 Expression
In Group 1, MMP-13 levels decreased significantly from 127.78 ± 13.15 ng/ml at baseline to 102.51 ± 13.23 ng/ml at the two-month follow-up (p < 0.001, paired t-test). In Group 2, a more pronounced reduction was observed, from 121.17 ± 12.36 ng/ml at baseline to 75.98 ± 12.80 ng/ml at the two-month follow-up (p < 0.001, Wilcoxon signed-rank test) (Figure 5).
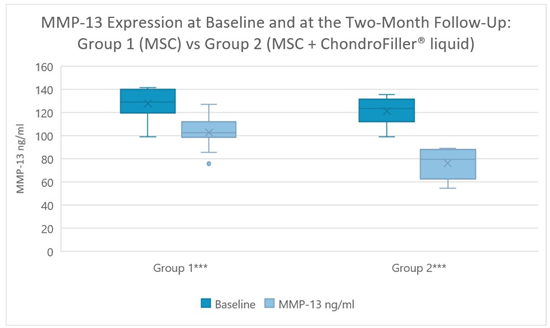
Figure 5: Matrix Metalloproteinase-3 (MMP-13) levels decreased significantly in both groups after two months (p < 0.001), with a more pronounced reduction in Group 2. *** highly significant.
MRI Outcomes
Significant reductions in bone marrow edema (BME), synovitis and effusion were observed in Group 1. BME was present in 75% (9 out of 12 patients) at baseline and decreased significantly to 41.67% (5 out of 12 patients) at the two-month follow-up (p < 0.046). 100% of patients (12 out of 12 patients) showed synovitis at baseline, which reduced significantly to 58.33% of patients (7 out of 12 patients) at the two-month follow-up (p < 0.025). Effusion was observed in 92% of patients (11 out of 12 patients) at baseline and lowered significantly to 58.33% of patients (7 out of 12 patients) at the two-month follow-up (p < 0.046) (Figures 6-8).
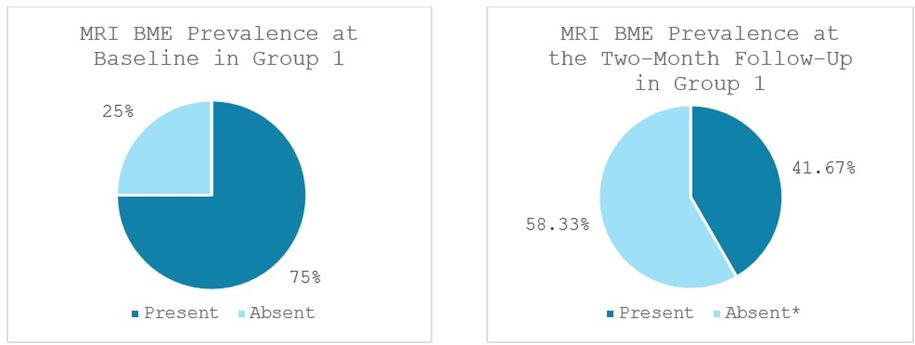
Figure 6: Bone Marrow Edema (BME) prevalence in Group 1 at baseline and at the two-month follow-up; *significant reduction. MRI: Magnetic Resonance Imaging. Values represent the portion of patients with BME (%).
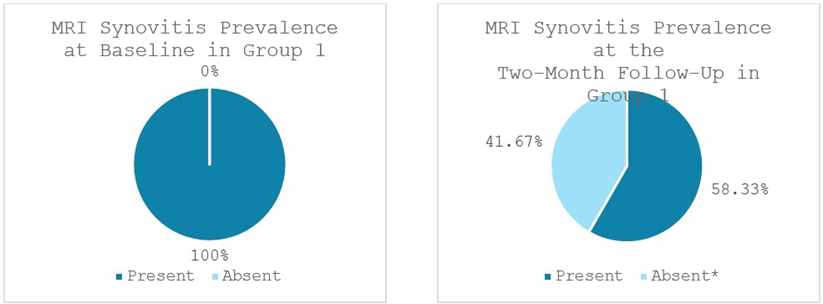
Figure 7: Synovitis prevalence in Group 1 at baseline and at the two-month follow-up; *significant reduction.
MRI: Magnetic Resonance Imaging. Values represent the portion of patients with synovitis (%).
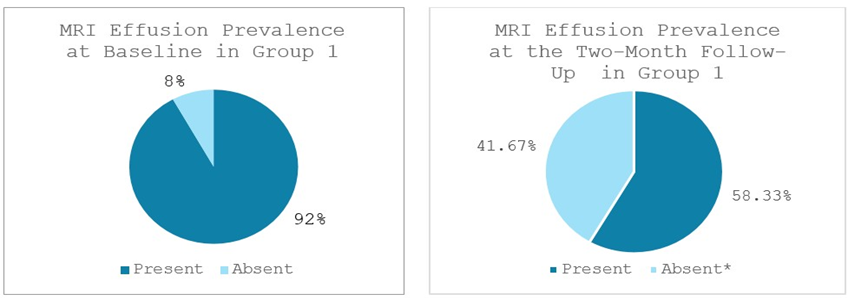
Figure 8: Effusion prevalence in Group 1 at baseline and at the two-month follow-up; *significant reduction. MRI: Magnetic Resonance Imaging. Values represent the portion of patients with effusion (%).
Group 2 exhibited significant reductions as well. Bone marrow edema (BME) was present in 69.23% of patients (9 out of 13 patients) at baseline and significantly lowered to 23.07% of patients (3 out of 13 patients) at the two-month follow-up (p < 0.014). 100% of patients (13 out of 13 patients) displayed signs of synovitis at baseline, statistically significantly decreasing to 23.07% of patients (3 out of 13 patients) at the two-month follow-up (p < 0.002). Effusion was observed in 92.31% of patients (12 out of 13 patients) at baseline and significantly dropped to 30.77% of patients (4 out of 13 patients) at the two-month follow-up (p < 0.011) (Figures 9-11).
