Intronic MicroRNA MiR-483-3p Modulates Oxidative Stress-induced Macrophage Apoptosis by Targeting MED1
by Xianlun Yin1#,Wenwen Teng2#, Xiaowei Wang1, Jing Gao1, Junjie Ma3*, Zhe Wang4*, Xiaoming Zhou5*
1State Key Laboratory for Innovation and Transformation of Luobing Theory; Key Laboratory of Cardiovascular Remodeling and Function Research of MOE, NHC, CAMS and Shandong Province; Department of Cardiology, Qilu Hospital of Shandong University, China.
2Shandong Provincial Hospital, Affiliated to Shandong First Medical University, China
3Department of Hematology, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, China
4Division of Geriatrics, Shandong Provincial Hospital affiliated to Shandong First Medical University, China
5Department of Research, Shandong Provincial Hospital Affiliated to Shandong First Medical University, China
# Equally contributed to this work
*Corresponding author: Xiaoming Zhou, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong Province, China. Zhe Wang, Division of Geriatrics, Shandong Provincial Hospital affiliated to Shandong First Medical University, China. Junjie Ma, Department of Hematology, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, China.
Received Date: 26 September 2025
Accepted Date: 08 October 2025
Published Date: 23 October 2025
Citation: Yin X, Teng W, Wang X, Gao J, Ma J, et al. (2025) Intronic MicroRNA MiR-483-3p Modulates Oxidative Stress-induced Macrophage Apoptosis by Targeting MED1. J Med Biomed Discoveries 7: 143. https://doi.org/10.29011/2688-8718.100143
Abstract
Background: Abdominal aortic aneurysm (AAA) is a life-threatening vascular disease. Oxidative stress, macrophage apoptosis, and miRNAs play important roles in its pathogenesis. This study investigated the role of miR-483-3p and its host gene IGF2 in oxidative stress-induced macrophage apoptosis during AAA progression, as well as the regulatory effects of γ-glutamylcysteine (γ-GC). Methods: AAA mouse models were established using Angiotensin II (Ang II) infusion. Macrophage apoptosis was quantified via co-localization of CD68 and TUNEL staining. RNA FISH, qPCR, and Western blot were employed to assess miR-483-3p, IGF2, MED1, p53, and p21 expression. The binding relationship between miR-483-3p and MED1 was verified via dual-luciferase assay. Flow cytometry analyzed the effects of H₂O₂ and γ-GC on macrophage apoptosis. Results: Macrophage apoptosis, intronic miR-483-3p and its host gene IGF2 expression in aneurysm tissue of AAA mice were significant increases, accompanied by a pronounced downregulation of γ-glutamylcysteine (γ-GC) synthetase. Although IGF2 markedly enhanced the pro-apoptotic effect of miR-483-3p, IGF2 alone had no significant effect on H₂O₂-induced macrophage apoptosis. Enrichment analysis revealed that miR-483-3p was involved in apoptosis process. MED1 was the target gene of miR-483-3p and inhibited the expression of pro-apoptotic genes p53 and p21. Furthermore, the pro-apoptotic effects of H₂O₂ and miR-483-3p on macrophages were significantly attenuated by γ-GC. Conclusion: In summary, we found that miR-483-3p promoted oxidative stress-induced macrophage apoptosis via MED1-p53/p21 axis with the collaboration of its host gene IGF2, which were significantly ameliorated by γ-GC.
Keywords: Abdominal aortic aneurysm; macrophage; miR-4833p; MED1; oxidative stress
Introduction
AAA is a severe vascular disease with >85% mortality upon rupture and rising incidence, lacking effective therapies [1]. Oxidative stress and infiltration of inflammatory cells, predominantly macrophages, are significant pathological changes in AAA [2,3]. Macrophage apoptosis triggers a complex immune-inflammatory cascade and plays a critical role in the initiation and progression of AAA [4,5]. Current research reveals macrophage apoptosis plays a paradoxical role in AAA development, showing protective roles in early stages but contributing to disease progression in advanced phases [6,7]. Nevertheless, research on macrophage death in the context of AAA remains relatively limited. The detail of modulating macrophage apoptosis in AAA has not been fully elucidated.
Oxidative stress and inflammation are critical factors in AAA Pathogenesis. Reactive oxygen species (ROS) play essential roles in maintaining cellular homeostasis and regulating various physiological functions at physiological concentration. For example, ROS can stimulate the differentiation of osteoclast through NF-κB Signaling [8]. However, excessive ROS triggers oxidative stress and leads to aberrant cellular functions including cell migration, proliferation, and apoptosis. These pathological alterations exacerbate inflammatory responses and facilitate vascular remodeling, thereby promoting the development of many vascular diseases including AAA [9]. Nevertheless, the precise mechanisms by which ROS induces macrophage apoptosis and its role in AAA pathophysiology remain to be fully understood.
Gamma-glutamylcysteine (γ-GC), a direct precursor of glutathione (GSH), is a critical intracellular antioxidant and plays a pivotal role in maintaining cellular antioxidant capacity and modulating oxidative stress responses [10]. Studies have demonstrated that γ-GC improves endothelial function and alleviates inflammatory injury by modulating antioxidant and signal transduction [11]. Additionally, GSH has been found to reduce the risk of thrombosis by attenuating platelet aggregation [12]. A decrease in γ-GC levels significantly elevated intracellular oxidative stress, contributing to the pathogenesis of various diseases. Supplementation with exogenous γ-GC has been shown to significantly alleviate oxidative stress and mitigate cellular damage [13]. γ-GC is efficiently absorbed by various cell types, including macrophages, where it promotes GSH synthesis and exerts anti-inflammatory effects [14,15]. However, the connection between γ-GC and AAA remains underexplored, and its role in AAA-associated inflammation and macrophage apoptosis is not yet fully understood.
