Interpregnancy Interval, Intention to Breastfeed and Breastfeeding Initiation Among Women with Class 3 Obesity
by Leandro Cordero1*, Michael R. Stenger1, Mark B. Landon2, Carl H. Backes1, Sara Conroy1, Craig A. Nankervis1
1Department of Pediatrics, The Ohio State University, Columbus, OH USA
2Department of Obstetrics and Gynecology, The Ohio State University, Columbus, OH USA
*Corresponding author: Leandro Cordero, Department of Pediatrics, The Ohio State University, 410 W. 10th Ave., Columbus, Ohio, USA
Received Date: 03 July 2025
Accepted Date: 10 July 2025
Published Date: 15 July 2025
Citation: Cordero L, Stenger MR, Landon MB, Backes CH, Conroy S, et al. (2025) Interpregnancy Interval, Intention to Breastfeed and Breastfeeding Initiation Among Women with Class 3 Obesity. Arch Pediatr 10: 328. https://doi.org/10.29011/2575-825X.100328
Abstract
Background: Interpregnancy intervals (IPI) relate to adverse perinatal outcomes, however, associations of IPIs, severe obesity and breastfeeding (BF) are underreported. Objective: To estimate differences in comorbidities and in intention to BF as declared prior to delivery with infant feeding at discharge among women with Class 3 obesity stratified by short (< 18 months), intermediate (18-59 months) or long (≥ 60 months) IPIs. Methods: Retrospective study of 339 women with Class 3 obesity who delivered live births at ≥ 34 weeks gestation. IPI was calculated from the first live birth to the conception of the next. Results: Overall, 95 (28%) women with short and 160 (47%) with intermediate IPI were similar. Conversely, 84 (25%) in the long group differed from the intermediate in intention to BF (55 vs 68%), prior BF (46 vs 61%), age (31 vs 29y), advanced maternal age (30 vs 13%), public healthcare assistance (70 vs 55%), A2 gestational diabetes (17 vs 6%), chronic hypertension on meds (21 vs 6%), median pregestational weight (129 vs 115kg) and weight at delivery (140 vs 126kg), primary cesarean (23 vs 10%), NICU admission (19 vs 13%) and hypoglycemia (29 vs 16%). At discharge, exclusive BF (21 vs 36%) and BF initiation (48 vs 66%) rates were lower, while formula feeding (52 vs 34%) was higher. Conclusions: Women with Class 3 obesity and long IPI are associated with severe comorbidities and lower intention to BF and BF initiation rates. Modifiable factors need to be addressed if the benefits of BF are to be achieved.
Keywords: Interpregnancy Interval; Breastfeeding; Class 3 Obesity.
Background
The relationship between birth spacing and perinatal outcomes has been a concern of healthcare providers for a long time [13]. Years ago, the World Health Organization (WHO) endorsed a 24-month interval between the delivery (previous) and the conception of the next (subsequent) pregnancy [4]. More recently, the American College of Obstetricians and Gynecologists (ACOG) recommended women avoid an interpregnancy interval (IPI) shorter than 6 months and longer than 60 months [5].
Many early reports focused on the association of short IPI (<18 months) with a high incidence of preterm birth and low birthweight while recent work highlights a risk of severe maternal morbidities and a higher risk of infant mortality after long (≥ 60 months) IPIs [6-9]. Other studies provided limited but consistent evidence that short IPI is associated with an increased risk of obesity and gestational diabetes (GDM) in the subsequent pregnancy [6,10].
Obesity is the most common medical condition that affects women of reproductive age globally [11-12]. Obesity before and during pregnancy are major risk factors for pregnancy loss, gestational diabetes mellitus (GDM) and pregestational diabetes mellitus (PGDM), hypertensive conditions, labour complications and severe maternal morbidities [11-14].
The benefits of lactation on short and long term maternal and infant health have been clearly documented [15-17]. Exclusive BF or any BF during birth hospitalization and through the first postpartum year are important for healthy women and for those with severe maternal comorbidities including obesity, chronic hypertension (CHTN), preeclampsia, GDM and PGDM that may interfere or delay BF initiation or BF duration [17-21]. Despite numerous publications on birth spacing and perinatal outcomes, associations between IPI, obesity and BF among sibships remain underreported [3,6,22].
Objective
The main objective was to estimate differences of IPIs stratified by short (< 18 months), intermediate (18-59 months) or long (≥ 60 months) with comorbidities and intention to BF by women with Class 3 obesity and infant feeding [exclusive BF (EBF), formula feeding (FF) or partial BF (BF/FF)] at discharge [4,5].
