Is Nephrolithiasis a Systemic Disease? - Prospective Evaluation of 531 Consecutive Non-Selected Kidney Stone Formers in a Single Stone Center
by Bernhard Hess1*, Juri Sromicki1,2
1Internal Medicine & Nephrology, KidneyStoneCenter Zurich, Klinik Im Park, Bellariastrasse 38, CH-8038 Zurich Switzerland
2Department of Cardiac Surgery, University Hospital, Rämistrasse 100, CH-8091 Zurich, Switzerland
*Corresponding author: Bernhard Hess, Internal Medicine & Nephrology, KidneyStoneCenter Zurich Klinik Im Park, Bellariastrasse
38, CH-8038 Zurich, Switzerland
Received Date: 23 April 2025
Accepted Date: 28 April 2025
Published Date: 30 April 2025
Citation: Hess B, Sromicki J (2025) Is Nephrolithiasis a Systemic Disease? - Prospective Evaluation of 531 Consecutive Non-Selected Kidney Stone Formers in a Single Stone Center. J Urol Ren Dis 10: 1418. https://doi.org/10.29011/2575-7903.001418.
Abstract
Purpose: We prospectively screened 531 non-selected consecutive renal Stone Formers (SF) for markers of systemic disease.
Methods: A systemic disease affects at least 2 organs/tissues or the whole body. We prospectively collected anthropometric and metabolic data from 531 non-selected SF over 11 years. Eight cystine stone patients and 131 calcium SF with various secondary causes were primarily classified as having systemic disease. The remaining 392 SF, 320 Idiopathic Calcium (ICSF), 63 uric acid (UA-SF) and 9 infection SF were screened for Metabolic Syndrome (MS), traits thereof, diabetes mellitus, gout, LDL- cholesterol > 3.0 mmol/l, proteinuria > 150 mg/d, urine volume < 1.2 L/d (reduced thirst sensitivity), and low bone mass.
Results: Systemic disease markers were more prevalent in ICSF (12%) than UA-SF (5%, p < 0.0001). Full MS was present in 21% of UA-SF vs. 13% of ICSF, p < 0.0025, whereas elevated LDL-C was more prevalent in ICSF (63% vs. 55% in UA-SF, p < 0.005). Very low urine volume occurred in 6% of ICSFs vs. none of UA-SFs (p < 0.0001). Three or more markers of systemic disease were present in 60% US-SF vs. 36% in ICSF, p < 0.0001. Overall, only 42 of 531 non-selected SF (7.9%) were without markers of systemic disease.
Conclusions: Nephrolithiasis should be considered a systemic disease, as 92% of SF exhibit markers of systemic disease. Therefore, recurrent ICSF and UA-SFs should primarily be referred to inter- nists/nephrologists for evaluation not only of urine chemistries, but also systemic diseases/risk factors which should be treated.
Keywords: Calcium vs. uric acid stone formers; Distal renal tubular acidosis; Metabolic evaluation; Markers of systemic disease; Non-selected kidney stone formers
Introduction
A systemic disease is defined as a disorder that affects several organs and tissues or even the whole body [1]. A typical example would be arterial hypertension which induces hypertensive damage to heart, kidneys, brain, retina and arteries, including coronaries [2]. Diabetes mellitus also can be seen as a systemic disease, because it comprises microvascular dysfunction that causes damage to many organ systems [3]. Further examples of systemic diseases are many autoimmune diseases. Previously, nephrolithiasis has already been named a systemic disorder, mainly in the context of metabolic syndrome [4]. More recently, nephrolithiasis has been associated in two ways with patterns of systemic cardio-metabolic disease: on the one hand, cardiovascular disease is associated with increased kidney stone risk. A meta-analysis of 25 observational studies with 934’588 participants [5] revealed significantly elevated odds ratios, between 1.53 and 1.77, for developing kidney stone disease in subjects with various cardiovascular and metabolic risk factors, i.e. hypertension, full metabolic syndrome, dyslipidemia, diabetes mellitus and obesity. The risk for developing nephrolithiasis increased progressively with increasing numbers of traits of metabolic syndrome [5]. On the other hand, nephrolithiasis appears to carry a risk for cardiovascular disease: based on a meta-analysis of 11 cohort studies with 3’658’360 participants free of coronary heart disease or stroke at inclusion to the cohorts [6], a history of nephrolithiasis was associated with elevated risks for developing coronary heart disease (odds ratio 1.24), myocardial infarction (odds ratio 1.24) and stroke (odds ratio 1.21). The large number of patients included in those meta-analyses has the disadvantage that the study population is not homogeneous: for instance, the meta-analysis of Peng and Zheng [6] analyzed subjects with very different ethnicities and lifestyles, i.e. North America (United States and Canada), Northern Europe (Sweden and Norway) and China.