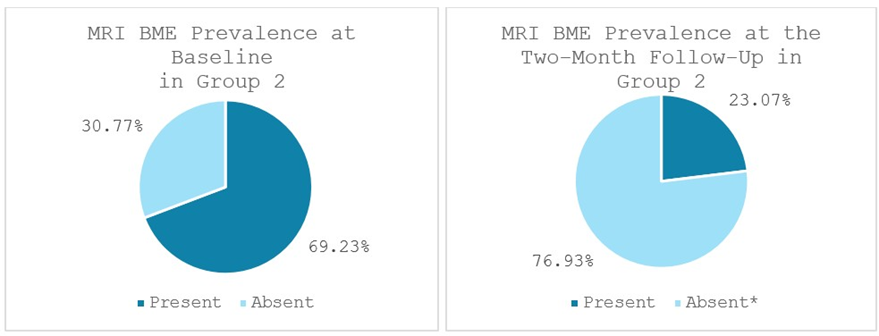
Figure 9: Bone marrow edema (BME) prevalence in Group 2 at baseline and at the two-month follow-up; *significant reduction. MRI: Magnetic Resonance Imaging. Values represent the portion of patients with BME. (%).
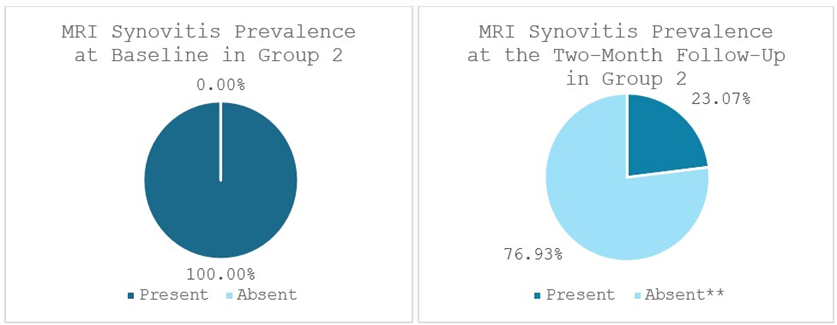
Figure 10: Synovitis prevalence in Group 2 at baseline and at the two-month follow-up; **statistically significant reduction. MRI: Magnetic Resonance Imaging. Values represent the portion of patients with synovitis (%).

Figure 11: Effusion prevalence in Group 2 at baseline and at the two-month follow-up; *significant reduction. MRI: Magnetic Resonance Imaging. Values represent the portion of patients with effusion (%).
MRI scans illustrating the improvements regarding Group 2 are presented below, showing pre-treatment images with evident cartilage defects, pronounced bone marrow edema, and synovial inflammation, followed by post-treatment scans demonstrating improved joint structure, reduced edema, and decreased inflammation (Figures 12-29).
MRIs of Group 2
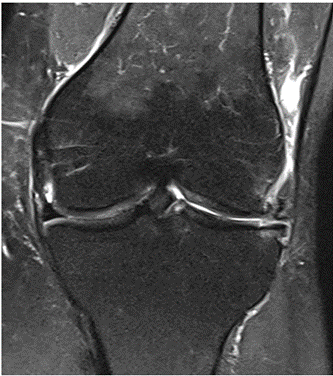
Figure 12: Female, 59 years, pre-intervention MRI.
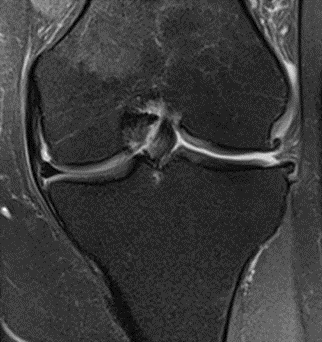
Figure 13: Female of figure 12, 59 years, two-month follow-up MRI: MSC plus ChondroFiller® liquid.

Figure 14: Male, 78 years, pre-intervention MRI.
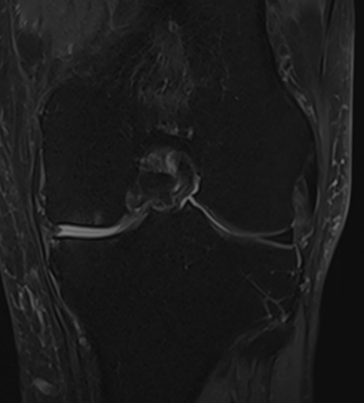
Figure 15: Male of figure 14, two-month follow-up MRI: MSC plus ChondroFiller® liquid.
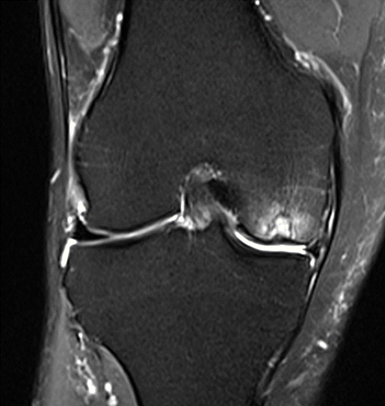
Figure 16: Female, 76 years, pre-intervention MRI.
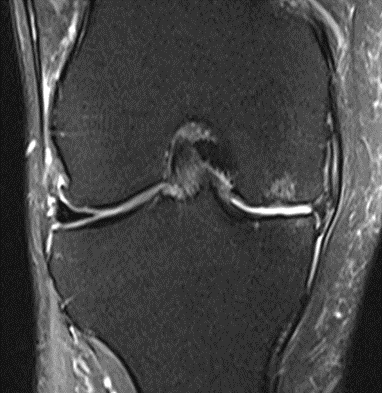
Figure 17: Female of figure 16, two-month follow-up MRI: MSC plus ChondroFiller® liquid.
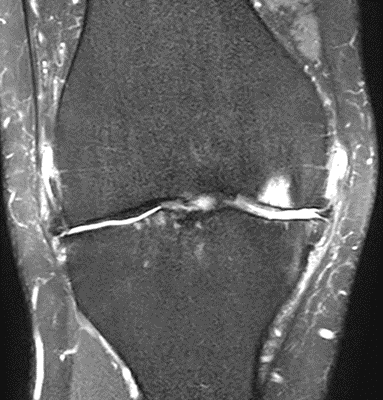
Figure 18: Female, 77 years, pre-intervention MRI.
