Is the Inner Ear the Brain’s Metronome? A New Vestibular Function in a Theoretical Model
by Gennaro Della Rocca
Consultant Physician, AORN Antonio Cardarelli Hospital – Emergency and Critical Care Department – UOC Neurology and Stroke Unit – Via Antonio Cardarelli 6, 80131 – Naples – Italy.
*Corresponding author: Gennaro Della Rocca, Consultant Physician, AORN Antonio Cardarelli Hospital Emergency and Critical Care Department – UOC Neurology and Stroke Unit – Via Antonio Cardarelli 6, 80131 – Naples – Italy.
Received Date: 04 September, 2025
Accepted Date: 18 September, 2025
Published Date: 22 September, 2025
Citation: Della Rocca G (2025), Is the Inner Ear the Brain’s Metronome? A New Vestibular Function in a Theoretical Model. J Psychiatry Cogn Behav 9: 208. DOI: https://doi.org/10.29011/2574-7762.0000208
Abstract
This paper proposes a theoretical model positioning the inner ear – particularly the vestibular system – as a key contributor to neural synchronization processes and cognitive network regulation. Traditionally viewed through its role in balance and spatial orientation, the vestibular system is here, reinterpreted as a sensory interface for temporal information, potentially modulating circadian rhythms and higher-order cognitive functions. From an evolutionary perspective, both the vestibular apparatus and cochlea respond to periodic stimuli – movement and sound, respectively – highlighting their shared capacity to detect rhythmic inputs. These cyclical inputs underpin distinct yet complementary functions: postural control and spatial representation in the vestibular system and language processing and social communication in the cochlear system. Critically, vestibular projections reach multiple cognitive domains, positioning the inner ear as a temporal coordinator of distributed neural networks. The timing signals it conveys may facilitate plasticity (via long-term potentiation), enable inter-areal communication (via coherence), and promote resonance-based synchronization even across anatomically unconnected regions. Visual and vestibular afferents both function as temporal and spatial reference frames, which are essential for the accurate reconstruction of external reality. These mechanisms suggest therapeutic potential: rhythmic stimulation – whether auditory, vestibular or multimodal – could enhance neuroplasticity and restore connectivity patterns in neuropsychological disorders. By framing rhythm as a shared medium between neural substrates and behavioral functions, this model supports the integration of vestibular-based interventions in cognitive rehabilitation, particularly in conditions involving dysregulated timing, such as Parkinson’s disease, neglect, or affective disorders.
Keywords: vestibular system; neural synchronization; temporal processing; circadian rhythms; neuroplasticity; cognitive rehabilitation.
Introduction
One of the most fascinating fields of neuropsychology concerns the intricate web of connections between neural networks, known as the connectome. This structure of the brain is neither tangible nor directly visible; it can only be investigated through instrumental methods capable of detecting dynamic state changes in cerebral areas, such as functional magnetic resonance imaging (fMRI) and magnetoencephalography (MEG); through metabolic changes, as in positron emission tomography (PET); or by means of tools that assess the function of specific areas, such as neuropsychological tests targeting cognitive abilities. Studying the brain during task performance with these techniques highlights the complexity of its functioning, revealing the involvement of numerous areas, even when not anatomically interconnected. The mathematical model that best accounts for this functional organization of brain networks is provided by Graph Theory [1]. Yet, despite our knowledge of various interaction phenomena at both neuronal (e.g., long-term potentiation, LTP) [2] and network levels (e.g., communication through coherence, CTC) [3], as well as large-scale neuronal organization (Large Scale Brain, LSB) [4], several elements remain elusive. These include, for instance, the role of inhibitory synapses, which increase during brain evolution [5], or the mechanisms underlying the global regulation of brain network activity. I would argue that the core issue resides in the very element that enables neuronal communication: resonance. By establishing synchrony between pre- and post-synaptic neurons, it ensures the effective transmission of information, which is subsequently integrated with inputs from other synapses and neurons. In short, the key aspect requiring further reflection is time and its properties – a subject that might help us take another step forward in understanding brain function. An example of the importance of timing in neuronal communication is provided by Melzack and Wall’s Gate Control theory [6], in which the differing conduction speeds of tactile and nociceptive nerve fiber can determine whether pain is perceived acutely or inhibited. Yet, to date, physics suggests that time does not exist [7], medicine argues that it emerges from the ordering of memories [8], while the sensory organ through which we perceive time remains unidentified [9]. This article proposes a novel theoretical perspective in which the inner ear, through its capacity to detect and process rhythmic stimuli, plays a central role in synchronizing cerebral electrical activity and regulating circadian rhythms. Its extensive anatomical connections and distinctive neurophysiological properties position it as a key modulator of cognitive plasticity and systemic biological functions.
To support this hypothesis, the paper integrates findings from multiple disciplines, including physics, phylogeny, anatomy, physiology, and neuropsychology, to construct a theoretical framework linking the inner ear to rhythm-based neuromodulation. It argues that rhythm, as perceived and transmitted by the inner ear, constitutes a fundamental physiological and therapeutic mechanism for addressing both cognitive and systemic disorders, with the potential to enhance existing pharmacological and rehabilitative interventions.
Finally, the paper considers how evolutionary processes may have assigned the inner ear a foundational role in maintaining brain connectivity and neural plasticity, reinstating a function that, while clinically observable, has been largely overlooked in contemporary neuroscience.