In this paper, we present all anthropometric and laboratory data from a prospective cohort study in 531 non- selected stone formers from Switzerland, referred to our Zurich stone center over 11 years for metabolic evaluation in a clinical routine setting. The main goal was to study the prevalences of markers of systemic disease in a homogenoeus Caucasian cohort, whereby more systemic markers than commonly investigated in patients with nephrolithiasis [5,6] were analyzed.
Subjects and Methods
Non-Selected Consecutive Stone Formers 2006-2016
From January 1, 2006, until December 31, 2016, we studied 531 consecutive non-selected renal stone formers (380 men, 151 women), aged 17 to 82 years, all of Caucasian origin. None of the stone formers suffered from malignant disease (carcinoma, lymphoma or myeloproliferative disorder). They were referred to us for metabolic evaluation of kidney stone disease and seen exclusively by B.H. in a single practice setting in Zurich/ Switzerland. This analysis includes all anthropometric as well as metabolic data which had been prospectively collected from this cohort.
Evaluation in the Stone Clinic
According to our standard protocol [7], patients had dinner not later than 7:00 p.m. The next morning, height and weight of patients were measured in the stone clinic, and body mass index was calculated as weight/height2 in kg per square meters. We measured systolic and diastolic blood pressures in all patients after they had been seated for 5 minutes, using a mercury sphygmomanometer (Riester empire N, Riester GmbH, Radolfzell/Germany). Fasting blood samples were drawn, and samples were analyzed for ionized calcium, sodium, potassium, glucose, urea and creatinine by a point-of-care blood gas analyzer (i-STAT1, Abbott, Baar/ Switzerland) and for cholesterol, HDL-cholesterol, LDLcholesterol and triglycerides in a central laboratory (UNILABS, Dübendorf/Switzerland). Due to restrictions in the Swiss health care system, systematic measurements of 25-OH- Vitamin D were not available. Fasting urines were tested for infection (dipstick), and pH was measured by ion- selective electrode (Metrohm 744 pH meter, Metrohm AG, Zofingen/Switzerland), as recently described [8]. All patients collected 24h-urines while on their free-choice diet [7]. Medications that could alter urinary factors associated with crystallization/stone formation in urine (diuretics, allopurinol) or urinary protein excretion (ACE inhibitors, AT1 receptor blockers, SGLT2 inhibitors) were stopped 2 weeks prior to metabolic evaluation. Urinary parameters associated with increased risk for crystallization/stone formation [8] as well as 24h excretions of protein, sodium and urea, markers of salt and protein intake, were measured in all stone patients by the central laboratory (UNILABS, Dübendorf/Switzerland). Daily salt and protein consumptions were calculated from 24h urine sodium and urea excretion as follows [9]:
- Urine-Na (mmol/d) x 0.058 = grams salt/day
- [Urine-Urea (mmol) x 0.18] + 13 = grams protein/day. This value was normalized 111 to kilograms normal body weight, using the Broca index: Height (cm) – 100 = kg 112 normal weight.
Stone Types
RResults of stone analyses (always performed by X-ray diffraction or infrared spectroscopy) were either provided by referring urologists or obtained from stones directly brought to us by the patients and analyzed by the central laboratory (UNILABS, Dübendorf/Switzerland). Stone types were classified according to the major component, if this amounted to > 65%, or named mixed stones, as recently described [8]. In a few cases where stone analysis had not been performed or was unavailable, stones were categorized as “calcium” according to the density on X- ray or CT scans. Among the 531 stone patients, 451 were calcium stone formers with the following stone compositions: calcium oxalate monohydrate 49.2%, calcium oxalate dihydrate 9.6%, mixed calcium oxalate mono- and -dihydrate 2.6%, calcium phosphate 3.9%, and mixed calcium oxalate/calcium phosphate 12.4%, as previously described [8]. In 22.3%, stones with missing stone analysis had to be classified as “calcium” due to their radiopaque appearance on X-ray or CT scans. Besides the 451 patients with calcium stones, there were 63 uric acid, 9 infection and 8 cystine stone formers.
Criteria Applied for Patterns of Systemic Disease
In a first step, we screened for predefined markers of systemic disease, as depicted in Figure 1. Eight cystine stone formers were defined as having a systemic disease, since this autosomal recessive disease consists of a defective transport of dibasic amino acids across epithelial cells in renal proximal tubules as well as in the small intestine [10]. Among the 451 calcium stone formers, 65 with (almost exclusively incomplete) distal renal tubular acidosis, as reported previously [8], were primarily considered having a systemic disorder, because chronic retention of insufficiently eliminated H+ ions affects different organ systems [8,11].
Furthermore, another 66 calcium stone formers were classified as having systemic disease: 20 post malabsorptive bariatric surgery [12], 16 with primary hyperparathyroidism [13], 15 with inflammatory bowel disease [14], 8 with medullary sponge kidney [15], 4 treated with carboanhydrase inhibitors [16], 2 with HIV disease [17], and 1 with biopsy-proven thin basement membrane disease with proteinuria/hematuria and arterial hypertension.