MiRNAs play critical roles in ROS-induced apoptosis, especially in the apoptosis of macrophage. For example, miR-21 targeted MKK3 and up-regulated pro-apoptotic p38-CHOP, thereby promoting macrophage apoptosis [16]. MiR-221-3p could target ADAM22 and suppress ox-LDL-induced macrophage apoptosis and foam cell formation [17]. Intronic miRNAs are located within the introns of protein-coding genes and usually share the same promoters with their host genes. MiR-483-3p is a typical intronic miRNA located in the second intron of IGF2 gene and participates in the development of various metabolic and malignant diseases. MiR-483-3p over-expression in murine 3T3-L1 cells could induce lipotoxicity and insulin resistance by impeding the lipid storage of adipocytes. MiR-483-3p inhibition increased the homing of endothelial progenitor cells in venous thrombosis rats [18]. In nephroblastoma, colon cancer, liver cancer, etc., miR-483-3p was up-regulated and inhibited tumor cell apoptosis, thereby promoting tumorigenesis [19]. However, a study also indicated that miR-4833p promoted apoptosis and inhibited tumor cell proliferation [20]. Nevertheless, the relationships among oxidative stress, miR-4833p, γ-GC, macrophage apoptosis, and AAA remain unclear.
Herein, we found significant increases in macrophage apoptosis, miR-483-3p and its host gene IGF2 expression in aneurysm tissues of AAA mice, accompanied by a pronounced downregulation of γ-glutamylcysteine synthetase (γ-GCS). H2O2 significantly upregulated the expression of miR-483-3p and its host gene IGF2 when inducing macrophage apoptosis, which were significantly ameliorated by γ-GC. MiR-483-3p upregulated p53/p21 by suppressing MED1, promoting macrophage apoptosis, while IGF2 enhances this process. γ-GC may be a potential therapeutic target for AAA.
Materials and Methods
Ethics Statement and Sample Collection
Blood and fecal samples were collected from 20 patients diagnosed with AAA and healthy individuals. AAA diagnosis was confirmed via CT angiography (CTA), with an abdominal aortic diameter exceeding 3 cm. Healthy individuals underwent abdominal CT scans to ensure the absence of AAA. Exclusion criteria for AAA patients included age above 80 years, the presence of severe hypertension, diabetes, or dyslipidemia, as well as other aneurysms, cardiovascular diseases, infectious diseases, gastrointestinal disorders, autoimmune conditions, hepatic or renal diseases, cancer, or a history of abdominal surgery. This study was approved by the Ethics Committee of Shandong University Qilu Hospital (2018-110) and verbal consent was obtained from the subjects. All procedures involving human participants adhered to the principles outlined in the Declaration of Helsinki and were conducted in accordance with applicable local laws and institutional guidelines. All animal procedures followed the US NIH Guide for the Care and Use of Laboratory Animals.
AAA Model Construction
ApoE-/- male specific pathogen-free mice, purchased from Beijing Vital River Laboratory Animal Technology Co, were housed in a controlled environment with 23-24°C, relative humidity of 50%, and a consistent 12:12-hour light-dark cycle. The mice were allowed free access to high-fat diet (containing 0.25% cholesterol and 15% cocoa butter) and water. The Ang II-induced AAA mice model was established according to previously published protocols [21]. Briefly, ApoE-/- mice were implanted subcutaneously with micro-osmotic pumps (Alzet, model 2004) to deliver either Ang II (1000 ng/min/kg) or PBS for 4 weeks. Upon completion of the experiment, mice were anesthetized, and tissue specimens were collected for other experiments.
Cell culture and treatment
HEK293T and RAW264.7 cells purchased from Shanghai Cell Bank of Chinese Academy of Sciences were cultured in DMEM (Gibco, Thermo Fisher Scientific, USA) containing 10% fetal bovine serum (Biological Industries, Israel) at 37 ºC with 5% CO2. RAW264.7 cells treated with 300 μM H2O2 were used as the experimental group, and RAW264.7 cells treated with H2O2 at physiological concentration (10 nM) were used as the control group. After treatment for 24 hours, cells were harvested for the following experiments. In some experiments, RAW264.7 cells were treated with recombinant IGF2 active protein (Abcam, USA) at 200 ng/ml for 48 hours.
Transfection
The expression plasmid of MED1 (pCMV6-MED1) was transfected into cells using Lipofectamine 3000 reagent (Life Technologies, USA) according to the manufacturer’s instructions. The mimic and inhibitor of miR-483-3p and the corresponding negative controls (Ribobio, China) respectively were transfected at a final concentration of 50 nM using Lipofectamine RNAiMAX reagent (Life Technologies, USA).
Target gene prediction and functional enrichment analysis
The target genes of miR-483-3p were predicted by TargetScan, miRWalk and miRDB. The target genes predicted by all three prediction tools were selected, and their functional enrichment analysis were performed by Enrichr (http://amp.pharm.mssm.edu/ Enrichr/) [22,23].
RNA isolation and Real-time quantitative PCR (qPCR)
Total RNA was extracted using TRIZOL reagent. Mir-X miRNA First-Strand Synthesis Kit (Clotech, Japan) and RT-PCR kit Mir-X miRNA qRT-PCR TB Green Kit (Clotech, Japan) were used for miRNA cDNA synthesis and PCR amplification. Human and murine RNU6B gene were used as endogenous standards. The mRNA expressions of MED1, IGF2, p53 and p21 were detected using PrimeScript RT Master Mix (Takara, Japan) and TB Green Premix Ex Taq II (Takara, Japan) in a CFX96 real-time PCR system (Bio Rad, USA). β-actin was used as an endogenous standard. The 2-ΔΔCt method was used for quantification of qPCR data. The primers in this study were listed in (Table S1).