Subject and Methods
This retrospective cohort investigation was approved by the Biomedical Sciences Institutional Review Board at The Ohio State University [IRB 2024H0198]. Electronic maternal and neonatal records (2013-21) were reviewed. In agreement with the WHO and the ACOG, we categorized women with Class 1 (29-34 kg/m2), Class 2 (35-39 kg/m2) and Class 3 obesity (low 40-49 kg/m2 and high ≥ 50 kg/m2) [4-5,22]. The study included women with Class 3 obesity who delivered at ≥ 34 weeks a first and second singleton live birth without major malformations. Advanced maternal age is defined as ≥ 35 years at delivery. Women with GDM, PGDM, CHTN, preeclampsia, anemia, polycystic ovary syndrome (PCOS), obstructive sleep apnea (OSA) and gastroesophageal reflux disease (GERD) were diagnosed and treated following established guidelines [5,23-26]. Infants born between 34 and 36 6/7 weeks of gestation were considered late preterm. Gestational weight gain (GWG) was defined as adequate, inadequate or excessive [27]. According to gestational age (GA) and birthweight, infants were categorized as small (SGA), appropriate (AGA), or large (LGA); those with birthweights ≥ 4000 g were considered macrosomic [28-29]. During the prenatal visit, on arrival to labor and delivery, and shortly after birth, women declared their past BF experience, if any, and their intended infant feeding choice (EBF, FF or BF/ FF) [20,30].
IPI was calculated as the time in months elapsed between the delivery of the first live birth (previous) and the start of the pregnancy that led to the next live birth (subsequent) [31]. The start of the subsequent pregnancy was estimated by subtracting the gestational age in weeks from the date of birth [1,6, 22]. As documented in the medical record, women with intervening obstetrical events such as miscarriages, ectopic pregnancies, and stillbirths between the previous and subsequent pregnancies were excluded [22,31-33].
Depending on the condition of the mother and their infant following delivery, maternal-infant interactions such as holding, skin to skin contact and BF were encouraged. Delivery room and postpartum maternal-infant interactions were observed and documented by the nursing staff [20,33].
Per our hospital practices, any symptomatic infants regardless of the mothers’ clinical condition, were directly transferred from the delivery room to the Neonatal Intensive Care Unit (NICU). Our family-centered care system has rooming-in available and fulltime lactation consultants whose services are offered to all women regardless of their infant feeding preference [20,33].
Screening for hypoglycemia (blood glucose < 40 mg/dl during the first 4 hours of life and < 45 mg/dl between four and twenty four hours of life) was done via serial point of care testing (AccuChek®) or by plasma glucose measurement in the laboratory (Beckman Coulter AU5800, Beckman Coulter Inc., Brea, CA, U.S.A.) starting within the first hour of life after the first feeding and every 2-4 hours thereafter as needed [33]. Asymptomatic infants in the Newborn Nursery with hypoglycemia were promptly BF or FF and those with recurrent hypoglycemia were treated with intravenous (IV) dextrose. On admission to the NICU, most infants were started on IV dextrose and those who were able to feed were BF or FF.
EBF was defined as direct feedings from the breast, expressed breast milk or donor human milk (DHM). Partial BF (BF/FF) was defined by direct BF, expressed breast milk or DHM supplemented with formula [20,33]. BF was considered initiated if during the 24 hours preceding hospital discharge, infants were EBF or BF/FF [20,33]. Due to the study design, information on infant feeding following discharge was not available.
Statistical analysis
Maternal and infant characteristics were summarized with count and percent for categorical variables and median interquartile ranges (IQR, 25th and 75th percentile) for continuous variables. Multinomial regression was used to estimate the association of IPI on both intention to BF and infant feeding type at discharge. Pregnancy, delivery and infant complications may be related to BF and occasionally may be confounders for the association of IPI on BF. These associations were explored with descriptive statistics [34-36].
Following best statistical practice and STROBE reporting guidelines, p-values are not presented in descriptive tables [3436]. For the main analyses, estimates and 95% CI intervals are presented, and the p-values are interpreted on a continuum with smaller p-values supporting evidence that the data are incompatible with the null hypothesis. All analyses were performed using R version 4.4.0 [37].
Results
Short IPI compared to intermediate IPI
Demographic and clinical maternal characteristics such as severe obesity, median age, advanced maternal age, white, African American, public healthcare assistance, current smokers, former smokers, GDM A1 and A2 combined, PGDM Type 1 and 2, CHTN on anti-hypertension medication, severe preeclampsia, vaginal delivery, primary and repeat cesarean, median pregestational weight, median weight at delivery and excessive gestational weight gain were similar between short and intermediate IPI (Table 1).
Neonatal outcomes of short vs intermediate IPI were similar in median GA, late preterm, median birth weight, admission to NICU and neonatal hypoglycemia (Table 2). However, infants in the short IPI have a higher prevalence of LGA (31 vs 17%) and macrosomia (24 vs 12%).
Table 1: Maternal and Clinical Demographics According to Interpregnancy Intervals.