The theoretical framework will be presented in five sections:
- The Ear and the Perception of Time
- Distance Senses: Vision and Hearing
- Time as a Functional Dimension of the Brain
- Temporal Dynamics in Brain Connectomics
- The Vestibular System and Circadian Regulation
Isolated clinical cases will then be discussed, demonstrating patient responses to vestibular stimulation as a potential means of modulating brain activity.
The Theoretical Model
a. The Ear and the Perception of Time
Time remains one of the most enigmatic constructs in contemporary science. Despite its constitutive role in all dynamic systems and processes, our understanding of time remains fragmented, limiting its integration into clinical and therapeutic paradigms.
Since Einstein’s Theory of Relativity, time has been understood as intrinsically linked to space, together forming the four-dimensional continuum of Minkowski’s space-time. More recent approaches, however, suggest that time may not exist as a fundamental entity [7] but rather emerges under specific conditions, such as memoryrelated ordering processes [8], pointing to its possible dependence on perception and cognition.
Its importance notwithstanding, there is currently no consensus on the existence of a dedicated sensory organ for time perception [9]. However, as early as Aristotle, philosophical insights proposed that time is not a substance or entity existing independently, but rather a measure of change and motion allowing for regular, cyclic assessment. Time, he argued, is defined by the sequence of events – by what comes ‘before’ and ‘after’.
From this perspective, perceiving time requires the ability to detect cycles, whether through pendulums or electronic oscillations. Among the sensory organs, the ear is uniquely equipped for this function. Sound waves stimulate the cochlea, while movementinduced vibrations, via activation of the striola in the otolithic organs (VEMPs) [10] and endolymphatic inertia, stimulate the vestibular system. Together, these structures allow the inner ear to perceive cyclic patterns, positioning it as a crucial contributor to temporal perception and rhythmic processing.
Phylogenetically, the cochlea evolved in aquatic animals through the modification of the vestibular system – most notably the saccule – as an adaptive response to environmental constraints such as the reduced density of air compared to water [11]. Although the vestibular and cochlear systems are functionally distinct today, both originated as mechanosensory structures and continue to rely on sensitivity to mechanical stimuli. This is further evidenced by the saccule’s responsiveness to low-frequency acoustic input [12], reinforcing the deep functional connection between auditory and vestibular functions. Mechano-transduction in both systems begins with the deflection of hair cells by mechanical forces, in the vestibular system through body movements, i.e. endogenous signals [13], and in the cochlea through sound waves, which represents an exogenous stimulus. In both cases, the input is cyclical, producing rhythmic patterns or action-pause sequences, essentially, time itself.
Accordingly the auditory and vestibular systems may serve as specialized detectors of temporal environmental variation, supporting time perception. The inner ear is extensively interconnected with all major cognitive hubs within the brain [14], including up to ten thalamic nuclei [15], a level of multisensory integration that is unparalleled among the sensory organs. Neurophysiological data indicate that the inner ear operates at high frequencies and exhibits high reflex gain, making it particularly effective at integrating input from vision (low frequency, high gain) and proprioception (low frequency, low gain) [16]. Based on the principle of resonance, which enhances synchrony and biological efficiency, it is plausible to propose that the inner ear contributes to the modulation of cerebral connectivity, with downstream effects on cognitive, emotional, and motor functions.
Although all sensory systems are designed to detect change, and therefore rhythm, none matches the temporal resolution of the inner ear. Unlike auditory and vestibular stimuli – both inherently cyclical – visual input may lack rhythmicity and is constrained by a flicker fusion threshold (50-60 hertz), with changes often irregular, as in drifting clouds. Olfactory and gustatory inputs typically fluctuate in a slow and unpredictable manner, often influenced by factors such as air currents. In contrast, somatosensory activation is driven by changes in pressure and temperature, which may follow irregular patterns. However, the somatosensory system can also detect vibrations, albeit at considerably lower frequencies than those processed by the inner ear, which responds to stimuli up to 800 hertz (Table 1).
|
Nature of the stimulus |
Sounds and vibration are, by its nature, rhythmic and cyclical |
Other senses: often less rhythmic or static (e. g., vision, smell, touch) |
|
Temporal precision |
Ear hair cells are highly sensitive to fast fluctuations |
Other senses: slower response to stimuli |
|
Environmental stability |
Many natural sounds and vibrations have a regular temporal patterns |
Other senses: stimuli can vary irregularly over time |
Table 1: Auditory and Vestibular Roles in the Perception of Time; Features of sounds and vibrations make them apt for detecting cycles, key to temporal perception.
The mechanoreceptors of the inner ear generate rhythmic neural signals directed to the Central Nervous System (CNS), resulting in two distinct sensory streams: vestibular input, continuously modulated by gravitational forces, and cochlear input, driven by sound. Integration with visual and somatosensory data enables the CNS to construct a coherent perceptual model of the external world. Over evolutionary time, the auditory and vestibular systems have developed complementary roles: aligning internal biological rhythms (e.g., circadian cycles) and enhancing environmental awareness (e.g., predator-prey detection) via high-frequency signal transmission to the CNS and increased sensory gain.
In conclusion, the vestibular system detects cyclical, bodygenerated movements, whereas the cochlea detects cyclical external stimuli, namely, sound. The shared core function is the detection of rhythmic variation. Both systems have evolved to collect cyclical, rhythmic stimuli and transmit temporal information to the CNS, modulating cerebral connectivity according to both internal and external rhythms (Figure 1).