|
Gene |
Primers |
Primer sequence (5′ → 3′) |
|
p53 |
forward |
5’- GCGTAAACGCTTCGAGATGTT-3’ |
|
reverse |
5’-TTTTTATGGCGGGAAGTAGACTG-3’ |
|
|
p21 |
forward |
5’-CGAGAACGGTGGAACTTTGAC-3’ |
|
reverse |
5’-CCAGGGCTCAGGTAGACCTT-3’ |
|
|
MED1 |
forward |
5’-GGACCTTTCTAAAATGGCTATTATGT-3’ |
|
reverse |
5’-CGGGGTGAGATAACCAACAC-3’ |
|
|
IGF2 |
forward |
5’-TCAGTTTGTCTGTTCGGACCG-3’ |
|
reverse |
5’-TAGACACGTCCCTCTCGGACTT-3’ |
|
|
Actin |
forward |
5’-AGAGAGGTATCCTGACCCTGAAGT-3’ |
|
reverse |
5’-CACGCA GCTCATTGTAGAAGGTGT-3’ |
|
|
MED1- 3’-UTR-wt |
SacI-forward |
5’-GAGCTCCAGCACCAGTCCCACAGAG-3’ |
|
XbaI-reverse |
5’-TCTAGA ATGCTAACTCCAACAACCTG-3’ |
|
|
MED1-3’-UTR-mut |
forward |
5’-ACCATAGGAATATTAGCGTTGAGTTACCT-3’ |
|
reverse |
5’-AGGTAACTCAACGCTAATATTCCTATGGT-3’ |
|
|
γ-GCS |
forward |
5’-CTGTCTGACCCCTGTGCTGAT-3’ |
|
reverse |
5’-GCAAACTAGAGAAGGGCAGGAA-3’ |
Table S1: PCR Primers usedin this study
Western Blot
Total proteins were isolated using RIPA buffer (Beyotime, China). Protein concentrations were determined by bicinchoninic acid (BCA) method. Then, 20µg total protein was separated by 10% SDS-PAGE and transferred to PVDF membrane (Bio-Rad, USA). The membrane was incubated with primary antibodies at 4 °C overnight and subsequently incubated with second antibody (Proteintech Group, USA) at room temperature for 2 h. The antibodies used in this study were listed in (Table S2).
|
Antibody Name |
Supplier |
Catalog Number |
Host species |
Dilution Ratio |
|
β-Actin Mouse mAb |
ABclonal |
AC004 |
Mouse |
1:10000 |
|
GAPDH Mouse mAb |
ABclonal |
AC033 |
Mouse |
1:100000 |
|
Anti-IGF2 antibody |
Abcam |
ab262713 |
Rabbit |
0.388888889 |
|
Anti-p53 antibody [PAb 240] |
Abcam |
ab26 |
Mouse |
0.736111111 |
|
Anti-p21 antibody [EPR3993] |
Abcam |
ab109199 |
Mouse |
0.736111111 |
|
Anti-MED1 (phospho T1457) antibody |
Abcam |
ab60950 |
Rabbit |
0.736111111 |
|
CD68 (KP1): sc-20060 |
Santa Cruz |
sc-20060 |
Mouse |
01:50 |
Table S2: Antibodies used in this study
Dual-Luciferase Reporter Assay
The 3’-UTR sequence of MED1 containing the wild-type (WT) or mutant (Mut) bases in the predicted binding site of miR-4833p was cloned into the SacI/XbaI restriction site of pmir-GLO (Promega, USA) to generate the plasmid pG-WT-MED1-3’-UTR or pG-Mut-MED1-3’-UTR, respectively. Then, HEK-293T cells were co-transfected, respectively, with the above vectors, miR-4833p mimics or miR-483-3p inhibitors and corresponding negative controls using Lipofectamine 3000 reagent and Lipofectamine RNAiMAX reagent (Life Technologies, USA). After 48h, the luciferase activity was determined using Dual-Luciferase Reporter Assay System (Promega, USA) on Centro LB 960 Microplate Luminometer (Berthold, Germany). Primers are listed in Table S1.
Flow Cytometry Analysis of Apoptosis
The apoptosis of RAW264.7 was detected using FITC Annexin V Apoptosis Detection Kit I (Becton Dickinson, USA) according to the manufacturer’s protocol. Cells with different treatments were resuspended in binding buffer to a concentration at 1×105/ ml. Next, the cells were stained with 5 µl FITC Annexin V and Propidium Iodide (PI), and incubated for 15 minutes in the dark at room temperature. The apoptotic rate was detected using the FACSCalibur (Becton Dickinson, USA) and data were analyzed using FlowJo X software (Flow Jo, USA).
Tunel Staining
AAA tissue sections were deparaffinized and washed with PBS, then immunolabeled with CD68 polyclonal antibody (Santa Cruz, USA) to identify macrophages. Macrophage apoptosis was subsequently detected using a TUNEL staining kit (Beyotime, China). Briefly, deparaffinized sections were incubated with TUNEL reaction mixture at room temperature for 30 min in a humidified chamber.
After three PBS washes, nuclei were counterstained with DAPI. Macrophage apoptosis was then observed and analyzed using fluorescence microscopy.
RNA Fish
The experiment was conducted in accordance with the manufacturer’s protocol for the RNA FISH paraffin section kit (GenePharma, China). The results were observed under a fluorescence microscope and RNA expression in the tissue was analyzed using Image pro plus 6.0 software (Media Cybernetics, USA).
Statistical analysis
Data are presented as mean ± SD. The normality was checked using Shapiro–Wilk test. T-test was used for significance analysis between two group. One-way ANOVA was used for statistical tests of multiple comparisons. Correlations were examined by the Spearman’s rank correlation coefficients with corresponding multiple comparison correction. All experiments were repeated at least three times independently and p < 0.05 was considered as statistically significant. Statistical analysis was performed using GraphPad Prism v8.0.2.
Results
Significant increases in levels of macrophage apoptosis, and the expression of miR-483-3p and its host gene IGF2 in AAA tissues
We found that compared to normal aortic tissues, the expression of γ-GCS, the key enzyme responsible for synthesizing the antioxidant γ-GC, was significantly reduced in the aneurysmal tissues of angiotensin II-induced AAA mice (Figure 1A). The co-localization area of CD68 and TUNEL staining was markedly enhanced in
AAA tissues compared to those in normal abdominal aorta tissues, indicating a significant increase in macrophage apoptosis (Figure 1B). RNA fluorescent in situ hybridization showed that miR-483-3p expression was significantly upregulated in AAA aneurysmal tissues (Figure 1C). The qPCR results further demonstrated the elevated levels of miR-483-3p and its host gene, IGF2, in macrophages isolated from aneurysmal tissues (Figure1D,1E). Moreover, correlation analyses revealed strong positive associations between macrophage apoptosis, miR-483-3p expression, and IGF2 expression levels (Figure 1F,1H). Additionally, targeted metabolomic analysis showed that serum γ-GC levels were significantly lower in AAA patients compared to healthy individuals (Figure1I). These findings suggest that oxidative stress is significantly elevated in AAA aneurysmal tissues and is closely associated with increased macrophage apoptosis and the upregulation of miR-483-3p and its host gene IGF2.