|
All Patients |
< 18 months (Short) |
18-59 Months (Intermediate) |
≥ 60 Months (Long) |
|
|
Study population no. (%) |
339 (100) |
95 (28) |
160 (47) |
84 (25) |
|
BMI 40-49 kg/m2 no. (%) |
179 (53) |
46 (48) |
90 (56) |
43 (51) |
|
BMI ≥ 50 kg/m2 no. (%) |
160 (47) |
49 (52) |
70 (44) |
41 (49) |
|
Mothers age (y) median [IQR] |
29 [26,32] |
28 [24,31] |
29 [26,32] |
31 [28,37] |
|
Advanced maternal age no. (%) |
60 (18) |
14 (15) |
21 (13) |
25 (30) |
|
Race: African American no. (%) |
99 (29) |
22 (23) |
47 (29) |
30 (36) |
|
Other no. (%) |
11 (3) |
0 (0) |
5 (3) |
6 (7) |
|
White no. (%) |
229 (68) |
73 (77) |
108 (68) |
48 (57) |
|
Public healthcare assistance no. (%) |
201 (59) |
54 (57) |
88 (55) |
59 (70) |
|
Current smokers no. (%) |
33 (10) |
11(12) |
9 (6) |
13 (15) |
|
Former smokers no. (%) |
79 (23) |
16 (17) |
38 (24) |
25 (30) |
|
Gestational diabetes - A1 no. (%) |
16 (5) |
4 (4) |
7 (4) |
5 (6) |
|
A2 no. (%) |
31 (9) |
7 (7) |
10 (6) |
14 (17) |
|
Pregestational diabetes - Type 1 no. (%) |
14 (4) |
4 (2) |
7 (4) |
5 (6) |
|
Type 2 no. (%) |
14 (4) |
5 (4) |
7 (4) |
3 (4) |
|
Gestational hypertension no. (%) |
59 (17) |
18 (19) |
30 (19) |
11 (13) |
|
Chronic hypertension no. (%) |
52 (15) |
18 (19) |
24 (15) |
10 (12) |
|
Chronic hypertension on medication no. (%) |
40 (12) |
12 (13) |
10 (6) |
18 (21) |
|
Preeclampsia with severe features no. (%) |
37 (11) |
10 (11) |
14 (9) |
13 (15) |
|
Polycystic ovarian syndrome no. (%) |
23 (7) |
6 (6) |
13 (8) |
4 (5) |
|
Obstructive sleep apnea no. (%) |
38 (11) |
7 (7) |
20 (13) |
11 (13) |
|
Gastroesophageal reflux no. (%) |
39 (12) |
9 (9) |
18 (11) |
12 (14) |
|
Asthma no. (%) |
59 (17) |
17 (18) |
24 (15) |
18 (21) |
|
Anemia no. (%) |
55 (16) |
18 (19) |
24 (15) |
13 (15) |
|
Postpartum hemorrhage no. (%) |
10 (3) |
3 (3) |
3 (2) |
4 (5) |
|
Delivery mode: Primary cesarean no. (%) |
48 (14) |
13 (14) |
16 (10) |
19 (23) |
|
Repeat cesarean no. (%) |
142 (42) |
39 (41) |
68 (43) |
35 (42) |
|
Vaginal no. (%) |
149 (44) |
43 (45) |
76 (48) |
30 (36) |
|
Pregestational weight (kg) median [IQR] |
117 [102, 139] |
115 [103,136] |
115 [97,138] |
129 [108,148] |
|
Weight at delivery (kg) median [IQR] |
131 [114, 151] |
130 [114,149] |
126 [113,151] |
140 [123,154] |
|
Weight gain: Inadequate no. (%) |
23 (6) |
7 (7.4) |
10 (6) |
6 (7) |
|
Adequate no. (%) |
77 (23) |
15 (16) |
38 (24) |
24 (29) |
|
Excessive no. (%) |
239 (71) |
73 (77) |
112 (70) |
54 (64) |
|
Mother hospital stay (d) median [IQR] |
3 [3,4] |
3 [3,4] |
3 [3,4] |
4 [3,5] |
Table 2: Neonatal Outcomes According to Interpregnancy Intervals.
|
All Patients |
< 18 Months (Short) |
18-59 Months (Intermediate) |
≥ 60 Months (Long) |
|
|
Study population no. (%) |
339 (100) |
95 (28) |
160 (46) |
84 (25) |
|
Infant sex (male) no. (%) |
175 (52) |
57 (60) |
74 (48) |
44 (52) |
|
Gestational age (w) median [IQR] |
39 [37,39] |
39 [38,39] |
39 [37,39] |
38 [37,39] |
|
Late preterm (34-36 wks.) no. (%) |
38 (11) |
8 (8) |
16 (10) |
14 (17) |
|
Birthweight (g) median [IQR] |
3458 [3133,3822] |
3470 [3198,3966] |
3540 [3203,3815] |
3345 [3023,3651] |
|
Intrauterine fetal growth |
||||
|
Small for gestation no. (%) |
12 (4) |
2 (2) |
7 (4) |
3 (4) |
|
Appropriate for gestation no. (%) |
253 (74) |
64 (67) |
126 (79) |
63 (75) |
|
Large for gestation no. (%) |
74 (22) |
29 (31) |
27 (17) |
18 (21) |
|
Macrosomia no. (%) |
55 (16) |
23 (24) |
19 (12) |
13 (15) |
|
Neonatal hypoglycemia no. (%) |