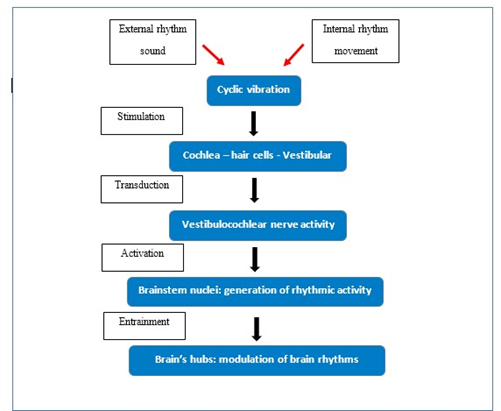
Figure 1: From Stimulus to the Modulation of Brain Rhythms; in the first phase, the stimulus - whether sound, vibration, or movement – reaches the receptor, generating a postsynaptic potential that rhythmically activates the synapses. This rhythmic synaptic activity reaches the brain, modulating its internal rhythms.
b. Distance Senses: Vision and Hearing
The earliest multicellular organisms evolved primary sensory organs such as touch, taste, and smell, which enabled them to explore their immediate environment. As body size increased, the ability to detect distant events became essential, leading to the evolution of vision and hearing approximately 500 million years ago [17]. These senses facilitated not only the perception of remote stimuli but also the recognition of recurring patterns through memory comparison. Attention, shaped by expectation, selectively focuses on these patterns, guiding appropriate behavioral responses and supporting the brain’s predictive capabilities [18].
In terms of ocular movement, the vestibular system’s role is crucial in maintaining visual targets on the fovea, the region of highest visual acuity, which covers roughly one degree of the visual field [19]. When an image shifts from the fovea, a series of compensatory reflexes activate to rapidly restore focus, involving vestibular reflexes such as the Vestibulo-Ocular Reflex (VOR), Vestibulo-Collic Reflex (VCR), and Vestibulo-Spinal Reflex (VSR), as well as visuo-vestibular reflexes including smooth pursuit, saccadic and optokinetic movements [20]. Effectively, the eye-ear system functions as an integrated apparatus, analogous to how space and time are understood as two inseparable components of reality.
In physics, relativity defines energy as E = mc², where one “c” is the constant of the system. Energy thus manifests either as mass (m) or as an electromagnetic wave – a photon (c). While Rovelli contends that time is not fundamental entity in quantum theory [7], photons – though massless – exhibit cyclical behavior, revealing an intrinsic temporal dimension [21]. Conversely, mass shapes quanta and is associated with space. This suggests a dual expression of energy: matter reflects spatial structure, while photons embody temporal cycles, implying a profound connection between massspace and rhythm-time.
However, quanta cannot exist without time, while photons, despite their cyclical nature, propagate through space. It is noteworthy that spatial referent, quanta, produce vibrations through movement that stimulate the ear, which perceives time, whereas temporal referents, photons, stimulate the eye, which perceives space. This reciprocal relationship between these elements and their opposing functions invites the proportional analogy:
m : c = eye : ear
which can be rearranged as:
m × ear = c × eye
Each term on both sides of the equation incorporates spatial and temporal components, reinforcing the concept that space and time are two sides of the same coin just as the eye and ear are both essential for constructing our internal representation of reality, similarly to the Cartesian coordinates of the x-axis and y-axis. It can thus, be argued that two referential components are necessary for perception and that the evolutionary emergence of distance sensory organs aligns with the fundamental laws of physics governing the universe itself.
c. Time as a Functional Dimension of the Brain
Lacquaniti et al. [22] argued that gravity functions as a temporal reference, assisting the brain in establishing directional orientation and sequential organization – mechanisms essential for higherorder cognitive processes such as prediction [23]. This temporal scaffolding is crucial for anticipating future events and allocating mental resources efficiently. Growing evidence suggests that time perception is not fixed, but is progressively supporting the notion of an active, dynamic construction of temporal perception by the brain through the rhythmic processing of both internal and external stimuli [24,25].
Due to resonance, a classic physical demonstration of synchronization involves metronomes placed on a shared movable platform: they gradually align in phase, whereas immobilizing the base prevents this synchronization. Vlasov and Bifone [26] showed that the phenomenon of remote synchronization is driven by hubs not directly connected to the synchronized clusters, in response to external stimuli or shifts in brain state. As these hubs receive vestibular input conveying environmental changes, the vestibular system may act as a shared mobile base, like that of synchronized metronomes, enabling dynamic reconfiguration of functional connectivity. Support for this model also comes from the space environment. Astronauts deprived of vestibular input in microgravity frequently experience behavioral disturbances, cognitive performance impairments [27], and circadian disruption [28], effects that parallel the desynchronization observed when a metronome’s base is fixed and unable to transmit oscillatory motion.