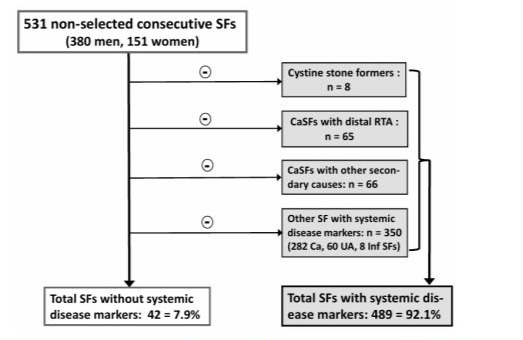
Figure 1: Flow-sheet of screening for markers of systemic disease in 531 consecutive non-selected kidney stone formers.
In a second step, the remaining 392 stone formers, i.e. 320 Idiopathic Calcium Stone Formers (ICSF), 63 uric acid stone formers (UA-SF) and 9 infection stone formers, were systematically screened for various markers of systemic disease, as depicted in Table 1. Metabolic syndrome was diagnosed according to the definition of the International Diabetes Federation [18], except that a body mass index >30 kg/m2 was used as an index of central obesity, as previously described by others [19]. Patients were screened for elevated fasting glucose as well as newly diagnosed/treated diabetes mellitus. Furthermore, a history of gout was considered a marker of systemic disease, since precipitation of monosodium urate crystals not only induces arthritis, but also is associated with distinctive immune responses and systemic inflammation [20]. The latter contributes to cardiovascular events as well as to progressive kidney disease [21], most likely due to a tubulointerstitial nephropathy because of intrarenal deposition of urate crystals [22]. This pattern correlates with the duration of the disease and is associated with decreasing GFR [22].
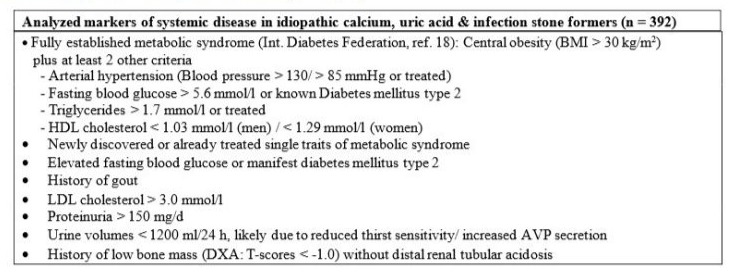
Table 1: Markers of systemic disease screened for in 392 kidney stone formers with idiopathic calcium, uric acid and infection stone disease.
LDL-cholesterol, which is atherogenic and strongly associated with cardiovascular disease [23], was chosen as another marker of systemic disease. A longitudinal study in 36’375 participants (72% men) without cardiovascular disease or diabetes mellitus and low 10-year cardiovascular risk (< 7.5%), followed for a median of 26.8 years [23], had revealed that LDL-cholesterol levels between 2.59-3.34 mmol/l were already associated with an age- adjusted 40% higher risk of death from cardiovascular disease (hazard ratio 1.4, 95% CI, 1.1-1.7) [23]. We therefore chose an LDL-cholesterol level > 3.0 mmol/l in untreated subjects as a marker of systemic risk. This is certainly appropriate, as the latest European Society of Cardiology guidelines on cardiovascular disease prevention even recommended an LDL-cholesterol goal < 2.6 mmol/l for primary prevention in apparently healthy people [24].
Furthermore, we introduced elevated urinary protein excretion (> 150 mg/d) in the absence of primary glomerular disease as a marker of systemic disease, as it has been shown that overt proteinuria is an independent predictor of cardiovascular morbidity and mortality [25] as well as of coronary risk [26]. Moreover, we had previously presented preliminary evidence for reduced thirst sensitivity and elevated vasopressin secretion during an osmotic challenge (5% saline) in kidney stone formers with urine volumes constantly below 1200 ml/d [27]. Thirst sensitivity, expressed as the slope of the linear regression line on a plot of thirst rating (visual analogue scale) against rising serum osmolality, was on average 1.4 times lower than in normal controls, and the total amount of vasopressin secreted during the hypertonic saline infusion was significantly higher in kidney stone formers [27]. Regardless of the primary event, this pattern involves more than one organ system. We therefore included a urine volume below 1200 ml/d in the list of markers of systemic disease. Finally, a low bone mass in the absence of distal renal tubular acidosis was also considered a marker of systemic disease, as lower lumbar bone mineral density is associated with a history of more active kidney stone formation [28].
Statistics
All data are means ± SD. For 24h urines, mean values of 2 collections were used. Comparisons within (paired t-test) and between (unpaired t-test) groups were performed by Student’s t-test. Frequencies of features between 2 groups were compared using Chi-square statistics. Linear regression analyses were performed using IBM SPSS statistics for Windows, Version 25 (IBM Corp., Armonk NY, USA). P values < 0.05 were considered statistically significant.