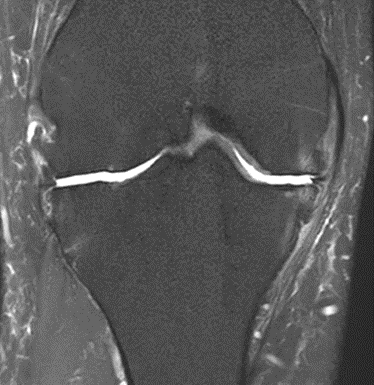
Figure 19: Female of figure 18, two-month follow-up MRI: MSC plus ChondroFiller® liquid.
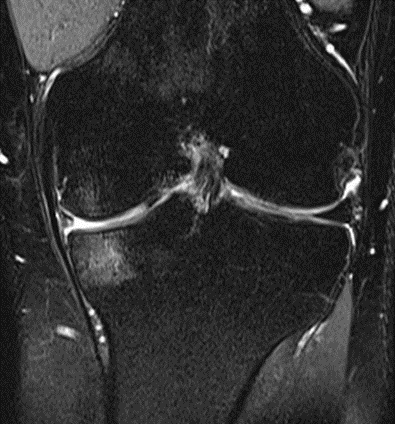
Figure 20: Male, 81 years, pre-intervention MRI.
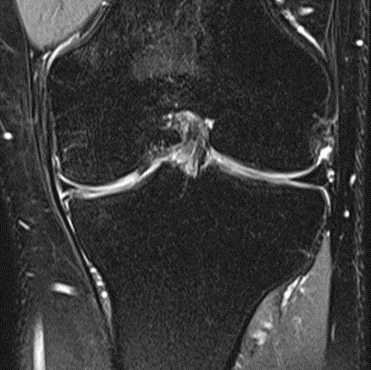
Figure 21: Male of figure 20, two-month follow-up MRI: MSC plus ChondroFiller® liquid.
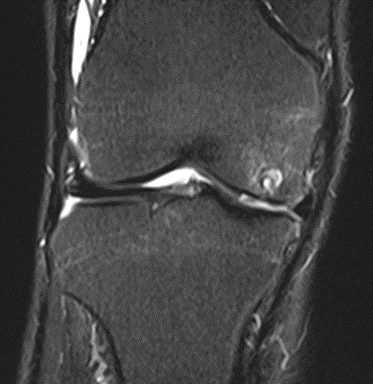
Figure 22: Male, 69 years, pre-intervention MRI.
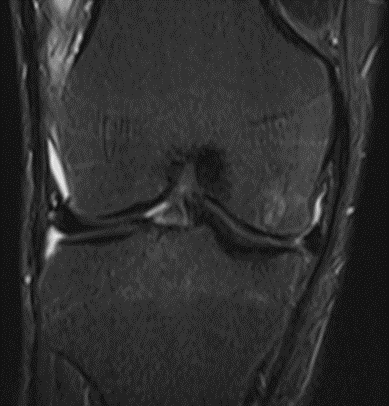
Figure 23: Male of figure 22, two-month follow-up MRI: MSC plus ChondroFiller® liquid.

Figure 24: Male, 67 years, pre-intervention MRI.
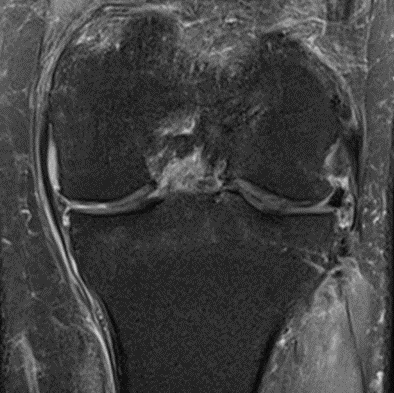
Figure 25: Male of figure 24, two-month follow-up MRI: MSC plus ChondroFiller® liquid.
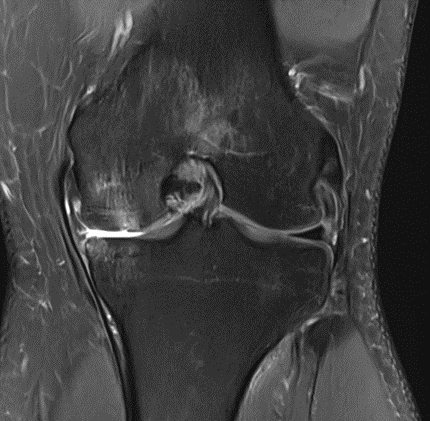
Figure 26: Female, 75 years, pre-intervention MRI.
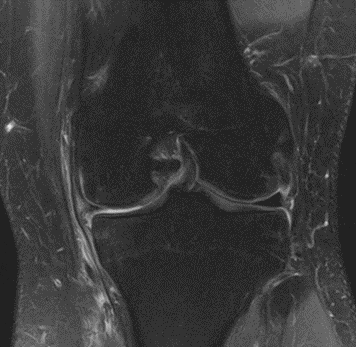
Figure 27: Female of figure 26, two-month follow-up MRI: MSC plus ChondroFiller® liquid.
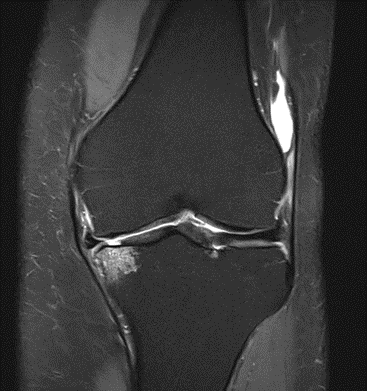
Figure 28: Female, 71 years, pre-intervention MRI.
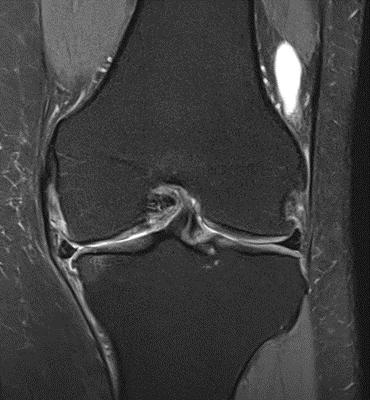
Figure 29: Female of figure 28, two-month follow-up MRI: MSC plus ChondroFiller® liquid.