In clinical contexts, a further illustration is found in Eye Movement Desensitization and Reprocessing (EMDR), a therapeutic technique which employs rhythmic eye movements to facilitate the reprocessing of traumatic memories [29]. Rhythmic stimulation appears to open temporal “windows” that permit synchronization among previously uncoupled neural networks, mediated in part by visuo-vestibular reflexes. This process enables the integration of new information with pre-existing memory traces, allowing for the deconditioning of the emotional responses associated with trauma. It is therefore essential to understand that time is not merely a passive datum perceived and transmitted to the brain. Rather, its modulation plays an active role in shaping neural plasticity and the formation of functional brain networks. In other words, the brain operates through temporal coding schemes, with the inner ear acting as a conductor within this complex orchestration. Indeed, vestibular activity has been closely linked to mechanisms of neuroplasticity, including LTP [30] and inter-network coordination via the principle of CTC [3]. Both phenomena can be conceptualized as expressions of resonance dynamics, potentially guided by vestibular input. This hypothesis is further supported by fMRI studies demonstrating widespread cerebral effects following caloric stimulation of the inner ear [31].
d. Temporal Dynamics in Brain Connectomics
The human brain constitutes a highly complex and flexible system in which multiple components interact dynamically, without reliance on a single primary driver. It operates through logarithmic dynamics and feedback loops, enabling stability and resilience even in the presence of partial damage. These mechanisms support the attainment of critical thresholds, behavioral modulation, memory consolidation, and learning, thereby allowing for progressively adaptive responses over time [32]. Graph Theory provides a framework for analyzing the flow of information by focusing on the topological relationships between components. This approach identifies nodes and links whose connectivity is independent of physical distance. Nodes with a greater number of connections are defined as central nodes or hubs. This model has been successfully applied to the anatomical and functional architecture of the human brain [1], which typically conforms to a “small-world” network configuration [33].
The principle of modularity refers to the brain’s capacity to form functional assemblies of nodes required for complex tasks. This can be compared to a 100-metre sprint: runners are initially held behind the starting line (cerebellar inhibition via GABA - stop) and then released by a signal (vestibular activation via NMDA - start). Similarly, the cerebellum and vestibular system jointly select and assemble task-specific networks, enabling both partial and complete dynamic reconfiguration. Through this mechanism, the vestibular system plays a pivotal role in organizing network architecture and shaping the human connectome [34].
The brain’s complex organization into distributed nodes and hubs constitutes what is termed the LSB network [4]. This dynamic intangible architecture is investigated through fMRI, which detects BOLD (Blood Oxygenation Level Dependent) signals linked to deoxyhaemoglobin fluctuations during neuronal activity, and MEG, which measures magnetic fields generated by neuronal currents and offers millisecond-level temporal resolution. Nonetheless, both methods exhibit certain limitations. For instance, information flow does not always follow anatomical pathways, and tracking the temporal evolution of cognitive processes becomes challenging when multiple, widely distributed regions are involved.
The Kuramoto model [35] offers a mathematical framework to explain how synchronization can emerge in a network of coupled oscillators. Remarkably, this phenomenon had already been described by Huygens in 1665, when he observed pendulum clocks synchronizing while mounted on a common wall. Such synchronization requires two fundamental properties: flexibility in oscillatory frequency and the presence of dominant rhythmic influences commonly referred to as “driving effect.” In this context, the inner ear assumes a strategic role in regulating the flow of cerebral information. Beyond supplying temporal information, it supports neuroplasticity mechanisms such as LTP and CTC, and facilitates synchronization across anatomically unconnected networks. In functional terms, it acts analogously to the wall supporting Huygens’ pendulums: a substrate enabling resonance, coherence, and temporal coordination across the brain.
e. The Vestibular System and Circadian Regulation
The vestibular system, beyond its recognized role in balance and spatial orientation, also contributes to circadian regulation. A direct pathway links the saccule to the medial vestibular nucleus (MVN), which projects to the Suprachiasmatic nucleus (SCN), the brain’s master circadian clock [36]. This connectivity allows vestibular input to influence rhythmic control alongside retinal signals.
While approximately 95 percent of SCN neurons are GABAergic and inhibitory, promoting network re-synchronization, the glutamatergic MVN demonstrates strong learning capacity and supports LTP [37]. The SCN also integrates input from the Ascending Reticular Activating System and limbic circuits, thereby participating in the broader regulation of thermoregulation, sleepwake cycles, and hormonal secretion [38].
Although some authors [39] have questioned the specific influence of gravity on these regulatory processes, the hypothesis that rhythm itself, transmitted via the saccule, serves as the primary variable appears both functionally consistent and evolutionarily plausible. The convergence of retinal and vestibular inputs within the SCN, alongside its connectivity with limbic regions, supports the conceptualization of circadian rhythm as an integrated cognitive executive function. This system orchestrates a wide array of hypothalamic-regulated physiological activities, particularly those linked to sleep, hormonal balance and immune function.
Disruptions to circadian rhythm are increasingly associated with the onset of both neurodegenerative [40] and inflammatory disorders [41]. Since executive functions are known to respond positively to cognitive rehabilitation [42], it may be hypothesized that the circadian system itself could be amenable to therapeutic intervention. A natural, yet illustrative example like rocking an infant illustrates how rhythmic vestibular input facilitates circadian and behavioral entrainment. These findings suggest that the vestibular system modulates not only spatial orientation but also the temporal coordination of physiological and cognitive functions, positioning it as a key regulator of the body’s internal clocks.
Preliminary Clinical Observations
Building upon the theoretical framework outlined above, preliminary observations were conducted to evaluate the clinical applicability of rhythmic stimulation using light and sound delivered via
a metronome. This intervention, based on simultaneous visual and auditory rhythmic input, was designed to engage both vestibular and visual pathways projecting to the Suprachiasmatic nucleus (SCN), the central circadian regulator. Simultaneous stimulation at a frequency of 1 Hz, with light and sound applied for five minutes before bedtime, was observed to shorten sleep onset latency, improve subjective sleep quality, reduce nocturnal awakenings, and promote more restorative sleep.