62 (18) |
12 (13) |
26 (16) |
24 (29) |
|
Admission to NICU no. (%) |
49 (14) |
12 (13) |
21 (13) |
16 (19) |
|
Infant length of stay (d) median [IQR] |
2 [2,3] |
2 [2,3] |
2 [2,3] |
2 [2,3] |
|
Prior breastfeeding experience no. (%) |
191 (56) |
54 (57) |
98 (61) |
39 (46) |
|
Lactation assistance no. (%) |
250 (74) |
75 (79) |
116 (73) |
59 (70) |
|
Maternal prenatal intention to feed |
||||
|
Breastfeeding no. (%) |
223 (66) |
69 (73) |
108 (68) |
46 (55) |
|
Partial breastfeeding no. (%) |
41 (12) |
8 (8) |
18 (11) |
15 (18) |
|
Formula only no. (%) |
75 (22) |
18 (19) |
34 (21) |
23 (27) |
|
Mothers time to the first breastfeeding |
||||
|
1 hour no. (%) |
44 (13) |
12 (13) |
23 (14) |
9 (11) |
|
2-6 hours no. (%) |
160 (47) |
49 (52) |
81 (51) |
30 (36) |
|
≥ 7 hours no. (%) |
38 (11) |
11 (12) |
15 (9) |
12 (14) |
|
Never breastfed no. (%) |
97 (29) |
23 (24) |
41 (26) |
33 (39) |
|
Infant feeding at discharge |
||||
|
Exclusive breastfeeding no. (%) |
111 (33) |
36 (38) |
57 (36) |
18 (21) |
|
Partial breastfeeding no. (%) |
95 (28) |
25 (26) |
48 (30) |
22 (26) |
|
Formula feeding only no. (%) |
133 (39) |
34 (36) |
55 (34) |
44 (52) |
|
Breastfeeding initiation no. (%) |
206 (61) |
61 (64) |
105 (66) |
40 (48) |
Long IPI compared to intermediate IPI
Women in the long IPI group tend to be older (31 vs 29y), of advanced maternal age (30 vs 13%), on public healthcare assistance (70 vs 55%), current smokers (15 vs 6%) and have CHTN on medication (21 vs 6%) (Table 1). Among dyads in the long IPI, vaginal delivery rate was lower (36 vs 48%) while A2 DM (17 vs 6%), median pregestational weight (129 vs 115kg) and weight at delivery (140 vs 126kg) were higher. The prevalence of low and high BMI in Class 3 obesity was similar. Compared to the intermediate group, newborns in the long IPI had similar rates of AGA, SGA, LGA and macrosomia (Table 2). Neonatal hypoglycemia (29 vs 16%) was higher in the long IPI group whereas admission to the NICU (19 vs 13%) was similar.
Intention to breastfeed and infant feeding at discharge
Intention to BF (73, 68 vs 55%) was lower and, consequently, intention to FF (19, 21 vs 27%) was higher in the long IPI as compared to the short and intermediate IPI groups (Table 2). At the time of discharge from the hospital, EBF rates were similar for the short and intermediate groups but were lower in the long IPI group (38, 36 vs 21%). While the rates of BF/FF were comparable (26, 30 vs 26%), those of FF only were higher in the long IPI group (36, 34 vs 52%).
From the multinomial regression models, estimated probabilities are shown below in Figure 1. Across all IPI, intention to EBF was higher than intention to FF or partial BF. At discharge, the distribution of feeding for short and intermediate IPI were similar, whereas long IPI had higher rates of FF as compared to BF/FF.
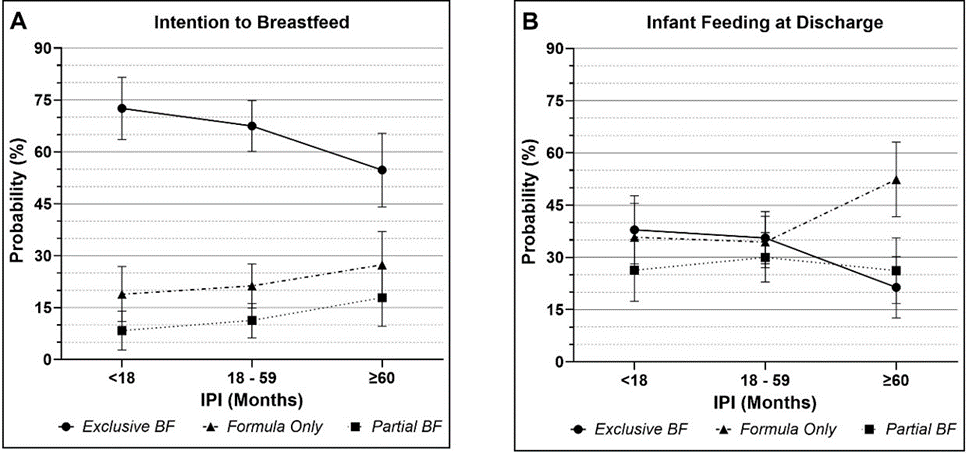
Figure 1: Estimated probabilities and 95% confidence intervals of Intention to Breastfeed (A) and Infant Feeding at Discharge (B) from multinomial logistic regression models.
The odds for intention to FF compared to EBF were lower for short vs intermediate IPI (aOR: 0.83 (95% CI 0.43, 1.58, p = 0.6)), but higher for long vs intermediate IPI (aOR: 1.59 (95% CI 0.84, 2.99, p = 0.2)). However, these estimates have wide confidence intervals. At discharge, the odds of FF vs EBF were higher for long compared to intermediate IPI (aOR: 2.53 (95% CI 1.31, 4.91, p = 0.006)).