MRIs of Group 1
To provide a comparative perspective, additional MRI scans from Group 1 are shown subsequently. While patients in this group also displayed reductions in bone marrow edema, effusion, and synovitis, the improvements appeared less pronounced than those seen with the combined therapy. Pre-treatment MRI scans revealed extensive edema and synovial inflammation, which were partially reduced at the 2-month follow-up. However, residual joint effusion and subtle signs of inflammation remained present in some cases. These findings further support the potential benefit of combining the ChondroFiller® liquid with MSC therapy to achieve more comprehensive structural improvements and facilitate enhanced cartilage regeneration (Figures 30-41).

Figure 30: Female, 72 years, pre-intervention MRI.
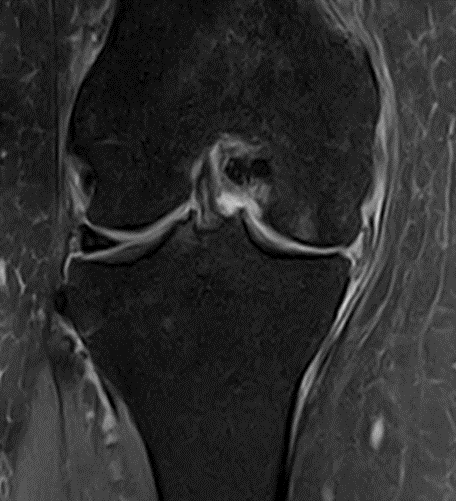
Figure 31: Female of figure 30, two-month follow-up MRI: MSC only.
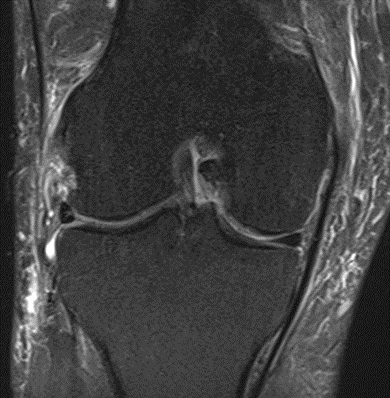
Figure 32: Male, 71 years, pre-intervention MRI.
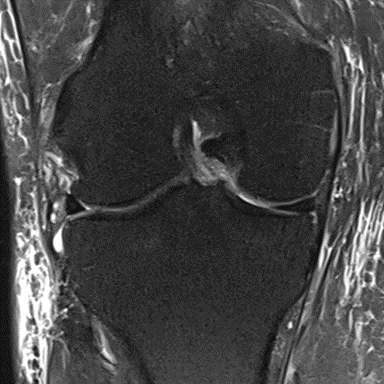
Figure 33: Male of figure 32, two-month follow-up MRI: MSC only. only.
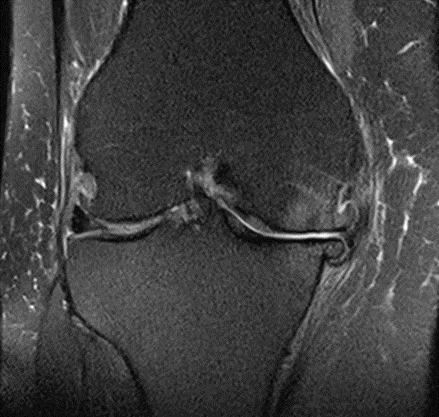
Figure 34: Female, 69 years, pre-intervention MRI.
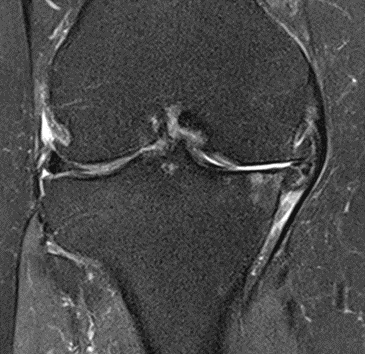
Figure 35: Female of figure 34, two-month follow-up MRI: MSC

Figure 36: Female, 82 years, pre-intervention MRI.
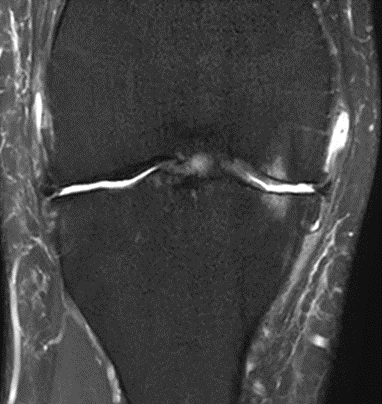
Figure 37: Female of figure 36, two-month follow-up MRI: MSC only.
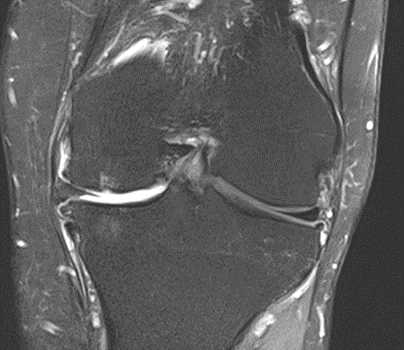
Figure 38: Male, 69 years, pre-intervention MRI.
Figure 39: Male of figure 38, two-month follow-up MRI: MSC only.
Figure 40: Male, 75 years, pre-intervention MRI.
Figure 41: Male of figure 40, two-month follow-up MRI: MSC only.
Discussion
The present study demonstrates that combining autologous Mesenchymal Stem Cells (MSCs) with an acellular type I collagen matrix (ChondroFiller® liquid) can safely yield notable clinical improvements in patients with end-stage (Kellgren-Lawrence grade IV) Knee Osteoarthritis (OA), exceeding the benefits of MSC therapy alone. Our results show, that even in end-stage knee OA, clinical improvement is possible. Both treatment groups showed significant reductions in pain and disability over the course of two months, consistent with the known efficacy of intra-articular MSCs in advanced OA. In line with our findings, a recent phase II randomized trial of adipose-derived MSC injections in knee OA reported significant pain relief versus placebo, and a 2020 meta-analysis confirmed that MSC therapies provide meaningful improvements in pain and function beyond standard care [17, 18].