Results Prevalences of Markers of Systemic Disease
Numbers of markers of systemic disease detected in calcium stone formers without secondary causes, having so- called idiopathic calcium stone disease (ICSF, n = 320), as well as with uric acid (UA-SF, n = 63) and infection stone disease (n = 9) are depicted in Figure 2. The most prevalent systemic marker among these 392 kidney stone formers was LDL-cholesterol > 3.0 mmol/l, present in 236 (60.0%) of stone formers, followed by systolic blood pressure > 130 mmHg in 154 (39.3%), triglycerides > 1.7 mmol/l in 127 (32.4%) and diastolic blood pressure > 85 mmHg in 124 (31.6%) of patients. In the small group of patients with infection stone disease, findings were similar to those in the whole group: 5 out of 9 had elevated LDL-cholesterol, 4 out of 9 elevated systolic blood pressure, 2 out of 9 patients exhibited elevated triglycerides or elevated fasting glucose, respectively, and 1 patient was without any systemic marker. Most likely due to urinary tract infection, elevated proteinuria was more prevalent (5 out of 9, 55.6%) among infection stone formers. Table 2 directly compares prevalences of markers of systemic disease in the 2 most frequently observed ICSF and UA-SF formers. The full metabolic syndrome as well as all its single traits were significantly more prevalent among UASF, whereby elevated triglycerides was the most prevalent single metabolic trait of the syndrome. Treated or newly discovered diabetes mellitus as well as elevated fasting glucose were also more prevalent among UA-SF. As could be expected, a history of gout also significantly more often affected UA-SF. On the other hand, LDL-cholesterol above 3 mmol/l occurred more often in ICSF. Analysis of 24h urines revealed that very low urine volumes, although not highly prevalent, were only present in ICSF, whereas elevated proteinuria was more common in UA-SF. Finally, the prevalence of patients without any abnormal systemic marker was significantly higher in ICSF.
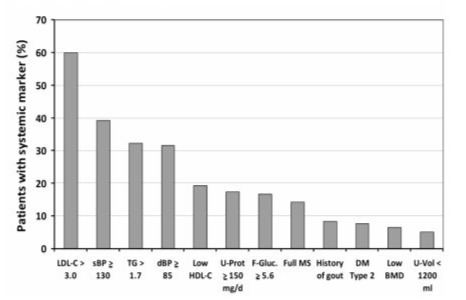
Figure 2: Prevalences (%) of various markers of systemic disease detected in patients with idiopathic calcium (n = 320), uric acid (n = 63) and infection stone disease (n = 9). Abbreviations: sBP and dBP; systolic and diastolic blood pressure, respectively; LDL-C and HDL-C: LDL-Cholesterol and HDL-Cholesterol, respectively; TG; triglycerides; F-Gluc: fasting glucose; DM: diabetes mellitus; Full MS: full metabolic syndrome; U-Protein: 24h proteinuria; U-Vol: 24h urine volume; BMD: bone mineral density.
|
Parameters |
ICSF (320) |
UA-SF (63) |
p value |
|
Full MS |
43/320
(13%) |
13/62
(21%) 41/62
(66%) 44/59
(75%) 28/59
(47%) 15/62
(24%) 33/62
(53%) 7/62
(11%) 17/60
(28%) 13/63
(21%) 34/62
(55%) 17/62
(27%) 0/63
(0%) 4/63
(6.4%) 3/63
(4.8%) |
< 0.0025 |
|
Any
feature of MS |
166/320
(52%) |
< 0.0001 |
|
|
sBP >
130 mmHg |
106/281
(38%) |
< 0.00001 < 0.0001 |
|
|
dBP >
85 mmHg |
96/281
(34%) |
||
|
Low
HDL-chol. |
61/315
(19%) |
< 0.05 |
|
|
High
triglycerides |
92/315
(29%) |
<
0.00001 |
|
|
Treated/newly
discovered Diabetes mellitus 2 |
23/320
(7%) |
< 0.005 |
|
|
Fast.
Gluc. > 5.6 mM |
47/306
(15%) |
<
0.0001 |
|
|
History of
gout |
20/320
(6.3%) |
<
0.00001 |
|
|
LDL-chol.
> 3.0 mM |
197/314
(63%) |
< 0.005 |
|
|
Proteinuria
> 150 mg/d |
46/320
(14%) |
<
0.0001 |
|
|
U-vol.