Breastfeeding initiation
BF initiation (EBF and BF/FF combined) was observed in 64% of the short, 66% of the intermediate and 48% of women in the long IPI group. The rate of women who were unable to BF exclusively or partially during their hospitalization was similar for those in the short and intermediate groups (24 vs 26%), but it was higher (39%) for those in the long IPI group. Prior BF experience was more common among women in the intermediate and short IPI than those in the long IPI group (Table 2). Lactation assistance, regardless of the IPI, was provided to a similar number of women.
Breastfeeding experience of the previous pregnancies
A review of the 191 women with prior BF experience with that of 148 without prior BF experience showed clinical and demographic similarities except for the lower prevalence of public healthcare assistance (52 vs 70%) and current smoking (6 vs 14%) (Table 3). Additionally, prior BF experience was similar in the short IPI, more common in the intermediate (51 vs 43%) and less frequent in the long IPI (20 vs 30%). Among women with prior BF experience, their prenatal choice of infant feeding for the subsequent pregnancy was: 87% intended to BF, 12% intended partial BF, while 1% intended to FF only. In contrast, of the women with no prior BF experience, 39% intended to EBF, 13% intended BF/FF and 49% intended FF. Consequently, at discharge, women with prior BF showed higher rates of exclusive BF (45% vs 17%), BF/FF (34 vs 22%), BF initiation (79 vs 39%) and lower rates of FF (21 vs 61%).
Table 3: Neonatal Outcomes Including Breastfeeding in the Subsequent Pregnancy
|
Prior Breastfeeding |
No Prior Breastfeeding |
|
|
Mother-Infant dyads no. (%) |
191 (56) |
148 (44) |
|
BMI 40-49 kg/m2 no. (%) |
102 (53) |
78 (53) |
|
BMI ≥ 50 kg/m2 no. (%) |
89 (47) |
70 (47) |
|
Short IPI no. (%) |
54 (28) |
41 (28) |
|
Intermediate IPI no. (%) |
98 (51) |
62 (42) |
|
Long IPI no. (%) |
39 (20) |
45 (30) |
|
Infant sex (male) no. (%) |
102 (53) |
73 (49) |
|
Gestational age (w) median [IQR] |
39 [37,39] |
38 [37,39] |
|
Late preterm (34-36 wks.) no. (%) |
17 (9) |
21 (14) |
|
Birthweight (g) median [IQR] |
3540 [3203,3886] |
3419 [3074,3772] |
|
Intrauterine fetal growth |
||
|
Small for gestation no. (%) |
6 (3) |
6 (4) |
|
Appropriate no. (%) |
143 (75) |
110 (74) |
|
Large for gestation no. (%) |
42 (22) |
32 (22) |
|
Macrosomia no. (%) |
35 (18) |
20 (14) |
|
Neonatal hypoglycemia no. (%) |
27 (14) |
35 (24) |
|
Admission to NICU no. (%) |
25 (13) |
24 (16) |
|
Infant length of stay (d) median [IQR] |
2 [2,3] |
2 [2,3] |
|
Lactation assistance no. (%) |
175 (92) |
76 (51) |
|
Maternal prenatal intention to feed |
||
|
Breastfeeding no. (%) |
167 (87) |
57 (39) |
|
Partial breastfeeding no. (%) |
22 (12) |
19 (13) |
|
Formula only no. (%) |
2 (1) |
72 (49) |
|
Mothers time to the first breastfeeding |
||
|
< 1 hour no. (%) |
31 (16) |
13 (9) |
|
2-6 hours no. (%) |
118 (62) |
43 (29) |
|
≥ 7 hours no. (%) |
21 (11) |
17 (11) |
|
Never breastfed no. (%) |
21 (11) |
75 (51) |
|
Infant feeding at discharge |
||
|
Exclusive breastfeeding no. (%) |
86 (45) |
25 (17) |
|
Partial breastfeeding no. (%) |
64 (34) |
32 (22) |
|
Formula only no. (%) |
41 (21) |
91 (61) |
|
Breastfeeding Initiation no. (%) |
150 (79) |
57 (39) |
Discussion
There are a variety of ways to measure the intervals between pregnancies [1-3, 22,31]. The most frequently used is the time in months elapsed from the date of the previous live birth to the onset of the pregnancy of the subsequent live birth while excluding all interpregnancy events such as stillbirth, miscarriages or induced abortions [2,6-10]. As reported by Consuelo et al. in 2018, this approach can distort the prevalence of short IPI length and its association with perinatal outcomes [1-2,31,38]. Thus, to facilitate interpretation of our data, we included only women without intervening obstetrical events between their previous and subsequent pregnancy that resulted in a live birth [22,31]. These women serve as their own control and report demographic and clinical variables that are more likely to remain constant over time, but which may highlight real increases and changes across IPIs [3,6]. Regardless of the methodology employed, like other investigators, we recognize that many adverse maternal and neonatal outcomes are associated with short and long IPIs [69,22]. It is worth noting that recent studies suggest that adverse associations of short IPIs from high-resource settings may be limited to shorter IPIs (< 6 months or possibly 6-11 months) as opposed to the 18 or 24 months IPIs from low-resource settings [1-3].