KOOS Subscores
Patients treated with MSC plus the ChondroFiller® liquid (Group 2) showed consistently better functional recovery than those receiving MSC only (Group 1). All five KOOS subdomains (Pain, Symptoms, ADL, Sport/Rec, QOL) improved in both groups, but the combination therapy consistently outperformed the MSConly group, with statistically significant advantages in the Pain and Sport/Rec domains. Notably, the ability to perform sports or recreational activities improved by ~34 points (from 34.1/100 ± 3.12 to 68.15/100 ± 4.71) in Group 2 vs ~26 points (from 37.5 ± 4.5 to 63.5 ± 5.0) in Group 1. This difference suggests the acellular collagen scaffold facilitated a faster and more robust return to high-demand knee function. Patients who received the collagen matrix felt more capable of strenuous tasks (running, pivoting, etc.) than those with MSCs alone, indicating an early functional gain attributable to the acellular collagen scaffold’s presence. These findings align with emerging evidence that biomaterial scaffolds can enhance the efficacy of cell therapies by providing a supportive milieu for joint repair [11,19]. A recent systematic review of MSCladen hydrogels for cartilage regeneration found that incorporating a 3D matrix consistently improved outcomes in animal models and even in early clinical trials [19]. Similarly, one-step cartilage repair techniques in focal cartilage lesions – which combine bone marrow-derived cells with an acellular collagen scaffold in a single procedure – have demonstrated superior hyaline cartilage formation and clinical scores at follow-up [20]. By providing a physical framework for the cells, the scaffold appears to act as a regenerative niche, enhancing cell retention, engraftment, and subsequent tissue repair beyond what MSCs achieve on their own. In essence, the combination therapy leverages both the biologic power of MSCs and the structural support of the collagen matrix to more effectively restore joint function.
A key role of the acellular collagen scaffold is likely its contribution to structural regeneration and cartilage protection. The ChondroFiller® liquid, composed of highly purified type I collagen, serves as a three-dimensional extracellular matrix mimic that fills cartilage defects or areas of degeneration. This scaffold can stabilize the MSC-rich concentrate at the target site and protect the reparative tissue from deleterious mechanical forces in the joint. In our study, the scaffold was injected intraarticularly, where it gelled within chondral defects and along damaged surfaces, forming a protective layer. Biomechanically, such a collagen gel may cushion impact and reduce shear stress on exposed subchondral bone or fragile repair tissue. A recent in vitro study confirmed that filling a cartilage defect with a collagen hydrogel can offload and preserve the opposing articular surface, whereas empty defects lead to greater wear of the intact cartilage [21]. In addition, the collagen matrix provides binding sites and a native-like architecture for cells to attach and organize. This ECM mimicry helps guide MSC differentiation towards chondrocytes and encourages the deposition of new cartilage matrix. Preclinical research has shown that MSCs embedded in collagen scaffolds exhibit enhanced chondrogenic differentiation and produce cartilage tissue of higher quality (with greater proteoglycan and type II collagen content) compared to MSCs delivered as a simple suspension [15,22]. In our context of diffuse osteoarthritic damage, the acellular collagen scaffold may have promoted a more homogeneous repair response, filling fissures and defects with a collagenous template that the MSCs (and perhaps endogenous progenitors) used to build new tissue. Although we did not obtain biopsy samples in this trial, the superior functional gains in Group 2 hint at improved structural repair. This is further supported by prior studies of collagen gel implants in joints – for instance, an arthroscopic study using ChondroFiller® liquid in focal knee lesions demonstrated MRI evidence of defect filling and congruent joint surfaces at 1–5 year follow-up [14]. Taken together, these data suggest that the acellular collagen scaffold provided more than just a passive filler role: it actively enhanced the cartilage regenerative process, resulting in better joint integrity and function than MSC injection alone.
Pain Relief and Anti-Inflammatory Effects
Both treatment arms achieved a dramatic reduction in knee pain by 2 months, with VAS scores dropping from severe (8.54/10 ± 0.97) at baseline to mild (2.08/10 ± 0.76) after two months in Group 2. Group 1 also showed significant improvement with VAS scores decreasing from severe (8.34/10 ± 1.07) at baseline to mild (2.42/10 ± 0.90) after two months. Interestingly, pain relief was equivalent between Group 1 and Group 2 at this early time point. This indicates that the potent analgesic effect observed is largely attributable to the MSC therapy, which is consistent with the known paracrine action of MSCs in arthritic joints. MSCs can secrete anti-inflammatory cytokines, growth factors, and extracellular vesicles that modulate the intra-articular environment and alleviate pain [10]. Experimental OA studies have shown that the MSC secretome reduces synovitis and even diminishes pain behavior and cartilage damage in vivo [10]. In our patients, the injected MSC-rich concentrate (derived from peripheral blood) likely delivered a cocktail of immunomodulatory factors that rapidly calmed synovial inflammation and nociceptive signaling, explaining the swift drop in pain in both groups. The acellular collagen scaffold, on its own, would not be expected to provide such an early analgesic effect, since it does not actively secrete factors. However, it is encouraging that adding the scaffold did not diminish the pain relief – indicating that the biomaterial is highly biocompatible and does not provoke inflammation or immune reactions that could counteract the MSCs’ benefits. In our trial we observed no inflammatory flares or effusion attributable to the scaffold, corroborating its bio-inertness.
Over the short term, the acellular collagen scaffold’s contribution may be more structural and protective than analgesic, but it could have indirect pain benefits by stabilizing the joint (preventing mechanical irritation) and by facilitating tissue regeneration that addresses the root cause of pain. Additionally, collagen itself may exert some anti-inflammatory influence once polymerized in the joint. There is evidence that injected collagen can act as a biological response modifier; for example, intra-articular polymerized type I collagen has been shown in a randomized trial to downregulate inflammation in knee OA, leading to reduced pain, improved function, and decreased inflammatory mediators compared to placebo [23]. Collagen matrices can bind or sequester pro-inflammatory molecules and create a microenvironment that favors reparative over destructive processes [23]. Thus, while the primary pain relief in our study is likely due to the MSCs blunting synovitis, the acellular collagen scaffold probably complemented this effect by providing a calm, biofriendly niche rather than triggering any additional inflammation. It is conceivable that over a longer time frame, as the scaffold integrally participates in cartilage repair, patients in the combination group might maintain pain reduction better than those with MSCs alone (especially if the latter’s effect wanes once the injected cells die off). Our 2-month data, however, confirm that both treatments are very effective for short-term pain relief in end-stage OA, with the combination therapy matching the MSC therapy in analgesic efficacy while conferring extra functional benefits.