< 1200 ml/d only |
20/320
(6.30%) |
<
0.0001 |
|
|
Low bone
mass (NO dRTA) |
22/320
(6.90%) |
NS |
|
|
NO
abnormalities |
38/320
(12%) |
<
0.00001 |
Table 2: Comparison of prevalences of markers of systemic disease ICSF vs. UA-SF. Abbreviations: sBP and
dBP; systolic and diastolic blood pressure, respectively; LDL-C and HDL-C: LDL-cholesterol and HDL-cholesterol, respectively; Fast. Gluc: fasting glucose; MS: metabolic syndrome; U-Vol: 24h urine volume; dRTA: distal renal tubular acidosis.
Figure 3depicts prevalences (%) of numbers of systemic markers found in ICSF and UA-SF. It is obvious that ICSF more often exhibit 1 or 2 abnormal systemic markers, whereas the distribution of numbers of systemic markers is shifted towards 3 or more per patient in UA-SF. Three or more markers of systemic disease were present in 60.3 % (38 out of 63) of UA-SF, compared with 35.6% (97 out of 320) among ICSF, p < 0.0001 (data not shown).
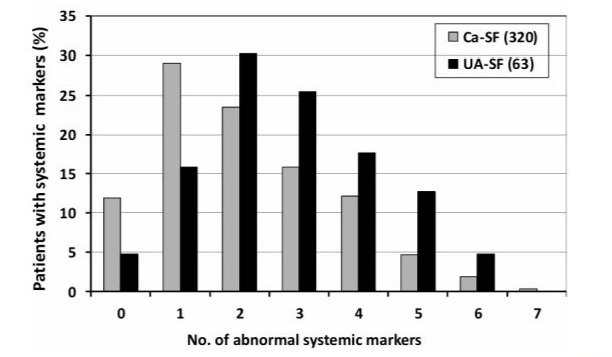
Figure 3: Prevalences (%) of numbers of systemic markers present in patients with idiopathic calcium nephrolithiasis (grey) and uric acid stone disease (black). For details, see text.
Linear Regression Studies
Only data of the 2 subgroups with relevant patient numbers, i.e. ICSF (n = 320) and UA-SF (n = 63), were considered for linear regression calculations. In ICSF, there were no significant correlations of numbers of single traits of metabolic syndrome as well as individual lipids (total cholesterol, HDL-and LDL-cholesterol, triglycerides) or fasting glucose with urinary stone parameters (U-calcium, U-oxalate, U-phosphate, U-uric acid, U-urea U-citrate, U-pH) or number of stones passed by patients. On the other hand (Table 3, upper part), urinary markers of salt (U-sodium) and protein intake (U-phosphate, U-urea) were positively correlated with body mass index, reflecting the fact that more overweight people consume more salt and protein. Urinary pH negatively correlated with body mass index, i.e. more overweight individuals exhibited higher urine acidity, most likely due to the higher acid load that goes along with increased protein intake. As could be expected, there was an inverse linear correlation of serum creatinine with creatinine clearance.
As depicted in Table 3 (bottom), numbers of stones ever passed by UA-SF were positively related to only one of measured blood parameters, i.e. triglycerides. There were no significant correlations of numbers of stones or urinary stone parameters (U-calcium, U-oxalate, U-phosphate, U-uric acid, U-urea U-citrate, U-pH) with any other metabolic parameter. As in ICSF, urinary urea as a marker of protein intake was positively related to body mass index, and serum creatinine was negatively correlated to creatinine clearance. Opposite to ICSF, UA-SF exhibited significant positive correlations of systolic and diastolic blood pressures with markers of salt (urinary sodium) and protein (urinary phosphate) intakes.
|
y-axis |
x-axis |
r value |
p value |
|
ICSF (n =
320) |
|
|
|
|
Urinary
sodium (mmol/d) |
Body mass
index (kg/m2) |
0.37 |
< 0.001 |
|
Urinary
urea (mmol/d) |
|
0.372 |
< 0.001 |
|
Urinary
phosphate (mmol/d) |
|
0.383 |
< 0.001 |
|
Urinary pH |
|
-0.226 |
< 0.001 |
|
Serum
creatinine (mmol/l) |
CCrea
(ml/min./1.73 m2) |
-0.498 |
< 0.001 |
|
UA-SF (n =
63) |
|
|
|
|
No. of
stones passed |
Serum
triglycerides (mmol/l) |
0.372 |
0.003 |
|
Urinary
urea (mmol/d) |
Body mass
index (kg/m2) |
0.332 |
0.01 |
|
Systolic
BP (mmHg) |
Urinary
sodium (mmol/d)) |
0.429 |
0.002 |
|
|
Urinary
phosphate (mmol/d) |
0.33 |
0.022 |
|
Diastolic
BP (mmHg) |
Urinary
sodium (mmol/d) |
0.336 |
0.022 |
|
|
Urinary
phosphate (mmol/d) |
0.308 |
0.033 |
|
Serum
creatinine (mmol/l) |
CCrea (ml/min./1.73 m2) |
-0.593 |
< 0.001 |
Table 3: Linear regression calculations in ICSF (n = 320) and UA-SF (n = 63). For details, see text.