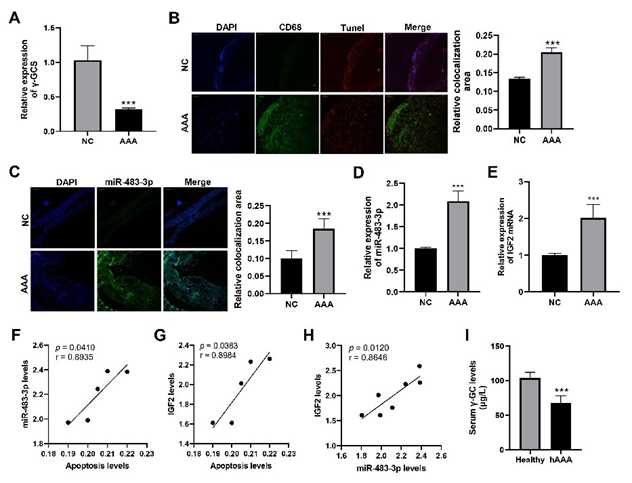
Figure 1: Significant changes in levels of γ-GCS, macrophage apoptosis, miR-483-3p expression and their relationships in AAA.
Comparison of expression levels of γ-glutamylcysteine synthetase (γ-GCS) mRNA in abdominal aorta tissues from normal mice (NC) and aneurysmal tissues from Ang II-induced AAA mice (AAA). (B) Representative immunofluorescence images of CD68 and TUNEL in abdominal aorta tissues of NC and AAA groups and their quantitative comparison. (C) Representative images of fluorescence in situ hybridization of miR-483-3p in abdominal aorta tissues of NC and AAA groups and their quantitative comparison. (D,E) Comparison of expression levels of miR-483-3p (D) and IGF2 (E) in abdominal aorta tissues of NC and AAA groups. (F,H) Correlation analyses between macrophage apoptosis, miR-483-3p and IGF2 expression levels. (I) Comparison of serum levels of γ-glutamylcysteine (γGC) in AAA patients (hAAA) and healthy individuals (healthy). Statistical analyses were performed using Student’s t-test for (A-E, I).
Spearman’s correlation analysis was used for (F,H). ***p < 0.001.
MiR-483-3p was predicted to be involved in multiple biological functions and significantly promoted macrophage
H2O2 is the main source of oxidative stress in cells. We found that high concentration of H2O2 induced the apoptosis of macrophages (Figure 2A). Compared to the macrophages treated by H2O2 at physiological concentration (10 nM), the expression of miR-483-3p was significantly up-regulated in macrophages treated with 300 μM H2O2 for 24 hours (Figure 2B). However, miR-483-3p mimic or inhibitor had no significant effects on the apoptosis of macrophages without H2O2 treatment (Figure 2C). Despite that, miR-483-3p mimic markedly enhanced the apoptosis of macrophages treated with 300 μM H2O2, while miR-483-3p inhibitor had no obvious effect on the H2O2-induced macrophage apoptosis (Figure 2D). To further unravel the mechanism by which miR-483-3p promoted H2O2-induced macrophage apoptosis, the target genes of miR-483-3p were predicted by three commonly used tools including TargetScan, miRWalk and miRDB. To reduce false positives in the predicted results, 45 target genes of the miR-483-3p predicted by all three tools were selected as the potential target genes of miR-483-3p (Figure 2E). The functional enrichment analysis of these 45 genes showed that miR-483-3p was involved in multiple important biological functions, including the regulation of apoptosis, intracellular lipid and cholesterol transport, LDL binding, cellular response to ROS and immunity (Figure 2F). Among them, the enrichment of regulation of apoptotic process was the most significant. Besides, these potential target genes were also significantly enriched in several important signaling pathways that regulate the cellular inflammation, proliferation, and apoptosis, such as NF-κB, Notch1, Wnt, P53 and IGF1 pathways (Figure 2G). These results suggested that miR-483-3p was associated with multiple biological functions and pathways including apoptosis, implying the importance of miR-483-3p in functional regulation.
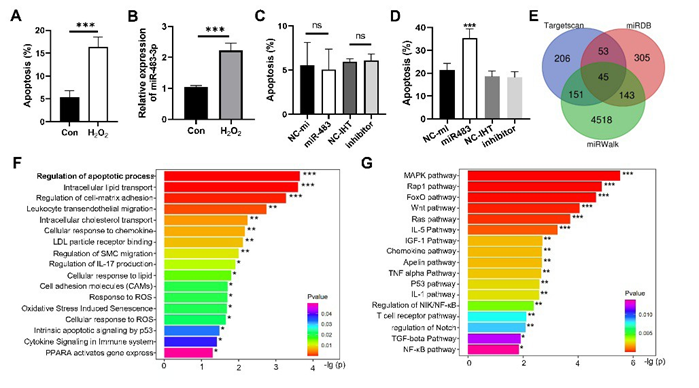
Figure 2: Hydrogen peroxide-induced apoptosis, miR-483-3p expression, and their interplay in macrophages and the predicted target genes of miR-483-3p. (A,B) Comparison of apoptosis rates (A) or miR-483-3p expression levels (B) in RAW264.7 cells treated with 10 nM (Con) or 300 μM H2O2 (H2O2). (C,D) Comparison of apoptosis rates in RAW264.7 cells (C) or 300 μM H2O2-treated RAW264.7 cells (D) with various treatments. NC-mi: treatment with negative control for miR-483-3p; NC-IHT: treatment with negative control for miR-483-3p inhibitor. Inhibitor: treatment with miR-483-3p inhibitor. (E) Venn diagram of the potential target genes of miR-483-3p predicted by TargetScan, miRWalk and miRDB. (F,G) Significantly enriched biological processes (F) and KEGG signaling pathways (G) of the potential target genes of miR-483-3p. Student’s t was used for (A,B). ANOVA test was used for (C,D). *: p < 0.05, **: p < 0.01, ***: p < 0.001. ns: no significance.