Imaging and Biochemical Findings
MRI evaluations in this study corroborated the clinical improvements and provided insight into the tissue-level effects of each therapy. Both groups demonstrated notable reductions in intra-articular pathology on MRI, including decreases in bone marrow edema (BME), synovitis, and joint effusion from baseline to 2 months. These MRI markers are important, as bone marrow lesions and synovial inflammation are associated with OA pain and progression. In Group 1, treated with MSCs, we observed a significant reduction in Bone Marrow Edema (BME), joint effusion, and synovitis over the 2-month followup period, consistent with the known anti-inflammatory and chondroprotective effects of cell-based therapy. At baseline, BME was present in 75% of patients (9/12), decreasing to 42% (5/12) at follow-up. Joint effusion was noted in 92% of patients (11/12) at baseline and declined to 58% (7/12) after two months. Synovitis was observed in all patients (100%, 12/12) at baseline and dropped to 58% (7/12) at follow-up. Group 2, treated with the combination of MSCs plus ChondroFiller® liquid, exhibited even more pronounced improvements in all MRI parameters. BME prevalence declined from 69% (9/13) at baseline to 23% (3/13) at follow-up. Joint effusion was initially present in 92% of patients (12/13) and reduced to 31% (4/13) after two months. Likewise, synovitis decreased dramatically from 100% (13/13) at baseline to just 23% (3/13) at follow-up. Post-treatment MRI scans from this group frequently demonstrated reduced subchondral bone signal intensity, lower joint fluid levels, and diminished synovial inflammation compared to Group 1. Although our sample size is limited, this trend suggests that the addition of the acellular collagen scaffold may enhance the disease-modifying impact of the treatment by further normalizing the joint environment. A plausible explanation is that the scaffold, by promoting new cartilage-like tissue formation, reduces mechanical stress on the subchondral bone, thereby alleviating marrow edema.
The more robust fill of cartilage defects could also decrease exposure of bone and attenuate the inflammatory crosstalk between bone and synovium. In line with this, we found that MMP-13 levels in synovial fluid dropped in both groups over 2 months, but the reduction was significantly greater with the combined therapy. MMP-13 (matrix-metalloproteinase-13) is a key catabolic enzyme responsible for degrading type II collagen in articular cartilage and is considered a critical mediator of OA progression [24]. Elevated MMP-13 drives cartilage breakdown and is associated with pain and structural worsening in OA [24]. Therefore, the ability of a treatment to suppress MMP-13 is indicative of a protective or regenerative mechanism at the molecular level. In Group 1, MMP13 decreased by roughly 20% from baseline (from 127.78 ng/ml ± 13.15 at baseline to 102.51 ng/ml ± 13.23 after two months), reflecting the general anti-catabolic effect of MSC secretions (e.g., MSCs can release TIMPs and anti-inflammatory cytokines that downregulate MMP expression [23]. Notably, in Group 2, MMP-13 levels fell by nearly 40% (from 121.17 ng/ml ± 12.36 at baseline to 75.98 ng/ml ± 12.8 after two months), suggesting a more pronounced halting of cartilage breakdown. The acellular collagen scaffold may contribute to this by both biochemical and biomechanical means: it can serve as a temporary substitute for cartilage, taking on some load (thus lessening stimuli for MMP13 production), and it might also directly interact with cells to modulate their phenotype. Chondrocytes and synoviocytes in a stabilizing collagen-rich milieu could receive signals to decrease matrix-degrading enzymes. Indeed, other investigators have reported that applying a collagen-based gel to arthritic joints leads to reduced markers of cartilage degradation (such as urinary CTXII) in patients with knee osteoarthritis compared to controls [23]. The greater decline of MMP-13 in our combination group dovetails with the MRI finding of greater BME reduction: both point to an enhanced joint preservation effect when the acellular collagen scaffold is included. In sum, these imaging and molecular results support the idea that while MSC injections provide a potent antiinflammatory and symptom-relieving effect, the acellular collagen scaffold fortifies the treatment by fostering tissue regeneration and suppressing the underlying drivers of OA, like subchondral bone stress and enzymatic cartilage breakdown.
Range of Motion
Another encouraging outcome was the improvement in knee Range of Motion (ROM). At baseline, many patients had ROM deficits due to pain, swelling, and osteophytes common in grade IV OA. After treatment, both groups showed gains in ROM, with knee extension improving to a near-normal 0° deficit on average. In Group 1, mean knee flexion increased from 115.4° at baseline to 123.8° at the two-month follow-up. In comparison, mean knee flexion improved from 120.4° at baseline to 128.5° at the twomonth follow-up in Group 2. Restoration of full extension is particularly important for gait and function, and this was achieved in both Group 1 and Group 2 patients. There was no significant between-group difference in ROM, likely because pain relief was comparable. The key point is that neither treatment caused any joint stiffness or other adverse effect that would limit motion; on the contrary, reducing pain and inflammation unlocked the knees to move more freely. The acellular collagen scaffold did not impede joint mobility – an important safety consideration given it solidifies inside the joint. Clinically, the ability to regain extension and maintain flexion range indicates improved functional capacity (reflected also in KOOS-ADL scores) and suggests that these biologic treatments can address not just pain but also stiffness and mechanical function in severe OA.
