Integrating Proctoscopic Assessment With Patient Experience of Micronized Purified Flavonoid Fraction in Treatment of Grade I–II Hemorrhoids: the IMPACT-HD
by Ashwin Porwal*
Healing Hands Clinic, Millennium Star Extension, Dhole Patil Road, Pune- 411001, Maharashtra, India
*Corresponding author: Ashwin Porwal, Consultant Proctologist & Surgeon, Healing Hands Clinic, Millennium Star Extension, Dhole Patil Road, Pune- 411001, Maharashtra, India
Received Date: 27 August 2025
Accepted Date: 03 September 2025
Published Date: 05 September 2025
Citation: Porwal A (2025) Integrating Proctoscopic Assessment With Patient Experience of Micronized Purified Flavonoid Fraction in Treatment of Grade I–II Hemorrhoids: the IMPACT-HD. J Surg 10: 11435 https://doi.org/10.29011/2575-9760.011435
Abstract
Background: Hemorrhoidal disease, characterized by symptomatic enlargement and distal displacement of anal cushions, leads to bleeding, discomfort, and reduced Quality Of Life (QoL). This study aimed to evaluate the effectiveness of Micronized Purified Flavonoid Fraction (MPFF) in alleviating clinical signs and improving patient-reported QoL in adults with Grade I–II hemorrhoids, integrating objective proctoscopic assessment with patient experience (IMPACT-HD).
Methods: This open-label, prospective pilot study enrolled 12 adults (≥18 years) with Grade I–II hemorrhoids and acute rectal bleeding (<2 days), confirmed by proctoscopy. Patients received MPFF (Daflon 1000 mg) at three tablets/day for 4 days, two/day for 3 days, and optionally one/day until Day 21. Assessments were performed at Days 0, 3, 7, 10, 14, and 21 using the HEMO-FISS-QoL questionnaire and proctoscopy to evaluate redness, swelling, bleeding, discharge, tenderness, and prolapse.
Results: Among 12 participants, 50% had Grade I and 50% had Grade II hemorrhoids. All reported constipation; 50% were overweight/obese, and 83% were non-vegetarian. At baseline, all had rectal bleeding, tenderness, discharge, and prolapse; 58% had moderate redness and 83% moderate swelling. Proctoscopic signs improved progressively: by Day 10, 25% had no redness and 75% had mild swelling; by Day 14, 50% had no redness; and by Day 21, 91% had no redness or swelling. QoL scores improved significantly across all domains (p < 0.05).
Conclusion: MPFF effectively reduced redness, swelling, and bleeding, and significantly improved QoL in patients with Grade I–II hemorrhoids. These findings support MPFF as a safe, non-invasive first-line therapy.
Keywords: Conservative Therapy; Hemorrhoids; HEMO-FISS; Micronized Purified Flavonoid Fraction; MPFF; Proctoscopy; Quality of Life
Introduction
Disorders of the anorectal region are a frequent cause of patient visits to both primary care and specialist clinics, impacting comfort, daily activities, and quality of life. Among these, Hemorrhoidal Disease (HD) is one of the most prevalent and distressing conditions, with an estimated point prevalence of 4–5% and a lifetime risk affecting up to half of the adult population. It represents one of the most common causes of anal pathology, with peak incidence reported between 45 and 65 years of age in both men and women [1]. The true incidence of HD is challenging to determine, as many affected individuals are reluctant to seek medical care. In France, for instance, 85% of patients visiting general practitioners with anal discomfort did not voluntarily disclose their symptoms [2]. Epidemiological data from Europe and Japan suggest an overall prevalence ranging from 3% to 27% [2,3]. In India, the prevalence of Grade II hemorrhoids has been reported at 42.9% [4], while a study from northeast India found that 64.5% of patients presented with third-degree hemorrhoids, 20.8% with second-degree, and 14.5% with fourth-degree [5]. Hemorrhoidal disease arises from the symptomatic enlargement and downward displacement of the normal anal cushions. When these cushions become engorged and lose their anatomical position, the resulting vascular congestion and mucosal trauma lead to bleeding, prolapse, pain, pruritus, and a marked reduction in quality of life [6]. Although rarely life-threatening, HD exerts a significant socio-economic burden through healthcare costs, work absenteeism, and reduced productivity [7]. Pathophysiologically, hemorrhoidal disease is driven by connective tissue degeneration, dilatation of the hemorrhoidal plexus, and microvascular hyperperfusion. Stasis within these engorged vessels triggers leukocyte activation and release of inflammatory mediators, which increase vascular permeability and fragility. As a result, the mucosa becomes oedematous, easily injured by stool passage, and prone to bleeding [6]. Factors such as constipation, straining, pregnancy, obesity, and prolonged sitting exacerbate these mechanisms, perpetuating venous engorgement and inflammation [8].