The following briefly outlines the experiences of subjects undergoing rehabilitation using non-invasive, low-risk vestibular stimulation techniques, with the aim of evaluating the predictability of the outcomes in support of this theory, rather than presenting a formal case-report study.
- Case 1: A 71-year-old man, three weeks postprostatectomy, reported nocturia 6-7 times per night, with minimal benefit from hypnotic therapy. Remarkably, after only a single session of metronome-based stimulation, nocturnal awakenings were reduced to once per night, and after four weeks, to twice weekly.
- Case 2: A 61-year-old female with widespread osseous metastases from breast carcinoma, including six vertebrae, pelvis, and femur, presented with refractory pain and four months of complete insomnia. Sleep returned by the third week of stimulation, and by the fourth week she reported being able to dance with her husband without significant discomfort.
- Case 3: A 23-year-old female with intellectual disability, insomnia, and severe dystonia of the cervical and truncal musculature had undergone 18 months of botulinum toxin therapy, discontinued owing to postural weakness and dysphagia. The resulting abnormal posture prevented self-feeding. Within one week of rhythmic metronome stimulation, she recovered sufficient motor control to maintain an upright position, enabling independent feeding, alongside an improvement in sleep. Integration of metronome-based exercises with vestibular stimulation also produced effects in various degenerative disorders, particularly parkinsonian syndromes and cerebellar diseases.
- Case 4: A 48-year-old woman, two years post-resection of the left cerebellar hemisphere for a grade IV astrocytoma and undergoing rehabilitation, was initially limited to slow indoor ambulation with bilateral support. Following vestibuloocular reflex (VOR) stimulation as a neuromodulator prior to physiotherapy, she regained functional independence, including driving, cycling, and returning to her work as a secretary within 30 days.
- Case 5: A 68-year-old man with a seven-year history of Parkinson’s disease developed severe freezing of gait over a two-week period. The symptom responded promptly after a single vestibular stimulation session followed by physiotherapy. The freezing recurred two months after physiotherapy was discontinued.
- Case 6: A 91-year-old man with mild dementia marked by apraxia, Parkinsonism with postural instability with suspected corticobasal degeneration, presented with nocturnal involuntary activity of the left hand, culminating in self-inflicting facial and cervical injuries in an attempt to self-strangulation. He further reported complete loss of functional use of the left hand in daily life. After one month of vestibular stimulation combined with gait rehabilitation and rhythmic metronome training, he regained autonomous ambulation, improved sleep continuity, and resumed playing the violin.
- Case 7: A 67-year-old male with Parkinsonism, marked postural instability, vertical gaze deficits, mild cognitive decline, and anxiety-related insomnia had been bedridden for two years following COVID-19 infection, requiring full caregiving support. After six weeks of vestibular therapy followed by cognitive and neuro-motor rehabilitation, he achieved independent ambulation, improved sleep, reduced anxiety, and resumed occupational activity. Residual impairments were confined to absent postural reflexes, while vertical gaze function showed partial recovery.
- Case 8: A 79-year-old male with moderate-to-advanced Alzheimer’s disease of over five years’ duration presented with Balint’s syndrome, including simultanagnosia that impaired his ability to pour liquids independently. Following eight weeks of vestibular stimulation integrated with cognitive and neuro-motor exercises, he regained autonomous performance of this task.
While these findings remain preliminary and are not yet validated by controlled trials, they offer initial support for the clinical plausibility of the proposed model. The observed improvements underscore the potential of rhythmic stimulation as a therapeutic tool in the regulation of biological timing and associated cognitive and behavioral processes.
Discussion
Understanding brain function continues to pose significant challenges; however, concepts related to time may offer valuable insights. This review suggests that time perception could reveal fundamental organizational principles underlying neural architecture and function. Although prior studies have reported relevant observations, they often lack coherent explanatory models. To the best of the author’s knowledge, no previous studies have tested this specific hypothesis, nor have they evaluated the predictability of the outcomes described here. Identifying the inner ear as the primary sensory organ for time perception introduces a potentially novel paradigm within medical science. Equally innovative is the proposal that its principal role may lie in time sensing, while auditory and vestibular (i.e. acceleration-related) functions constitute secondary correlates. Such a reinterpretation may offer a more coherent framework for explaining the known neuroanatomical and neuro-functional connections of the inner ear, without the need for speculative associations with higherorder processes, such as language, thermoregulation, or sleep.
Forbes et al. [43] noted the persistence of vestibular tone in postural muscles even when not engaging in postural control, suggesting an additional, previously unaccounted function of the vestibular system. Visual and vestibular signals converge upon major integrative brain hubs, enabling the simultaneous processing of spatial and temporal information. Notably, the hippocampus, central to spatial navigation and memory, receives vestibular input but operates primarily within the immediate present (hic et nunc) [44]. Other multimodal cortical regions also receive combined visuo-vestibular input, supporting the hypothesis that all cognitive domains – including linguistic, attentional, spatial, motor, executive, and hypothalamic functions - are structured fundamentally around dual spatial-temporal frameworks to facilitate effective neural representation.