Comparison with Other Regenerative Strategies
Our findings extend the growing body of literature on orthobiologic interventions for knee OA. Past studies have primarily focused on MSCs or platelet-rich plasma alone, reporting modest to substantial short-term benefits in pain and function. For instance, in addition to the adipose MSC trials noted above, allogeneic bone marrow MSCs have shown improvements in WOMAC scores over placebo [25], and various uncontrolled series have reported pain relief with autologous MSC injections at 6–12 months [26]. However, regeneration of cartilage in advanced OA using cells alone remains challenging, partly due to the hostile degenerative environment and cell loss after injection. The concept of augmenting cell therapies with scaffolds is rooted in tissue engineering principles and has been explored mostly in focal cartilage repair scenarios. One notable parallel is the matrix-assisted chondrocyte implantation (MACI) technique, where autologous cultured chondrocytes are seeded onto a collagen membrane and implanted into focal defects – this approach yields superior cartilage quality than injection of cells alone because the matrix keeps cells localized and supports their phenotype [27,28]. By analogy, our injectable collagen gel (ChondroFiller® liquid) plays a similar role for MSCs delivered into an arthritic joint. There is sparse literature directly examining MSC + acellular collagen scaffold versus MSC alone in osteoarthritic human joints, making our study relatively novel. Nevertheless, evidence from related strategies is supportive. Gobbi et al. reported that a one-step procedure using bone marrow aspirate concentrate (which contains MSCs) embedded in a collagen matrix led to hyaline-like cartilage repair in full-thickness knee lesions and excellent clinical outcomes at 2 years [20]. In a randomized comparison of microfracture (which relies on bone marrow stem cells and a fibrin clot scaffold) versus a collagen gel implant for chronic cartilage defects, the collagen scaffold was found to be as effective as microfracture in improving function and MRI appearance of the repair tissue [29].
Limitations
This study represents the first prospective clinical investigation evaluating the combined use of autologous mesenchymal stem cells (MSCs) and an acellular collagen scaffold (ChondroFiller® liquid) specifically in patients with Kellgren–Lawrence grade IV knee osteoarthritis. As a proof-of-concept trial, it provides valuable early evidence that joint preservation is achievable even in end-stage disease. Nevertheless, certain limitations must be acknowledged. First, the relatively small sample size (n = 25) and short follow-up period (2 months) limit the generalizability of our findings and preclude definitive conclusions regarding long-term structural outcomes. However, the consistency of improvements across multiple outcome domains, including KOOS, VAS, ROM, MRI, and MMP-13, strengthens the internal validity of the results and supports the biological plausibility of the observed effects. Second, the absence of a placebo or sham control arm restricts interpretation of treatment efficacy relative to natural history. Yet, the magnitude of improvement-particularly the dramatic VAS and KOOS changes-exceeds what is typically observed in placebo-controlled OA trials, and both groups received an active biologic intervention rather than standard care. Third, the nonrandomized design and lack of blinding may introduce potential bias. Future studies should therefore aim to include evaluator blinding and, if feasible, random allocation to reduce selection and performance effects. In sum, while these limitations warrant cautious interpretation, they do not diminish the clinical and mechanistic significance of the observed outcomes. This study lays the groundwork for future randomized, controlled trials and provides a compelling rationale to further explore MSC– scaffold combinations as a joint-preserving option in late-stage osteoarthritis.
Clinical Implications
For patients with advanced knee osteoarthritis who are poor candidates for surgery or seeking to delay TKA, the outcomes of this study offer a potential alternative. The combined MSC + acellular collagen scaffold therapy was delivered via intra-articular injection, an outpatient procedure with minimal downtime, and resulted in significant pain relief and functional gains by two months. Importantly, it did so without serious adverse events or inflammation, signaling a favorable risk-benefit profile. If such improvements can be sustained longer-term, this approach could bridge the treatment gap for end-stage OA patients who have exhausted conservative measures but are hesitant or highrisk for joint replacement. Our findings suggest that even when radiographic OA is severe, the joint retains some capacity for repair that can be unlocked with the right combination of therapies. The acellular collagen scaffold in particular may help stabilize the disease by reinforcing cartilage structure and mitigating further degeneration. There is evidence that collagen-based injectables can extend the time to knee replacement – one long-term observational study reported that repeated intra-articular polymerized collagen injections delayed the need for TKA by a median of about 5 years in patients with symptomatic knee OA [30]. While our trial was short, it sets the stage for exploring whether a one-time MSC + acellular collagen scaffold treatment (or a series of injections) can defer the “endpoint” of arthroplasty in a similar manner. Moreover, the improvements in high-level activities (KOOS Sport/Rec) seen with the combined therapy raise the possibility that certain patients could return to recreational exercise or physical jobs that would have been untenable with their previous pain and stiffness. From a healthcare system perspective, effective regenerative injections could reduce the burden of surgeries and associated costs if they enable patients to cope with their native joints longer.
Future Directions
The positive outcomes of this preliminary study justify further research on MSC + acellular collagen scaffold combination therapy in osteoarthritis. Longer-term trials are a top priority. A followup duration of at least 1–5 years is needed to determine if the structural benefits (as suggested by MRI and MMP-13 reduction) translate into slowed disease progression. Future studies should incorporate advanced imaging modalities such as quantitative MRI to objectively assess cartilage regeneration. Imaging at serial time points can track whether new cartilage matrix is forming and being maintained in areas treated with the acellular collagen scaffold. Larger randomized controlled trials with adequate power should be conducted to confirm these findings.
Conclusion
In conclusion, the integration of an acellular collagen scaffold with MSC therapy represents a promising frontier in OA treatment. Ongoing and future studies will determine if this combined regenerative approach can fulfill its potential as a joint-preserving, disease-modifying therapy for the millions suffering from advanced knee osteoarthritis. The groundwork laid by this trial provides a strong impetus for these next steps. While our early outcomes are encouraging, further research is warranted to confirm these benefits in larger patient populations and to determine the longevity of the repair. Future randomized trials with extended follow-up and detailed imaging evaluations will help establish whether this approach can achieve sustained cartilage regeneration and alter the long-term trajectory of knee osteoarthritis. If validated, such therapy could shift the paradigm of OA management toward less invasive, tissue-preserving interventions and offer patients an alternative path to pain relief and joint restoration short of artificial joint replacement.
Ethical Considerations
All participants provided written informed consent prior to enrollment. As a non-interventional observational study, the treatments were administered based on clinical decisions made independently of the study protocol.