Whole Cohort - Prevalences of Markers of Systemic Disease
Overall, as depicted in Figure 1, our analysis demonstrates that 489 out of 531 consecutive non- selected patients with nephrolithiasis, i.e. 92.1% of all stone formers, exhibit markers of systemic disease.
Discussion
This prospective study of a homogeneous cohort of 531 kidney stone patients is unique in that it presents data collected in an everyday practice setting in one single center where patients were always seen by the same physician. The main finding of the present analysis is that 92.1% of the whole nephrolithiasis cohort exhibited between 1 and 7 well-defined markers of systemic disease, and that these systemic markers should routinely be searched for and treated. At first sight, 139 stone formers were obviously affected by systemic disease, such as cystinuria, distal renal tubular acidosis or various other pathologies (malabsorptive bariatric surgery, primary hy perparathyroidism, inflammatory bowel disease, medullary sponge kidney, treatment with carboanhydrase inhibitors, HIV disease, and glomerular disease with proteinuria/hematuria). Thus, screening for various other predefined markers of systemic disease (Table 1) focused on the remaining 392 stone formers of whom only 42 patients finally turned out to be free of any systemic marker.
As depicted in Figure 2, the most prevalent systemic markers in stone formers were elevated blood pressures and abnormalities of lipid parameters. In a meta-analysis of observational studies including a total of 313’222 participants, patients with nephrolithiasis had a 43% higher risk for arterial hypertension, compared with non-stone formers [29]. The pathophysiologic mechanisms for this association are not fully understood, but have been speculated to be part of the metabolic syndrome and its traits [30]. Indeed, the UA-SF in our cohort were significantly more often affected by metabolic syndrome or its traits and had more often elevated systolic and diastolic blood pressures, as compared with ICSF. This is in accordance with previous data from Sakhaee et al. [19] who demonstrated that in calcium stone formers, the risk for calcium oxalate crystal precipitation was not associated with the numbers of traits of metabolic syndrome.
Among the lipid parameters, elevated LDL-cholesterol was most prevalent, observed significantly more often in ICSF than UASF. We were not able to find any correlation of elevated LDLcholesterol with neither anthropometric data nor blood or urine parameters. A small Turkish study had claimed that patients with combined calcium oxalate monohydrate-dihydrate stones (n =27) had higher LDL-cholesterol levels (3.67 + 1.04 mmol/l) than those with pure calcium oxalate monohydrate stones (2.72 + 1.04 mmol/l, n = 10, p < 0.05) [31], a distinction that could not be made in our database. Data from the NHANES 2007-2020, analyzing 3689 subjects with a history of kidney stone disease versus 34’928 subjects without kidney stones, did not show any association of LDL-cholesterol with a history of stone disease [32]. Thus, elevated LDL-cholesterol appears to be an incidental finding with respect to stone disease, however highly important for the cardiovascular risk profile.
The prevalence of elevated triglycerides ranked third among the markers of systemic disease in our cohort (Figure 2). Moreover, hypertriglyceridemia was clearly more prevalent among UA-SF whose activity of stone disease, i.e. numbers of stones passed, was positively correlated with serum triglycerides (Table 3). This is in accordance with previous findings by others who found that elevated triglyceride is independently associated with uric acid stone disease [33,34]. A link between hypertriglyceridemia and uric acid stone formation appears to be insulin resistance. Insulin resistance produces compensatory hyperinsulinemia that will induce hypertriglyceridemia and low HDL-cholesterol, key criteria of the metabolic syndrome [18,35]. Performing an euglycemic hyperinsulinemic clamp study, Abate et al [36] have provided evidence that UA-SF are more insulin resistant than normal controls and have lower urinary pH values. Low urine pH is the main trigger of the crystallization and stone formation of uric acid, because uric acid at pH values below 5.5 is present as hardly soluble undissociated uric acid [36]. As insulin stimulates ammonium production from L-glutamine in the proximal renal tubule, insulin resistance at the renal level will produce less ammonium and thereby reduce trapping of free H+ ions excreted by the kidney into the urine. Thus, Abate et al. concluded that low urine pH (high concentration of free H+ ions) in UA-SF arises at least partly as a consequence of renal tubular insulin resistance [36]. The same group more recently studied UA-SF on isocaloric metabolic diet and demonstrated that treating insulin resistance directly by the thiazolidindione pioglitazone significantly improved features of the metabolic syndrome and increased urine pH in comparison with placebo treatment which did not alter these parameters significantly [37].
The relatively low prevalences of elevated fasting glucose, full metabolic syndrome, diabetes mellitus and history of gout among all 382 stone formers are most likely due to the fact that the group consisted of significantly more ICSF than UA-SF. As depicted in Table 2, these pathologies were indeed significantly more prevalent in UA-SF. This is in accordance with Cho et al. [38] who found that the odds ratios for forming uric acid stones were 1.8-fold higher for stone formers with the full metabolic syndrome or impaired fasting glucose, respectively, and even 2.1-fold higher for those with hypertriglyceridemia [33].