MiR-483-3p targeted MED1 gene to inhibit its expression in macrophages
MED1 was predicted to be a target gene of miR-483-3p. The results of RNAhybrid also indicated a binding site of miR-483-3p seed sequence in the 3’-UTR sequence of MED1, and the binding energy was as low as -22.4 kcal/mol (Figure 3A). In addition, the highconcentration H2O2 significantly reduced MED1 expression at mRNA and protein level in macrophages (Figure 3B,3C). Moreover, MED1 expression levels were significantly negatively correlated with miR-483-3p expression levels (Figure 3D). At the mRNA level, miR-483-3p mimic markedly down-regulated MED1 expression in macrophages whereas the effect of miR-483-3p inhibitor was opposite (Figure 3E). At the protein level, miR-483-3p mimic also remarkably decreased MED1 expression, but miR-483-3p inhibitor had no obvious effect on MED1 expression (Figure 3F). Additionally, the pmir-GLO dual luciferase reporter vector containing wild-type (WT) or mutated (Mut) 3’-UTR sequence of MED1 were constructed and respectively co-transfected with miR-483-3p mimic or its negative control (NC) into 293T cells. The results showed that the co-transfection of WT dual luciferase reporter vector and miR-4833p mimic significantly decreased the luciferase activity whereas co-transfection of Mut dual luciferase reporter vector and miR-483-3p mimic had no influence on the luciferase activity (Figure 3G). These results demonstrated that MED1 is a target gene of miR-483-3p, and its expression is down-regulated by miR-483-3p.
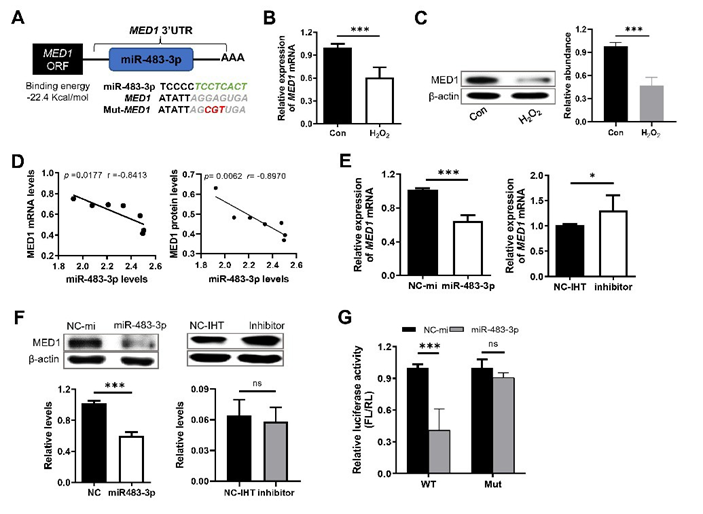
Figure 3: MED1 was the target gene of miR-483-3p. (A) The predicted binding site and binding energy of miR-483-3p in 3’-UTR of MED1 gene. Green letters indicate the sequence in MED1 3’-UTR that complementarily binds to the miR-483-3p seed region. Red italic letters: the mutation of the binding sequence used to construct the dual luciferase reporter vector. (B,C) Comparison of MED1 expression at mRNA (B) and protein (C) level in RAW264.7 cells treated with 10 nM (Con) or 300 μM H2O2 (H2O2). (D) Spearman correlation between the expression levels of MED1 mRNA (left panel) or protein (right panel) and miR-483-3p levels. (E, F) Relative expression of MED1 mRNA (E) and protein (F) in RAW264.7 cells with various treatments. (G) Relative luciferase active (FL/RL) detected by dual luciferase reporter assay. H293T cells were respectively co-transfected with the dual luciferase reporter vector containing the widetype (WT) or mutant (Mut) sequence of the miR-483-3p binding site in MED1 3’-UTR and miR-483-3p mimic or its negative control. NC-mi: treatment with negative control for miR-483-3p; NC-IHT: treatment with negative control for miR-483-3p inhibitor. Inhibitor: treatment with miR-483-3p inhibitor. Statistical analysis was performed using student’s t. Spearman’s correlation analysis was used for (D). *: p < 0.05, ***: p < 0.001. ns: no significance.
miR-483-3p enhanced H2O2-induced macrophage apoptosis through MED1-p53/p21 pathway in concert with its host gene IGF2
MiR-483-3p is a typical intron miRNA located in the second intron of IGF2 gene (Figure 4A). Our results showed that high-concentration H2O2 significantly increased expression of IGF2 mRNA and protein in macrophages (Figure 4B). IGF2 expressions were positively correlated with levels of miR-483-3p expression (Figure 4C). These results indirectly implied that miR-483-3p and its host gene IGF2 might share the same transcriptional regulatory elements. Additionally, miR-483-3p mimic remarkably upregulated the expression of p53 and p21 in macrophages. However, the overexpression of MED1 significantly diminished the above effects of miR-483-3p mimic and reduced the apoptosis of macrophages induced by H2O2 (Figure 4D,4F). We found that as a secreted protein, while IGF2 treatment alone did not influence H2O2 -induced macrophage apoptosis, the combination of IGF2 and miR-483-3p could significantly increase the apoptosis of macrophage induced by H2O2. Moreover, MED1 overexpression also significantly abrogates the synergistic effect of IGF2 and miR-483-3p in promoting hydrogen peroxide-induced macrophage apoptosis. The results indicated a synergistic effect of miR-4833p and its host gene on regulating macrophage apoptosis (Figure 4G). The above results demonstrated that miR-483-3p could enhance H2O2-induced macrophage apoptosis through its target gene MED1 and downstream p53/p21 in concert with its host gene IGF2.