The trophic effects of visual and vestibular pathways on multimodal cortical areas have been well documented by Tsai and colleagues. Their research employed rhythmic auditory and visual stimulation in het mice genetically predisposed to Alzheimer’s disease and lacking otoconia. In a landmark study, Martorell et al. [45] found that stimulated mice performed significantly better than controls in maze navigation tasks and exhibited almost no amyloid deposits post-mortem. In a subsequent investigation, Murdock et al. [46] reported enhanced glymphatic clearance following stimulation: although no plaques were found in brain tissue, amyloid residues were detected in cervical lymph nodes, suggesting active systemic removal.
Therapeutically, numerous studies have demonstrated that visual or auditory cues can enhance motor, spatial, and cognitive performance [47]. Improvements have been documented in posture and spatial orientation [48], as well as in language acquisition and processing [49]. Rhythmic stimulation methods – including electrical neuro-modulation [50], virtual reality [51], music therapy, mindfulness and tai chi – also show promising effects, though the specific neural pathways involved remain unclear.
Noisy Galvanic Vestibular Stimulation (nGVS) has demonstrated potential in enhancing visuospatial memory in healthy individuals, through stimulus-dependent modulation of brain oscillatory activity, producing linear effects on cerebral rhythms measurable via MEG [52]. Therapeutic benefits of vestibular stimulation have also been reported in unilateral neglect [53], prosopagnosia [54], central post-stroke pain [55], and Parkinson’s disease, with improvements in both motor and autonomic symptoms [56] despite an incomplete understanding of the underlying mechanisms.
Smith [57] proposed that vestibular dysfunction contributes to Parkinson’s pathology, as a possible therapeutic strategy for both motor and non-motor symptoms supported by findings of Lewy bodies in vestibular nerve roots [58] and reduced neurofilaments in Deiters’ nucleus [59]. Furthermore, the vestibular system has been shown to interact with the orexinergic system [60] and modulate sympathetic activity, giving rise to the concept of a vestibulosympathetic reflex [61], with potential implications for neurorehabilitation.
These concepts highlight a form of cerebral activity that, although not directly observable, is fundamental to behavior and to the functional integrity of the human organism: electrical brain activity. This underpins the organization of cognitive processes, language, motor control, social interaction, and the homeostatic regulation of autonomic functions. It can be primarily affected, for example, by prolonged exposure to magnetic fields, as described by Rosati [62] in Macomer, Sardinia, Italy, where a higher incidence of multiple sclerosis was noted following the installation of an industrial power facility. Despite these encouraging findings, our understanding of how vestibular input influences cortical and subcortical brain networks remains incomplete. Nonetheless, a recurring theme across the literature is the pivotal role of synchronization processes, supporting the central hypothesis in this paper, namely, that the vestibular system plays a strategic role in mediating temporal coherence across distributed neural systems.
Conclusions
The complexity and breadth of this topic have required an interdisciplinary approach, integrating clinical rehabilitation insights with a critical appraisal of the literature, and it warrants a more comprehensive discussion [63]. Reframing the inner ear as a sensory organ for time perception clarifies its integrative function in orchestrating neural synchrony across networks. Particularly significant is the vestibular system’s role in regulating circadian rhythms, a key determinant of systemic homeostasis mediated by sleep, which is often impaired in inflammatory [64] or neurodegenerative conditions [65]. This overlooked temporal role complements its established function in spatial orientation. Throughout history, humans have instinctively exploited the inner ear’s wiring potential through natural vestibular and auditory stimulation. Rocking soothes infants; flowing water triggers urination and playful movements such as jumping or swinging engage vestibular circuits. In adolescence, musical involvement stimulates cochlear pathways, fostering social bonding and linguistic development. These behaviors preceded scientific understanding but reflect an evolutionary utilization of temporal sensory input for neurodevelopment.
This model identifies three core time-related properties of neural organization, conceptually parallel to spatial constructs:
- Rhythm, the firing pattern of individual neurons (a line);
- Synchrony, alignment between pre- and post-synaptic activity (a two-dimensional plane);
- Network harmony where large-scale assemblies oscillate at distinct frequencies to support complex cognition (3D spatial structures).
This theoretical model supports the growing view that cognition lies at the heart of all behavior and functional activity and is not localized in neurons but emerges from dynamic interactions within temporally coordinated networks. Thought, unlike matter, exists outside the laws of physical extension - Descartes’s res extensa - resides in a temporal domain and can only be accessed through a temporal key: rhythm. From this perspective, rhythm serves as the interface between neural hardware and cognitive software, a principle with tangible implications for rehabilitation. Through rhythmic modulation and synchronization, the brain adapts to environmental demands with remarkable flexibility enabling flexible, context-dependent responses. This approach also offers an evolutionary rationale for the proliferation of inhibitory synapses, which may enhance timing precision and functional adaptability. While remaining largely conceptual, the proposed construct draws on established neurophysiological principles and is supported by convergent empirical findings. It is intended not as a definitive conclusion, but as a stimulus for continued clinical investigation and validation, particularly in the context of neuro-rehabilitation.
Acknowledgements
This work was made possible through ongoing intellectual exchange with Domenico Petriccione, psychiatrist and psychoanalyst, whose critical insights and deep cultural perspective significantly shaped the development of the theoretical model. His ability to identify conceptual tensions and stimulate reflection, even in the absence of clear solutions, provided essential guidance and contributed meaningfully to the refinement of the manuscript.
I also wish to thank Germana Pastore who, through her careful translation, succeeded in making complex and intertwined arguments highly readable, showing a clear understanding of the message and spirit of this article.