Urine volume below 1200 ml/d, found to possibly be caused by lower thirst sensitivity in a preliminary study [27], was present in only 5.1% of all stone formers and in none of the UA-SF. Reasons for that are unknown, and the set-up of our daily routine metabolic unit did not allow for further investigating the exact cause of the underlying pathophysiology. Overall, this prospective analysis of 531 consecutive non-selected patients with nephrolithiasis in a single center by one physician over 11 years is unique, as it ensures the best possible homogeneity in data management and interpretation. The fact that there is no control group may be seen as a limitation. However, our intention was never to perform a study comparing stone formers with healthy controls, but primarily reflect everyday clinical routine where physicians always compare their findings with normal values published in clinical guidelines. All 24h urine parameters, anthropometric as well as serum markers that we screened for are well defined with published normal values (see method section).
In conclusion, our prospectively collected data in a cohort of 531 consecutive non-selected patients with nephrolithiasis reveals that 92% of all patients exhibit patterns of systemic disease. The fact that kidney stone formers have a high prevalence of markers of systemic disease does not at all imply causality, but is a strong reminder that these associations carry risks (cardiovascular, metabolic) that go far beyond the risk of recurrent stone formation. Therefore, these systemic markers have to be included in the work-up of stone patients. This implies that stone patients should primarily be seen by internists or nephrologists for evaluation not only of 24h- urine chemistries, but systemic pathologies as described above. This is especially true for the so-called “idiopathic” calcium stone formers and patients with uric acid stone disease in whom high prevalences of systemic markers carry an increased risk for cardiovascular and other pathologies.
Ethics Approval: Approval for this long-term study was obtained from the ethics committee of the University of Zurich, as mentioned in a previous publication [7]. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Funding: No funding was received for conducting this data collection in clinical practice.
Disclosure of Interest: The authors have no conflicts of interest to declare that are relevant to the content of this article.
Authors’ Contributions: J. Sromicki: Data analysis, data management/statistics, manuscript editing B. Hess: Protocol/ project development, data collection, data management, data analysis and manuscript writing/editing.
References
- https://www.sciencedirect.com/topics/medicine-and-dentistry/systemic-disease.
- Kucmierz J, Frak W, Mlynarka E, Franczyk B, Rysz j. Molecular interactions of arterial hypertension in its target organs. Int J Mol Sci 2021; 22: 9669. doi: 10.3390/ijms22189669.
- Horton WB, Barrett EJ. Microvascular dysfunction in diabetes mellitus and cardiometabolic disease. Endocr Rev 2021; 42: 29-55.
- Sakhaee K. Nephrolithassis as a systemic disorder. Curr Opin Nephrol Hypertens 2008; 17: 304-309.
- Rahman IA, Nusaly IF1, Syakri Syahrir S, Nusaly H, Mansyur MA. Association between metabolic syndrome components and the risk of developing nephrolithiasis: A systematic review and bayesian metaanalysis. F1000Research 2021, 10:104, doi.org/10.12688/f1000research.28346.1.
- 6. Peng J-P, Zheng H. Kidney stones may increase the risk of coronary heart disease and stroke. A PRISMA-compliantmeta-analysis Medicin e2017;96:e7898,DOI:10.1097/MD.
- Hess B, Hasler-Strub U, Ackermann D, Jaeger Ph. Metabolic evaluation of patients with recurrent idiopathic calcium nephrolithiasis. Nephrol Dial Transplant 1997; 12: 1362-1368.
- Sromicki J, Kacl G, Föhl M, Hess B. Prospective long-term evaluation of incomplete distal renal tubular acidosis in idiopathic calcium nephrolithiasis diagnosed by low-dose NH4CL loading –gender prevalences and impact of alkali treatment. J Nephrol 2022; 35: 1619–1626.
- Hess B. “Bad dietary habits” and recurrent calcium oxalate nephrolithiasis. Nephrol Dial Transplant 1998; 13: 1033-1038.
- Moussa M, Papatsoris AG, Abou Chakra M, Moussa Y, Update on cystine stones: current and future concepts in treatment. Intractable Rare Dis Res. 2020; 9: 71-78
- Sromicki J, Hess B. Abnormal distal renal tubular acidification in patients with low bone mass: prevalence and impact of alkali treatment. Urolithiasis 2017; 45: 263-269
- Kinlen D, Cody D, O’Shea D. Complications of obesity. QJM 2018: 111: 437-443.
- Smita Jha 1, William F Simonds, Molecular and Clinical Spectrum of Primary Hyperparathyroidism. Endocr Rev 2023 Mar 24; bnad009. doi: 10.1210/endrev/bnad009.