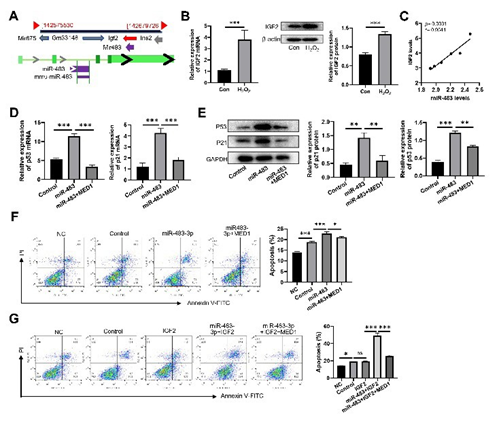
Figure 4: miR-483-3p promoted H2O2-induced macrophage apoptosis through MED1-p53/p21 in concert with its host gene IGF2. (A) The genomic positions of miR-483-3p and its host gene IGF2. (B) Comparison of expression levels of IGF2 mRNA (left panel) and protein (right panel) in RAW264.7 cells treated with 10 nM H2O2 (Con) and 300 μM H2O2 (H2O2). (C) Spearman correlation between the relative expression levels of IGF2 mRNA and miR-483-3p. (D, E) Comparison of relative expression of p53 and p21 at mRNA (D) and protein levels (E) in RAW264.7 cells with various treatments. (F) Comparation of apoptosis rate of RAW264.7 cells with various treatments. NC: without treatment. (G) Comparation of apoptosis rate of RAW264.7 cells with various treatments. NC: without treatment; Control: treatment with 10 nM H2O2; miR-483-3p: treatment with 300 μM H2O2 and miR483-3p; IGF2: treatment with 300 μM H2O2 and IGF2; miR4833p+MED1: treatment with 300 μM H2O2, miR-483-3p and MED1; miR483-3p+IGF2: treatment with 300 μM H2O2, miR-483-3p and IGF2 protein; miR483-3p+IGF2+MED1: treatment with 300 μM H2O2, miR-483-3p, MED1 and IGF2 protein. Statistical analysis was performed using student’s t (B) and ANOVA test (DG). Spearman’s correlation analysis was used for (C). *: p < 0.05. **: p < 0.01. ***: p < 0.001. ns: no significance.
γ-GC alleviated H2O2-induced macrophage apoptosis by down-regulating miR-483-3p
Known for its antioxidant properties, the effects of γ-GC on the oxidative stress-induced macrophage apoptosis and the miR-4833p pathway were examined. Our results demonstrated that γ-GC treatment significantly reduced the H2O2-induced macrophage apoptosis (Figure 5A). Additionally, qPCR analysis revealed that γ-GC substantially suppressed the upregulation of miR-4833p induced by high-concentration H2O2 (Figure 5B). γ-GC also notably restored MED1 expression levels while reversing the H2O2-induced elevation of P53 expression (Figure 5C). These data indicate that γ-GC can alleviate oxidative stress-induced macrophage apoptosis through the inhibition of the miR-483-3p pathway.
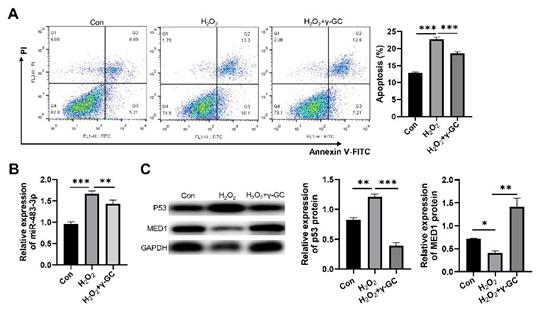
Figure5: γ-GC alleviated H2O2-induced macrophage apoptosis through inhibiting miR-483-3p pathway. (A) Comparison of apoptosis rates of RAW264.7 cells with various treatments. (B) Comparison of relative expression levels of miR-483-3p in RAW264.7 cells with various treatments. (C) Comparison of relative expression levels of p53 and MED1 protein. Con: treatment with 10 nM H₂O₂; H₂O₂: treatment with 300 μM H₂O₂; H₂O₂+γGC: treatment with 300 μM H₂O₂ and 4 mM γ-GC. Statistical analysis was performed using ANOVA tests. n ≥ 3. *: p < 0.05. **: p < 0.01. ***: p < 0.001.
Discussion
Our study revealed that serum γ-GC levels were significantly reduced in AAA patients. The levels of macrophage apoptosis, intronic miR-483-3p and its host gene IGF2 expression were increased in aneurysm tissue of AAA mice. Moreover, miR-4833p significantly enhanced H2O2-induced macrophage apoptosis by down-regulating its target gene MED1 and up-regulating downstream p53/p21 expression in concert with its host gene IGF2. Notably, γ-GC suppressed miR-483-3p expression and its downstream signaling induced by H2O2, thereby attenuating oxidative stress-induced macrophage apoptosis.
MiR-483-3p widely regulates apoptosis in various cell types, with its effects varying significantly among different cell types. In hepatocellular carcinoma cells, miR-483-3p inhibits apoptosis by targeting PUMA while in squamous carcinoma cells, it promotes apoptosis Our study showed that miR-483-3p promoted H2O2induced macrophage apoptosis through its target gene MED1 and downstream p53/p21. This functional diversity suggests that miRNA activity is regulated by multiple factors, including the cellular microenvironment and genetic context. Notably, although IGF2, the host gene of miR-483-3p, plays a critical role in cell growth, its role in oxidative stress-induced macrophage apoptosis remains unclear. Our findings in this study suggest that IGF2 enhanced the pro-apoptotic effect of miR-483-3p on macrophages. In general, intronic miRNAs are normally expressed in coordination with host genes via sharing transcription elements. However, the mechanism of regulation among miRNAs and host genes is complex. Some miRNAs can directly activate host gene transcription through mRNA interactions, for instance, miR483-5p binds to the 5′-UTR of IGF2 mRNA and enhances its expression [24]. Alternatively, they may indirectly upregulate host genes by targeting negative transcriptional regulators. miR-499 upregulates the expression of the host gene Myh7b by targeting the transcription factor Sox6 [25]. Conversely, there are some intronic miRNAs whose expression and function are not consistent with their host genes. In glioblastoma, miR-744 targets the TGFB1 gene and negatively regulates the function of the host gene MAP2K4 [26]. MiR-483-3p expression is reported to be increased in many tumors, however its host gene IGF2 does not always have the same trend. In HepG2 cells cultured in a low-glucose medium, the expression of miR-483-3p and IGF2 changed in the opposite direction [27]. These seemingly contradictory results illustrate that the regulation mechanism of intronic miRNAs and their host genes might be more complicated than we now know.