Conflict of Interest
The study was financially supported by Meidrix Biomedicals GmbH. The sponsor had no role in the study design, data collection, data analysis, interpretation of results, or manuscript preparation. The authors declare that there are no other conflicts of interest relevant to this work
References
- Steinmetz JD, Culbreth GT, Haile LM, (2023) Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. The Lancet Rheumatology 5:e508-e522.
- (2025) Osteoarthritis.
- Hunter DJ, Bierma-Zeinstra S (2019) Osteoarthritis. Lancet 393:17451759.
- Meehan JP, Danielsen B, Kim SH, Jamali AA, White RH (2014) Younger age is associated with a higher risk of early periprosthetic joint infection and aseptic mechanical failure after total knee arthroplasty. J Bone Joint Surg Am 96: 529-535.
- Wu K-C, Chang Y-H, Ding D-C, Lin S-Z (2024) Mesenchymal Stromal Cells for Aging Cartilage Regeneration: A Review. Int J Mol Sci 25:12911.
- Barry F, Murphy M (2013) Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol 9: 584-594.
- Caplan AI, Correa D (2011) The MSC: an injury drugstore. Cell Stem Cell 9:11-15.
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284: 143-147.
- Maumus M, Jorgensen C, Noël D (2013) Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. Biochimie 95: 2229-2234.
- Khatab S, van Osch GJ, Kops N, Bastiaansen-Jenniskens YM, Bos PK, et al (2018) Mesenchymal stem cell secretome reduces pain and prevents cartilage damage in a murine osteoarthritis model. Eur Cell Mater 36:218-230.
- Liang J, Liu P, Yang X, Liu L, Zhang Y, et al (2023) Biomaterial-based scaffolds in promotion of cartilage regeneration: Recent advances and emerging applications. J Orthop Translat 41: 54-62.
- Meidrix Biomedicals (2025) GmbH ChondroFiller® liquid – Product Information.
- Simeonov E (2024) Implantation of ChondroFiller Liquid® as a scaffold material for the treatment of chondral lesions of the knee joint. J of IMAB 30: 5936-5941.
- Mazek J, Gnatowski M, Salas AP, O’Donnell JM, Domżalski M, et al (2021) Arthroscopic utilization of ChondroFiller gel for the treatment of hip articular cartilage defects: a cohort study with 12- to 60-month follow-up. Journal of Hip Preservation Surgery 8:22- 27.
- Xing D, Liu W, Wang B, Li JJ, Zhao Y, et al (2020) Intra-articular Injection of Cell-laden 3D Microcryogels Empower Low-dose Cell Therapy for Osteoarthritis in a Rat Model - PMC. 29:0963689720932142.
- Vega A, Martín-Ferrero MA, Del Canto F, et al (2015) Treatment of Knee Osteoarthritis With Allogeneic Bone Marrow Mesenchymal Stem Cells: A Randomized Controlled Trial. Transplantation 99:1681-1690.
- Jo CH, Lee YG, Shin WH (2014) Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells 32:1254-1266.
- Song Y, Zhang J, Xu H, Lin Z, Chang H, et al (2020) Mesenchymal stem cells in knee osteoarthritis treatment: A systematic review and meta-analysis. J Orthop Translat 24:121-130.
- Manferdini C, Gabusi E, Saleh Y, Lenzi E, D’Atri G, et al (2022) Mesenchymal Stromal Cells Laden in Hydrogels for Osteoarthritis Cartilage Regeneration: A Systematic Review from In Vitro Studies to Clinical Applications. Cells 11:3969.
- Gobbi A, Karnatzikos G, Scotti C, Mahajan V, Mazzucco L, et al (2011) One-Step Cartilage Repair with Bone Marrow Aspirate Concentrated Cells and Collagen Matrix in Full-Thickness Knee Cartilage Lesions: Results at 2-Year Follow-up. Cartilage 2: 286-299.
- Pieringer AM, Milz S, Imhoff AB, Vogt S (2024) Influence of cartilage defects and a collagen gel on integrity of corresponding intact cartilage: a biomechanical in-vitro study. Arch Orthop Trauma Surg 144:4309-4317.
- FAQ ChondroFiller ® liquid. meidrix biomedicals GmbH.
- Furuzawa-Carballeda J, Lima G, Llorente L, Nuñez-Álvarez C, Ruiz-Ordaz BH, Echevarría-Zuno S, et al (2012) Polymerized-type I collagen downregulates inflammation and improves clinical outcomes in patients with symptomatic knee osteoarthritis following arthroscopic lavage: a randomized, double-blind, and placebo-controlled clinical trial. ScientificWorldJournal 2012:342854.
- Wang M, Sampson ER, Jin H, Li J, Ke QH, et al (2013) MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther 15: R5.
- Lee B-W, Lee JJ, Jung J-Y, Ju JH (2025) Intra-Articular Injection of Human Bone Marrow-Derived Mesenchymal Stem Cells in Knee Osteoarthritis: A Randomized, Double-Blind, Controlled Trial. Cell Transplant 34: 9636897241303275.
- Lee W-S, Kim HJ, Kim K-I, Kim GB, Jin W (2019) Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl Med 8: 504-511.
- Brittberg M, Recker D, Ilgenfritz J, Saris DBF, on behalf of the SUMMIT Extension Study Group (2018) Matrix-Applied Characterized Autologous Cultured Chondrocytes Versus Microfracture: Five-Year Follow-up of a Prospective Randomized Trial. Am J Sports Med 46:1343-1351.
- Schuette HB, Kraeutler MJ, McCarty EC (2017) Matrix-Assisted Autologous Chondrocyte Transplantation in the Knee: A Systematic Review of Mid- to Long-Term Clinical Outcomes. Orthop J Sports Med 5: 2325967117709250.
- Kim MS, Chun CH, Wang JH (2020) Microfractures Versus a PorcineDerived Collagen-Augmented Chondrogenesis Technique for Treating Knee Cartilage Defects: A Multicenter Randomized Controlled Trial. Arthroscopy 36:1612-1624.
- Borja-Flores A, Macías-Hernández SI, Hernández-Molina G (2020) Long-Term Effectiveness of Polymerized-Type I Collagen IntraArticular Injections in Patients with Symptomatic Knee Osteoarthritis: Clinical and Radiographic Evaluation in a Cohort Study. Adv Orthop 2020: 9398274.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.