In the current study, demographics and clinical maternal variables were similar between short and intermediate IPI except for the higher prevalence of LGA and macrosomia in the short IPI. These observations are not unusual considering that all women in our study had Class 3 obesity which is a known predictor of excessive fetal growth and macrosomia [12,21,29].
The American Academy of Pediatrics and the Academy of Breastfeeding Medicine recommend exclusive BF for all infants during birth hospitalization and beyond [17-20]. In 2019, of all infants discharged home from the hospital in the U.S., 62.3% were BF exclusively, in contrast to this sample population with 38% in the short, 36% in the intermediate and 21% in the long IPI group [17,19]. Concurrently, the national data quoted above showed that 83.6% of the general population initiated BF whereas this sample showed 64% in the short, 66% in the intermediate and 47% in the long IPI group [17,19]. Obstacles known to be associated with low EBF and BF/FF that affected our study population included lack of intention to BF, lack of prior BF experience, preeclampsia, CHTN, GDM, PGDM, obesity, complications of labor, cesarean deliveries, premature birth, neonatal hypoglycemia, admission to the NICU, formula supplementation, delayed lactogenesis II and maternal infant separation among others [18,21,26,32,39].
Women in the long and the intermediate IPI group differed in the prevalence of advanced maternal age, smoking and public healthcare assistance. Older women, especially those with obesity and those who smoke are less likely to initiate BF or to BF longer than women who do not smoke [21,23,39-41]. Efforts aimed to prevent or stop smoking during pregnancy are clearly described in the literature [40-41]. In a recent study we reported that almost 25% of women with Class 3 obesity had previously smoked and that 10% continue to smoke during the pregnancy [21]. The difference between former and current smokers observed in the long IPI group may imply some success, at least temporary, of smoking cessation programs [40-41]. Promoting BF initiation and duration in both women who smoke and do not smoke could have valuable health benefits for mothers and infants [21,40-41].
Public healthcare assistance was higher in the long IPI as compared to the intermediate IPI group, while EBF and BF initiation were lower. Recently, we reported that women with Class 3 obesity who were receiving public healthcare assistance were less likely to exclusively BF (aOR 0.521 CI 95% 0.403,0.675) or to initiate any BF (aOR 0.484 CI 95% 0.362,0.646) [21]. We agree with Greiner et al. that more research is needed to identify risk factors which affect perinatal outcomes for women with severe obesity who receive public healthcare assistance [42-43].
Earlier studies recognized a robust association of short IPI with GDM. Recently Dude et al. reported that a long IPI of > 60 months is linked with the development of GDM or PGDM on the subsequent pregnancy (aOR 2.13 CI 90% 1.44,3.15) [6,10]. Our investigation showed that at the initiation of the subsequent pregnancy the rate of A2 GDM was higher in the long IPI as compared to the short and intermediate IPI groups. This observation is in line with an earlier study where the prevalence of women with Type 2 DM increased from 59% in the short, to 68% in the intermediate and to 82% in the long IPI [22]. The critical importance of GDM in pregnancy lies in its potential for recurrence, development of severe obesity, progression to Type 2 diabetes and their effects on their offspring [13,44].
The exponential rise in the prevalence of CHTN during pregnancy seen in recent decades is largely secondary to the obesity epidemic and to increasing maternal age [11-12,21,26]. A recent study of 317 women with CHTN and 106 others with CHTN superimposed on diabetes led to lower rates of exclusive BF (19%) as well as BF initiation (63%) at discharge from the hospital [45]. Thus, the higher rates of severe CHTN observed for women in the long IPI group that coexisted with obesity, diabetes and advanced age over time are not unexpected [21,45].
Cesarean delivery regardless of its medical indication has an unavoidable effect on women who intended to BF not only due to their health condition but also to the physical separation from their infant [46]. Neonatal hypoglycemia is more common in infants with prematurity, macrosomia or born to women with obesity and diabetes, and has a negative effect on BF due to the psychological impact on the mother, the need for treatment with formula if BF alone does not correct and for the potential admission to the NICU for further treatment [21,33].
Although intention to BF is common among healthy as well as high-risk obstetrical populations, successful BF initiation rates vary significantly [18-21,33]. Our data showed that women in the long IPI not only intended to BF less often but also initiated BF at a lower rate than those in the short and intermediate IPI.
The importance of a successful prior BF experience is highlighted by the fact that 85% of women who BF their first child are likely to BF their second, whereas 78% of women who did not BF their first child, are unlikely to BF the next [42-47]. Relevant to our study prior BF experience occurs with less frequency among women in the long IPI group. Despite significant comorbidities 87% of women with prior BF experience intended to BF the subsequent infant in contrast to 39% of those women who did not BF the first infant. Considering the above, it is not unexpected that exclusive BF and BF initiation were more common among women with prior BF experience. Furthermore, a positive BF experience improves attitude, confidence, self-efficacy, motivation and renewed intention to BF [20-22, 45-48]. In contrast, a negative BF experience is usually related to maternal or neonatal morbidities or to the difficulties of lactation such as poor sucking or latch problems, perception of low milk supply, mastitis or nipple fissures [46-48].