References
- Sporns O (2011) Networks of the Brain. MIT press.
- Bliss TVP, Collingridge GL (1993) A sinaptic model of memory longterm potentiation in the hippocampus. Nature 361: 31-39.
- Fries P (2015) Rhythms for Cognition: Communication through Coherence. Neuron 88: 220-235.
- Bressler SL, Menon V (2010) Large-scale brain networks in cognition:
emerging methods and principles. Trends Cogn Sci 14: 277-290.
- Raghanti MA, Spocter MA, Butti C, Hof PR, Sherwood CC (2010) A Comparative Perspective on Minicolumns and Inhibitory GABAergic Interneurons in the Neocortex. Front Neuroanat 4: 3.
- Melzack R, Wall PD (1965) Pain Mechanism: a New Theory. Science 150: 971-979.
- Rovelli C (2017) L’ordine del tempo. Piccola Biblioteca, Adelphi.
- Benini A (2017) Neurobiologia del tempo. Milano 36.
- Le Poidevin, R (2019) The Experience and Perception of Time. The Stanford Encyclopedia of Philosophy (Summer 2019 Edition).
- Curthoys IS, Vulovic V, Burgess AM, Manzari L, Sokolic L, et al. (2014) Neural basis of new clinical vestibular test: otholitic neural responses to sound and vibrations. Clin Exp Pharmacol Physiol 41: 371-380.
- Graf W, Klam F (2006) Le système vestibulaire: anatomie fonctionnelle et comparée, évolution et développement. Comptes Rendus Palevol 5: 637-655.
- Papathanasiou ES (2013) Saccular sensitivity to sound. J Laringol Otol Jan 127 :107.
- Lopez C (2015) Making Sense of the Body: the Role of Vestibular Signals. Multisens Res 28: 525-557.
- Hitier M, Besnard S, Smith PF (2014) Vestibular pathways involved in cognition. Front Integr Neurosci 8: 59.
- Lopez C, Blanke O (2011) The thalamo-cortical vestibular system in animals and human. Brain Res Rev 67: 119-146.
- Hwang S, Agada P, Grill S, Kiemel T, Jeka JJ (2016) A central processing sensory deficit with Parkinson’s disease. Exp Brain Res 234: 2369-2379.
- Del Sorbo A (2018) Ascoltando la pelle. Macro Cesena 29-41.
- Goffman E (1986) Frame Analysis: An Essay of the Organization of Experience. Northeastern University Press.
- Holladay JT (2004) Visual acuity measurements. J Cataract Refract Surg 30: 287-290.
- Rossi G (1994) Manuale di otorinolaringoiatria. Minerva Medica Torino.
- Heeck J (2013) How stable is the Photon? Physical Reviews Letters 111: 801-804.
- Lacquaniti F, Bosco G, Gravano S, Indovina I, La Scaleia B, et al. (2015) Gravity in the brain as a reference for space and time perception. Multisens Res 28: 397-426.
- Friston K (2010) The free-energy principle: a unified brain theory? Nat Rev Neurosci 11: 127-138.
- Craig AD (2009) How do you feel — now? The anterior insula and human awareness. Nat Rev Neurosci 10: 59-70.
- Wittmann M (2013) The inner experience of time. Philos Trans R Soc Lond B Biol Sci 364: 1955-1967.
- Vlasov V, Bifone A (2017) Hub-driven remote synchronization in brain networks. Sci Rep 7: 10403.
- Roma PG, Schneiderman JS, Schorn JM, Whiting SE, Landon LB, et al. (2021) Assessment of Spaceflight Medical Conditions and Treatments’ Potential Impacts on Behavioral Health and Performance. Life Sci Space Res 30: 72-81.
- Barger LK, Flynn-Evans EE, Kubey A, Walsh L, Ronda JM, Wang W, et al. (2014) Prevalence of sleep deficiency and use of hypnotic drugs in astronauts before, during, and after spaceflight: an observational study. Lancet Neurol 13: 904-912.
- Chamberlin DE (2019) The Predictive Processing Model of EMDR. Front Psychol 10: 2267.
- Tai SK, Leung LS (2012) Vestibular stimulation enhances hippocampal long-term potentiation via activation of cholinergic septo-hippocampal cells Behav Brain Res 232: 174-182.
- Black RD, Bell RP, Riska KM, Spankovich C, Peters RW, et al. (2021) The Acute Effects of Time-Varying Caloric Vestibular Stimulation as Assessed With fMRI. Front Syst Neurosci Aug 15: 648928.
- Casati G (1991) Il Caos. Le leggi del disordine. Le Scienze Editore Milano.
- Watts DJ, Strogaatz SH (1998) Collective dynamics of “small-word” networks. Nature 393: 440-442.
- Sporns O, Tononi G, Kötter R (2005) The human connectome: a structural description of the human brain. PLoS Comput Biol 1: 42.
- Kuramoto Y (1984), Chemical Oscillations, Waves and Turbulence.
- Horowitz SS, Blanchard JH, Morin LP (2004) Intergeniculated leaflet and ventral lateral geniculate nucleus afferent connections: an anatomical substrate for functional input from the vestibulo-visuomotor system. J Comp Neurol 474: 227-245.
- Liu C, Reppert SM (2000) GABA synchronizes clock cells within the Suprachiasmatic circadian clock. Neuron 25: 123-128.
- Saper CB, Scammell TE, Lu J (2005) Hypothalamic regulation of sleep and circadian rhythms. Nature 437: 1257-1263.