ROS are major contributors to oxidative stress and play a critical role in the pathogenesis of AAA. Under normal physiological conditions, macrophages produce ROS against invading microorganisms [28]. However, excessive ROS can lead to macrophage apoptosis. For example, oxidative stress mediated by ox-LDL activates the endoplasmic reticulum stress-CHOP pathway, thereby inducing macrophage apoptosis [29]. Macrophage apoptosis also promotes the formation of necrotic cores in advanced plaques, leading to plaque rupture and acute thrombosis [30]. Research has shown that pyroptosis of macrophages is associated with the formation and progression of AAA, where the purinergic receptor P2X7 in macrophages activates caspase-1, triggering the pyroptotic pathway and promoting AAA development [31]. The role and mechanism of miRNA in the apoptosis of macrophages induced by oxidative stress have not been fully elucidated. It has been shown that miR-140-5p targets TLR4 and inhibits oxLDL-induced oxidative stress and macrophage apoptosis [32]. Furthermore, miR-146a-5p/TRAF6 axis mediates the inhibitory effect of Pterostilbene (PTE) on macrophage pyroptosis, providing a protective effect in AAA [33]. However, there is still a lack of reports on the regulation of macrophage apoptosis by miRNAs under oxidative stress in the context of AAA. Our findings revealed that miR-483-3p promoted oxidative stress-induced macrophage apoptosis by targeting MED1. Nonetheless, to fully elucidate the role of miRNA in oxidative stress-induced macrophage apoptosis, it is necessary to discover more related miRNAs.
GSH is a pivotal endogenous antioxidant that plays a fundamental role in maintaining oxidative-reductive homeostasis, protecting tissues and organs from oxidative stress-induced damage. However, the extremely low oral bioavailability of GSH greatly limits its clinical application. γ-Glutamylcysteine, a direct precursor of GSH, exhibits high oral bioavailability. Unlike GSH, γ-GC demonstrates a dose-dependent capacity to effectively scavenges ROS, mitigate oxidative stress and regulate various cellular functions [34]. Additionally, γ-GC could suppress cadmium-induced PC12 cell apoptosis by attenuating oxidative stress and inhibiting MAPK and PI3K/Akt signaling pathways [10]. Our study revealed that γ-GC alleviated oxidative stress-induced macrophage apoptosis by downregulating miR-483-3p expression and restoring the levels of its target gene, MED1. As a life-threatening large vessel disease, the main treatments for AAA remain insufficient to effectively slow disease progression. Given the critical role of oxidative stress in inflammation and AAA pathogenesis, our findings suggest that γ-GC has therapeutic potential for AAA, offering a valuable avenue for developing novel anti-inflammatory and AAA therapies.
In conclusion, our study observed significant increases in macrophage apoptosis, intronic miR-483-3p and its host gene IGF2 expression in AAA tissue, accompanied by a pronounced downregulation of γ-GCS. Oxidative stress significantly upregulated the expression of miR-483-3p and IGF2 in macrophages. Moreover, miR-483-3p promoted H2O2-induced macrophage apoptosis by regulating the expression of its target gene MED1 and downstream p53/p21. IGF2 facilitated the pro-apoptotic effects of miR-483-3p, while IGF2 alone did not influence macrophage apoptosis. Furthermore, γ-GC can effectively alleviate macrophage apoptosis induced by oxidative stress through inhibiting miR-4833p expression. Our findings elucidate the role and mechanism of intronic miRNAs in oxidative stress-induced macrophage apoptosis, deepening our understanding of AAA pathogenesis and identifying potential targets for therapeutic strategies.
Ethics Statement
This study was approved by the Ethics Committee of Shandong University Qilu Hospital (Approval No. 2018-110). All procedures involving human participants adhered to the principles outlined in the Declaration of Helsinki and were conducted in accordance with applicable local laws and institutional guidelines. All animal procedures followed the US NIH Guide for the Care and Use of Laboratory Animals.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Funding
This work was supported by Shandong Province Health Science and Technology Innovation Team Construction Project.
Authors’ Contributions
Zhe Wang, Xiaoming Zhou designed the study. Xianlun Yin and Wenwen Teng conducted the study and collected data. Xiaowei Wang and Jing Gao analysed the data. Junjie Ma interpretated the data. Xianlun Yin and Wenwen Teng wrote the manuscript. Zhe Wang, Xiaoming Zhou and Junjie Ma reviewed and revised the manuscript. All authors read and approved the final manuscript.
References
- Antoniou GA, Antoniou SA, Torella F (2025) Editor’s Choice - Endovascular vs. Open Repair for Abdominal Aortic Aneurysm: Systematic Review and Meta-analysis of Updated Peri-operative and Long Term Data of Randomised Controlled Trials. Eur J Vasc Endovasc Surg 59(3): 385-397.
- Zhao G, Lu H, Chang Z, Zhao Y, Zhu T, et al. (2021) Single-cell RNA sequencing reveals the cellular heterogeneity of aneurysmal infrarenal abdominal aorta. Cardiovasc Res 117(5): 1402-1416.
- Yuan Z, Lu Y, Wei J, Wu J, Yang J, et al. (2021) Abdominal Aortic Aneurysm: Roles of Inflammatory Cells. Front Immunol 11: 609161.
- Castaneda OA, Lee SC, Ho CT, Huang TC (2017) Macrophages in oxidative stress and models to evaluate the antioxidant function of dietary natural compounds. J Food Drug Anal25(1): 111-118.
- Xu JM, Shi GP (2012) Emerging role of mast cells and macrophages in cardiovascular and metabolic diseases. Endocr Rev 33(1): 71-108.
- Zhang X, Li F, Wang W, Xiao X, Liu B, et al. (2020) Macrophage pyroptosis is mediated by immunoproteasome subunit β5i (LMP7) in abdominal aortic aneurysm. Biochem Biophys Res Commun 533(4): 1012-1020.
- Fujii T, Yamawaki-Ogata A, Terazawa S, Narita Y, Mutsuga M (2024) Administration of an antibody against apoptosis inhibitor of macrophage prevents aortic aneurysm progression in mice. Sci Rep 14(1): 15878.