Limitations of this study are those inherent to a retrospective design and the lack of information regarding contraception, pregnancy intention and follow-up information on infant feeding following discharge. The strength of this investigation is the methodology used to define IPI and the reporting of associations of IPI and BF initiation in a population of women with Class 3 obesity who delivered consecutive infants. Additionally, the data regarding mothers and infants was obtained directly from medical records and not from maternal recall questionnaires. Finally, to the best of our knowledge this is one of the few studies which related BF to IPI among women with Class 3 obesity.
Conclusion
Regardless of IPI group, women with Class 3 obesity demonstrate lower intention to BF and BF initiation compared to the general population. Women in the short IPI and in the intermediate IPI groups, are similar in prevalence of morbidities and in intention to BF and BF rates at discharge. Women in the long IPI group have a higher incidence and severity of morbidities and comorbidities and consequently have lower rates of intention to BF and BF initiation. However, a long IPI, if associated with BF, provides opportunities to maximize interpregnancy care and to improve the quality of life for women with Class 3 obesity and the future health of their children.
Data Availability
The data sets generated during and/or analyzed during the current study are not publicly available due to patient privacy but are available from the corresponding author on reasonable request.
Authors’ contributions
LC: Conceptualized and designed the original study, collected data, assisted with data analysis, and drafted the initial and subsequent manuscripts
CAN/MRS: Assisted with the study design and data analysis and critically reviewed and revised several drafts of the manuscript
SC: Designed and implemented statistical analysis and revised the manuscript accordingly
MBL/CHB: Reviewed and revised the manuscript for important intellectual contents
All authors approved the final manuscript as submitted and agreed with its publication
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article. Competing Interests
The authors declare no conflict of interest.
Ethics approval and consent to participate
The Ohio State University Biomedical Science IRB (#2010H0198) approved through this retrospective study with waivers of informed consent and HIPAA research authorization through 4/17/2025. All methods were performed in accordance with the relevant guidelines and regulations of the declaration of Helsinki.
References
- Ahrens KA, Hutcheon JA, Ananth CV, Basso O, Briss PA, et al. (2018) Report of the office of population affairs’ expert work group meeting on short birth spacing and adverse pregnancy outcomes: Methodological quality of existing studies and future directions for research. Paediatr Perinat Epidemiol 33: 1-10.
- Hutcheon JA, Moskosky S, Ananth CV, Basso O, Briss PA, et al. (2019) Good practices for the design, analysis, and interpretation of observational studies on birth spacing and perinatal health outcomes. Paediatr Perinat Epidemiol 33: O15-O24.
- Klebanoff MA (2019) Interpregnancy interval and outcomes beyond the neonatal period: More complicated than it seems. Paediatr Perinat Epidemiol 33: 371-373.
- World Health Organization. Report of a WHO technical consultation on birth spacing: Geneva, Switzerland 13-15 June 2005. Reference Number: WHO/RHR/07.1
- ACOG Committee Opinion ACOG Committee Opinion No. 736: Optimizing Postpartum Care (2018) Obstet Gynecol 131: e140-e150.
- Hanley GE, Hutcheon JA, Kinniburgh BA, Lee L (2017) Interpregnancy interval and adverse pregnancy outcomes: An analysis of successive pregnancies. Obstet Gynecol 129: 408-415.
- McKinney D, House M, Chen A, Muglia L, DeFranco E (2017) The influence of interpregnancy interval on infant mortality. Am J Obstet Gynecol 216: 316.e1-9.
- Thoma ME, Rossen LM, De Silva DA, Warner M, Simon AE, et al. (2019) Beyond birth outcomes: Interpregnancy interval and injuryrelated infant mortality. Paediatr Perinat Epidemiol 33: 360-370.
- Garg B, Darney B, Pilliod RA, Caughey AB (2021) Long and short interpregnancy intervals increase severe maternal morbidity. Am J Obstet Gynecol 331: e1-8.
- Dude AM, Battarbee A, Yee LM (2019) Interdelivery interval and diabetes mellitus in a subsequent pregnancy. Am J Perinatol 36: 10391044.
- ACOG Practice Bulletin #230. Obesity in pregnancy. Obstet Gynecol 2021;137: e128-37.
- Creanga AA, Catalano PM, Bateman BT (2022) Obesity in pregnancy. N Engl J Med. 387: 248-259.
- Langley-Evans SC, Pearce J, Ellis S (2022) Overweight, obesity and excessive weight gain in pregnancy as risk factors for adverse pregnancy outcomes: A narrative review. J Hum Nutr Diet 35: 250-264.
- Fox R, Kitt J, Leeson P, Aye CYL, Lewandowski AJ (2019) Preeclampsia: Risk factors, diagnosis, management, and the cardiovascular impact on the offspring. J Clin Med 8: 1625.
- Steube A (2015) Associations between lactation, maternal carbohydrate metabolism, and cardiovascular health. Clin Obstet Gynecol 2015;58: 827-839.
- Nguyen B, Jin K, Ding D (2017) Breastfeeding and maternal cardiovascular risk factors and outcomes: A systematic review. PLoS ONE 12: e0187923.
- Barrera CM, Beauregard JL, Nelson JM, Perrine CG (2019) Association of maternity care practices and policies with in-hospital exclusive breastfeeding in the United States. Breastfeed Med 14: 243-248.