- Smith PF, Zheng Y (2013) From ear to uncertainty: vestibular contributions to cognitive function. Front Int Neurosci 7: 84.
- Irwin MR, Vitiello MV (2019) Implications of sleep disturbance and inflammation for Alzheimer’s disease dementia. Lancet Neurol Mar 18: 296-306.
- Mogavero MP, DelRosso LM, Fanfulla F, Bruni O, Ferri R (2021) Sleep disorders and cancer: State of the art and future perspectives. Sleep Med Rev Apr 56: 101409.
- Della Rocca G, Conchiglia G, Visciglio A, Russo P, Grossi D (2016) Quanto strategiche sono le “aree cognitive strategiche” nella riabilitazione del paziente con deficit cognitivo? Un’esperienza con la Riabilitazione Cognitiva Integrata (RCI). Psicogeriatria 2: 19-27.
- Forbes PA, Siegmund GP, Schouten AC, Blouin JS (2015) Task, muscle and frequency dependent vestibular control of posture. Front Integr Neurosci 8: 94.
- Moser EI, Kropff E, Moser M-B (2008) Place cells, grid cells and the brain’s spatial representation system. Annu Rev Neurosci 31: 69-89.
- Martorell AJ, Paulson AL, Suk H-J, Abdurrob F, Drummond GT, et al. (2019) Multi-sensory Gamma Stimulation Ameliorates Alzheimer’sAssociated Pathology and Improves Cognition. Cell Apr 177: 256-271.
- Murdock MH, Yang C-Y, Sun N, Pao P-C, Blanco-Duque C, et al. (2024) Multisensory gamma stimulation promotes glymphatic clearance of amyloid. Nature Mar 627: 149-156.
- BA English AM Howard AM (2017) The effects of auditory and visual cues on timing synchronicity for robotic rehabilitation. IEEE Int Conf Rehab Robot 682-688.
- Lopez C (2016) The vestibular system: balancing more than just the body. Curr Opin Neurol Feb 29: 74-83.
- Zamuner TS, Rabideau T, McDonald H, Yeung HH (2023) Developmental change in children’s speech processing of auditory and visual cues: An eyetracking study. J Chil Lang 50: 27-51.
- Beretta VS, Santos PCR, Orcioli-Silva D, Zampier VC, Vitório R, et al. (2022) Transcranial direct current stimulation for balance rehabilitation in neurological disorders: A systematic review and meta-analysis. Ageing Res Rev 81: 101736.
- Demeco A, Zola L, Frizziero A, Martini C, Palumbo A, et al. (2023) Immersive Virtual Reality in Post-Stroke Rehabilitation: A Systematic Review. Sensor 23: 1712.
- Kim DJ, Yogendrakumar V, Chiang J, Ty E, Wang ZJ, et al. (2013) Noisy galvanic vestibular stimulation modulates the amplitude of EEG synchrony patterns. PLoS One 8: e69055.
- Rorsman I, Magnusson M, Johansson BB (1999) Reduction of visuospatial neglect with vestibular galvanic stimulation. Scand J Rehabil Med 31: 117-124.
- Wilkinson D, Ko P, Kilduff P, McGlinchey R, Milberg W (2005) Improvement of a face perception deficit via subsensory galvanic vestibular stimulation. J Int Neuropsychol Soc 11: 925-929.
- McGeoch PD, Williams LE, Lee RR, Ramachandran VS (2008) Behavioural evidence for vestibular stimulation as a treatment for central post-stroke pain. J Neurol Neurosurg Psychiatry 79: 12981301.
- Yamamoto Y, Struzik ZR, Soma R, Ohashi K, Kwak S (2005) Noisy vestibular stimulation improves autonomic and motor responsiveness in central neurodegenerative disorders. Ann Neurol 58: 175-181.
- Smith PF (2018) Vestibular Functions and Parkinson’s disease Front Neurol 9: 1085.
- Seidel K, Mahlke J, Siswanto S, Krüger R, Heinsen H, et al (2015) The brainstem pathologies of Parkinson’s disease and Dementia with Lewy bodies. Brain Pathol 25: 121-135.
- Wellings TP, Brichta AM, Lim R (2017) Altered neurofilament protein expression in the lateral vestibular nucleus in Parkinson’s disease. Exp Brain Res 235: 3695-3708.
- Horowitz SS, Blanchard J, Morin LP (2005) Medial vestibular connections with the hypocretin (orexin) system. J Comp Neurol 487: 127-146.
- Yates BJ, Bolton PS, Macefield VG (2014) Vestibulo-sympathetic responses. Compr Physiol 4: 851-887.
- Rosati G, Aiello I, Granieri E, Pirastru MI, Becciu S, et al. (1986) Incidence of multiple sclerosis in Macomer, Sardinia, 1912-1981: onset of the disease after 1950. Neurology Jan 36: 14-19.
- Della Rocca G (2024) Is Time born from the Brain or is it the other way around? Giammarino Editore 1-269.
- Huang BH, Duncan MJ, Cistulli PA, Nassar N, Hamer M, et al. (2022) Sleep and physical activity in relation to all-cause, cardiovascular disease and cancer mortality risk. Br J Sports Med 56: 718-724.
- Mattis J, Sehgal A (2016) Circadian Rhythms, Sleep, and Disorders of Aging. Trends Endocrinol Metab 27: 192-203.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.