- Wang X, Chen B, Sun J, Zhang P, Zhang H,et al. (2018) Iron-induced oxidative stress stimulates osteoclast differentiation via NF-κB signaling pathway in mouse model. Metabolism 83: 167-176.
- Chen Q, Wang Q, Zhu J, Xiao Q, Zhang L (2018) Reactive oxygen species: key regulators in vascular health and diseases. Br J Pharmacol 175(8): 1279-1292.
- Bi A, Guo Z, Yang G, Huang Y, Yin Z, et al. (2022) γ-glutamylcysteine suppresses cadmium-induced apoptosis in PC12 cells via regulating oxidative stress. Toxicology 465: 153029.
- Nakamura YK, Dubick MA, Omaye ST (2012) γ-Glutamylcysteine inhibits oxidative stress in human endothelial cells. Life Sci 90(3-4): 116-121.
- Coppola L, Grassia A, Verrazzo G, Tirelli A, Giunta R, et al. (1992) Glutathione (GSH) improved haemostatic and haemorheological parameters in atherosclerotic subjects. Drugs Exp Clin Res 18(11-12): 493-498.
- Liu Y, Chen Z, Li B, Yao H, Braidy N, et al. (2021) Supplementation with γ-glutamylcysteine (γ-GC) lessens oxidative stress, brain inflammation and amyloid pathology and improves spatial memory in a murine model of AD. Neurochem Int 144: 104931.
- Yang Y, Li L, Hang Q, Luo L, Yin Z, et al. (2019) γ-glutamylcysteine exhibits anti-inflammatory effects by increasing cellular glutathione level. Redox Biol 20: 157-166.
- Zhang H, Zhang SJ, Lyn N, Forman HJ, Davies Kj, et al. (2020) Down regulation of glutathione and glutamate cysteine ligase in the inflammatory response of macrophages. Free Radic Biol Med 2020;158: 53-59.
- Canfrán-Duque A, Rotllan N, Zhang X, Zhang X, Busto R, et al. (2017) Macrophage deficiency of miR-21 promotes apoptosis, plaque necrosis, and vascular inflammation during atherogenesis. EMBO Mol Med 9(9): 1244-1262.
- Zhuang X, Li R, Maimaitijiang A, Shi H, Gao X, et al. (2019) miR-2213p inhibits oxidized low-density lipoprotein induced oxidative stress and apoptosis via targeting a disintegrin and metalloprotease-22. J Cell Biochem 120(4): 6304-6314.
- Pepe F, Visone R, Veronese A (2018) The Glucose-Regulated MiR483-3p Influences Key Signaling Pathways in Cancer. Cancers (Basel) 10(6): 181.
- Veronese A, Lupini L, Consiglio J, Vison R, Croce CM, et al. (2010) Oncogenic role of miR-483-3p at the IGF2/483 locus. Cancer Res 70(8): 3140-3149.
- Bertero T, Bourget-Ponzio I, Puissant A, Laubat A, Ponzio G, et al. Tumor suppressor function of miR-483-3p on squamous cell carcinomas due to its pro-apoptotic properties. Cell Cycle 12(14): 2183-2193.
- Tian Z, Zhang Y, Zheng Z, Zhang M, Zhang T, et al. (2022) Gut microbiome dysbiosis contributes to abdominal aortic aneurysm by promoting neutrophil extracellular trap formation. Cell Host Microbe 30(10): 1450-1463.
- Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, et al. (2013) Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14: 128.
- Kuleshov MV, Jones MR, Rouillard AD, Jenkins SL, Wang Z, et al. (2016) Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 44(W1): W90-W97.
- Liu M, Roth A, Yu M, Haber DA, Shioda T,et al. (2013) The IGF2 intronic miR-483 selectively enhances transcription from IGF2 fetal promoters and enhances tumorigenesis. Genes Dev 27(23): 2543-2548.
- Yeung F, Chung E, Guess MG, Bell ML, Leinwand LA (2012) Myh7b/ miR-499 gene expression is transcriptionally regulated by MRFs and Eos. Nucleic Acids Res40(15): 7303-7318.
- Hübner M, Hinske CL, Effinger D, Kreth S, Thon N, 92018) et al. Intronic miR-744 Inhibits Glioblastoma Migration by Functionally Antagonizing Its Host Gene MAP2K4. Cancers (Basel) 10(11): 400.
- Lupini L, Pepe F, Ferracin M, Braconi C,Visone R, et al. 92016) Overexpression of the miR-483-3p overcomes the miR-145/TP53 proapoptotic loop in hepatocellular carcinoma. Oncotarget 7(21): 3136131371.
- Tran N, Mills EL (2024) Redox regulation of macrophages. Redox Biol 72: 103123.
- Yao S, Tian H, Zhao L, Qin S, Yang N, et al. (2017) Oxidized high density lipoprotein induces macrophage apoptosis via toll-like receptor 4-dependent CHOP pathway. J Lipid Res 58(1): 164-177.
- De Meyer GRY, Zurek M, Puylaert P, Martinet W (2024) Programmed death of macrophages in atherosclerosis: mechanisms and therapeutic targets. Nat Rev Cardiol 221(5): 312-325.
- Sun L, Li X, Luo Z, Li J, Shu C, et al.(2023) Purinergic receptor P2X7 contributes to abdominal aortic aneurysm development via modulating macrophage pyroptosis and inflammation. Transl Res 258: 72-85.
- Liu H, Mao Z, Zhu J, Shen M, Chen F (2021) MiR-140-5p inhibits oxidized low-density lipoprotein-induced oxidative stress and cell apoptosis via targeting toll-like receptor 4. Gene Ther 28(7-8): 413421.
- Cai H, Huang L, Wang M, Liu R, Yao X, et al. (2024) Pterostilbene alleviates abdominal aortic aneurysm via inhibiting macrophage pyroptosis by activating the miR-146a-5p/TRAF6 axis. Food Funct 15(1): 139-157.
- Quintana-Cabrera R, Fernandez-Fernandez S, Bobo-Jimenez V, Escobar J, Sastre J, et al. (2012) γ-Glutamylcysteine detoxifies reactive oxygen species by acting as glutathione peroxidase-1 cofactor. Nat Commun 3: 718.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.