- Cordero L, Stenger MR, Landon MB, Nankervis CA (2021) Breastfeeding initiation among women with preeclampsia with and without severe features. J Neonatal Perinatal Med 14: 419-426.
- Centers for Disease Control and Prevention (2022). Breastfeeding report card.
- Cordero L, Stenger MR, Landon MB, Nankervis CA (2022) Exclusive breastfeeding among women with type 1 and type 2 diabetes mellitus. BMC Pregnancy Childbirth 22: 69.
- Cordero L, Stenger MR, Landon MB, Needleman BJ, Noria S, et al. (2024) Breastfeeding initiation according to the severity of Class 3 obesity. J Neonatal-Perinatal Med 18: 70-78.
- Cordero L, Stenger MR, Landon MB, Nankervis CA (2020) Interpregnancy interval, intention to breastfeed and breastfeeding initiation among women with pregestational diabetes mellitus. Int J Women’s Health Wellness 6: 114.
- Ramji N, Challa S, Murphy PA, Quinlan J, Crane JMG, et al. A comparison of breastfeeding rates by obesity class. J Matern Fetal Neonatal Med 2018;31: 3021-3026.
- ACOG Practice Bulletin Summary #222. Gestational hypertension and preeclampsia. Obstet Gynecol 2020;135:1492.
- ACOG Practice Bulletin #201. Pregestational diabetes mellitus. Obstet Gynecol 2018;132:e228-48.
- ACOG Practice Bulletin #203. Chronic hypertension in pregnancy. Obstet Gynecol 2019;133:e26-50.
- Champion ML, Harper LM (2020) Gestational weight gain: Update on outcomes and interventions. Curr Diab Rep 20: 11.
- Olsen IE, Groveman SA, Clark RH, Zemel BS (2010)New intrauterine growth curves based on United States data. Pediatrics 125: e214-e224.
- ACOG Practice Bulletin #216 (2020) Macrosomia. Obstet Gynecol 135: e18-e35.
- Cordero L, Stenger MR, Blaney SD, Finneran MM, Nankervis CA, et al. (2020) Prior breastfeeding experience and infant feeding at discharge among women with pregestational diabetes mellitus. J Neonatal Perinatal Med 13: 563-570.
- Conzuelo-Rodriguez G, Naimi AI (2018) The impact of computing interpregnancy intervals without accounting for intervening pregnancy events. Paediatr Perinat Epidemiol 32: 141-148.
- ACOG Practice Bulletin #756 (2018). Optimizing support for breastfeeding as part of obstetrical practice. Obstet Gynecol 132: e187.
- Cordero L, Ramesh S, Hillier K, Giannone PJ, Nankervis CA. (2018) Early feeding and neonatal hypoglycemia in infants of diabetic mothers. SAGE Open Med 1: 2050312113516613.
- Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, et al. (2007) Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. Epidemiology 18: 805-835.
- Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, et al. (2016) Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol 31: 337-350.
- VanderWeele TJ (2019) Principles of confounder selection. Eur J Epidemiol 34: 211-219.
- R Core Team (2024) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing.
- Ahrens KA, Hutcheon JA (2018) Optimal birth spacing: What can we measure and what do we want to know? Paediatr Perinat Epidemiol 32: 149-151.
- ACOG 202 Society for maternal-fetal medicine (2022) Pregnancy at age 35 years or older. Obstet Gynecol 140: e221-e228.
- ACOG Committee Opinion #807 (2020). Tobacco and nicotine cessation during pregnancy. Obstet Gynecol 135: e221.
- Godleski SA, Shisler S, Eiden RD, Schuetze P (2020) Maternal Smoking and Psychosocial Functioning: Impact on Subsequent Breastfeeding Practices. Breastfeed Med 15: 246-253.
- Greiner KS, Speranza RJ, Rincón M, Beeraka SS, Burwick RM (2020) Association between insurance type and pregnancy outcomes in women diagnosed with hypertensive disorders of pregnancy. J Matern Fetal Neonatal Med 33: 1427-1433.
- Cordero L, Stenger MR, Landon MB, Needleman BJ, Noria S, et al. (2023) Bariatric Surgery to Conception Intervals and Breastfeeding Initiation. Arch Pediatr 8: 244.
- Walker E, Flannery O, Mackillop L (2020) Gestational diabetes and progression to type two diabetes mellitus: missed opportunities of follow up and prevention? Primary Care Diabetes 14: 698-702.
- Cordero L, Stenger MR, Landon MB, Nankervis CA (2022) Breastfeeding Initiation Among Women with Chronic Hypertension Superimposed on Pregestational Diabetes Mellitus. J NeonatalPerinatal Med 15:171-177.
- Columbo L, Cripp BL, Consonni D, Bettinelli ME, Agosti V, et al. (2018) Breastfeeding determinants in healthy term newborns. Nutrients 10: 48.
- Huang Y, Ouyang Y, Redding SR (2019) Previous breastfeeding experience and its influence on breastfeeding outcomes in subsequent births: A systematic review. Women Birth 32: 303-309.
- Schafer EJ, Campo S, Colaizy TT, Mulder PJ, Breheny P, et al. (2017) First-time mothers’ breast-feeding maintenance: role of experiences and changes in maternal perceptions. Public Health Nutr 20: 30993108.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.