- Rogler G, Singh A, Kavanaugh A, Rubin DT. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021; 161: 1118-1132.
- Fabris A, Anglani F, Lupo A, Gambaro G. Medullary sponge kidney: state of the art. Nephrol Dial Transplant 2013; 28: 1111-1119.
- Sacré A, Jouret F, Manicourt D, Devuyst O. Topiramate induces type 3 renal tubular acidosis by inhibiting renal carbonic anhydrase. Nephrol Dial Transplant 2006: 21: 2995-2996.
- Luca S, Nelson Am. HIV and the spectrum of human disease. J Pathol 2015; 235: 229-241.
- Alberti KGMM, Paul Zimmet P, Jonathan Shaw J, IDF Epidemiology Task Force Consensus Group. The metabolic syndrome--a new worldwide definition. Lancet 2005; 366: 1059-1062.
- Sakhaee K, Capolongo G, Maalouf NM, Pasch A, Moe OW, Poindexter J, Adams-Huet B. Metabolic syndrome and the risk of calcium stones. Nephrol Dial Transplant 2012: 27: 3201-3209.
- Gu H, Yu H, Qin L, Yu H, Song Y, Chen G et al. MSU crystal deposition contributes to inflammation and immune responses in gout remission. Cell Rep 2023; 42: 113139.
- Johnson RJ, Sanchez Lozada LG, Lanaspa MA, Piani F, Borghi C. Uric Acid and Chronic Kidney Disease: Still More to Do. Kidney Int Rep 2022; 8: 229-239.
- Bardin T, Nguyen QD, Tran KM, Nghia HL, Minh DD, Richette P et al. A cross-sectional study of 502 patients found a diffuse hyperechogenic medulla pattern in patients with severe gout. Kidney Int 2021; 99: 218-226.
- Abdullah SM, Defina LF, Leonard D, Barlow CE, Radford NB, Willis B et al. Long-term association of low-density lipoprotein cholesterol with cardiovascular mortality in individuals at low 10-year risk of atherosclerotic cardiovascular disease. Circulation 2018; 138: 2315-2325.
- Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021; 42: 3227-3337.
- Segura j, Campo C, Ruilope LM. Proteinuria: an underappreciated risk factor in cardiovascular disease. Curr Cardiol Rep 2002; 4: 458-462.
- Perkovic V, Verdon C, Ninomiya T, Barzi F, Cass A, Patel A et al. 2008. The relationship between proteinuria and coronary risk: a systematic review and meta-analysis. PLoS Med; 5(10): e207. doi: 10.1371/journal.pmed.0050207.
- Hess B, Takkinen R, Farina K, Jaeger Ph. Altered vasopressin (AVP) release and thirst sensitivity in male recurrent renal stone formers with low urine volume (LVSF). Kidney Int 1997; 52: 1426(abstract).
- Li Z, Li L, Zheng J, Li M, Wu S, Xin K, Li R, Bai S, Chen X. Associations between lumbar bone mineral density, serum 25-hydroxyvitamin D and history of kidney stones in adults aged 30-69 years in the USA (NHANES 2011-2018). BMJ Open. 2023 May 23; 13(5): e070555. doi:10.1136/bmjopen-2022-070555.
- Shang W, Li Y, Ren Y, Yang Y, Li H, Dong J. Nephrolithiasis and risk of hypertension: a meta-analysis of observational studies. BMC Nephrol 2017: 18: 344, DOI 10.1186/s12882-017-0762-8
- Cupisti A, D’Allessandro C, Samoni S, Meola M, Egidi MF. Nephrolithiasis and hypertension: possible links and clinical implications. J Nephrol 2014; 27: 477-482.
- Inci M, Demirtas A, Sarli B, Akinsal E, Baydilli N. Association between body mass index, lipid profiles and types of urinary stones. Renal Failure 2012; 34: 1140-1143.
- Gao M, Liu M, Zhu Z, Chen H. The association of dyslipidemia with kidney stone: result from the NHANES 2007-2020. Int Urol Nephrol 2023; 56: 35-44.
- Cho ST, Jung SI, Myung SC, Kim TH. Correlation of metabolic syndrome with urinary stone composition. Int J Urol 2013; 20: 208-213.
- Torricelli FC, De SK, Gebreselassie S, Li I, Sarkissian C, Monga M. Dyslipidemia and kidney stone risk. J Urol 2014; 191: 667-672.
- Reaven GM. Insulin resistance, the insulin resistance syndrome, and cardiovascular disease. Panminerva Med. 2005; 47: 201-210.
- Abate N, Chandalia M, Cabo-Chan Jr AV, Moe OW, Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int 2004; 65: 386-392.
- Maalouf NM, Poindexter JR, Adams-Huet B, Moe OW, Sakhaee K. Increased production and reduced urinary buffering of acid in uric acid stone formers is ameliorated by pioglitazone. Kidney Int 2019: 95: 1262-1268.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.