Patients with early-stage hemorrhoids are often hesitant to undergo invasive procedures such as rubber band ligation, injection sclerotherapy, or infrared coagulation. While effective, these interventions are frequently associated with postoperative pain, short-term functional limitations, and the need for surgical expertise [9]. This creates a practical need for rapid, effective, and well-tolerated conservative therapies that can be used as first-line or adjunctive approach to allow for postponement or even avoiding the surgical treatment. Micronized Purified Flavonoid Fraction (MPFF) offers a pharmacological approach that directly addresses the underlying pathophysiological pathway of HD [10]. Composed of 90% diosmin and 10% hesperidin in micronized form to improve bioavailability, MPFF increases venous tone, reduces capillary permeability, enhances lymphatic drainage, and inhibits leukocyte–endothelial adhesion [11]. These combined effects improve haemodynamic function, alleviate oedema, and suppress inflammatory cascades within hemorrhoidal tissue, thereby reducing bleeding, swelling, and discomfort. By targeting both vascular and inflammatory pathways, MPFF provides a sound and patient-friendly alternative or adjunct to invasive interventions, particularly in early-stage disease [12]. In a multicentre randomised controlled trial, Godeberg et al. (2021) reported significant reductions in bleeding, pain, and oedema and decreased analgesic use in the MPFF group compared with placebo [13]. A Cochrane systematic review by Sheikh P et al. (2020) further concluded that flavonoids, particularly MPFF, significantly reduce the risk of persistent symptoms and recurrence [14]. On the basis of such evidence, several European and American clinical guidelines recommend MPFF as a first-line adjunct to conservative management of HD [15,16]. Despite its widespread use in Europe and Latin America, data on MPFF in the Indian population remain limited, especially from real-world clinical practice. Local dietary patterns, bowel habits, and healthcare-seeking behaviour may influence therapeutic outcomes. This study was therefore undertaken to evaluate the efficacy and safety of MPFF in patients with HD under routine practice conditions, aiming to provide context-specific evidence that can inform treatment guidelines and optimise patient care in the region.
Materials and Methods
Study Design and Setting
This was a prospective, single-centre, observational study conducted at the Healing Hands Clinic, DP Road, Pune, Maharashtra, India. The study aimed to evaluate the clinical efficacy and patient-reported outcomes of Micronised Purified Flavonoid Fraction (MPFF) in the management of early-grade hemorrhoids. The study was carried out over a period of three weeks, with patient enrolment and follow-up visits. All procedures adhered to the ethical principles outlined in the Declaration of Helsinki, and written informed consent was obtained from all participants prior to inclusion.
Study Population
Eligible participants were newly diagnosed adult patients (≥18 years) with Grade I–II hemorrhoids, confirmed through detailed clinical history and proctoscopic examination. All enrolled patients presented with acute rectal bleeding of less than two days’ duration at the time of screening. Exclusion criteria included the presence of comorbid conditions likely to influence hemorrhoidal disease or treatment outcomes (e.g., inflammatory bowel disease, chronic liver disease), recurrent hemorrhoids, concomitant anorectal disorders such as fissures, fistulas, or abscesses, and current use of medications known to interfere with MPFF efficacy or safety. Pregnant and lactating women were also excluded.
Treatment Regimen
All participants received MPFF in the form of Daflon 1000 mg tablets. The dosing regimen was in accordance with standard prescribing practices for acute hemorrhoidal episodes: 3 tablets daily for the first four days, followed by 2 tablets daily for the subsequent three days. Based on the patient’s clinical response, treatment could be optionally continued at a reduced dose of one tablet daily until Day 21. Adherence during the first seven days was actively monitored through scheduled telephone calls, during which patients were also encouraged to report any adverse effects.
Clinical Assessment And Follow-Up
Baseline evaluation included a comprehensive history, clinical examination, and proctoscopy to assess the degree of hemorrhoidal prolapse, inflammation, discharge, and tenderness. Follow-up assessments were conducted on Days 3, 7, 10, 14, and 21. At each visit, both physician-reported clinical parameters and patient-reported Quality Of Life (QoL) scores were recorded. QoL was measured using the validated HEMO-FISS-QoL questionnaire, administered in the patient’s preferred language, to capture symptom severity, functional limitations, and psychosocial impact. Proctoscopic examination at follow-up visits was performed by the same investigator to minimise inter-observer variability.
Outcome Measures
The primary outcome was the change in HEMO-FISS-QoL score from baseline to Day 21. Secondary outcomes included the proportion of patients achieving complete resolution of rectal bleeding, reduction in clinical signs of inflammation and prolapse, and overall physician-assessed improvement.
Statistical Analysis
All data were entered into a predesigned case record form and subsequently analysed using Statistical Package For Social Sciences (SPSS), version 22.0 (IBM Corp., Armonk, NY, USA). The Shapiro–Wilk test was applied to assess normality of continuous variables. Parametric data were analysed using Student’s t-test, while non-parametric or ordinal data were assessed using the Mann–Whitney U test. Categorical variables were compared using the χ² test or Fisher’s exact test, as appropriate. Results were expressed as mean ± Standard Deviation (SD) or median (interquartile range) for continuous variables, and as frequencies and percentages for categorical variables. A two-tailed p-value of less than 0.05 was considered statistically significant.
Results
Patient Demographics and Baseline Characteristics
A total of 12 patients were enrolled in the study, comprising 8 males (66.7%) and 4 females (33.3%), with a mean age of 36.75 ± 10.75 years (range: 18–54 years). The mean weight and height were 61.07 ± 8.38 kg and 164.71 ± 15.84 cm, respectively, corresponding to a mean Body Mass Index (BMI) of 22.86 ± 3.85 kg/m². Half of the cohort (50%) were overweight or obese, 83% reported a non-vegetarian diet, and 17% had a history of hypertension. All participants had a history of constipation, and none reported cardiovascular disease or other significant comorbidities. The duration of acute rectal bleeding prior to presentation was one day in 7 patients (58%) and two days in 5 patients (42%). At baseline, 6 patients (50%) had Grade I hemorrhoids and 6 (50%) had Grade II (Table 1).
|
S. no. |
Parameter |
Value |
|
1 |
Age, years (mean ± SD) |
36.75 ± 10.75 |
|
2 |
Age range (years) |
18–54 |
|
3 |
Sex, n (%) |
Male: 8 (66.67%); Female: 4 (33.33%) |
|
4 |
Weight (kg, mean ± SD) |
61.07 ± 8.38 |
|
5 |
Height (cm, mean ± SD) |
164.71 ± 15.84 |
|
6 |
BMI (kg/m², mean ± SD) |
22.86 ± 3.85 |
|
7 |
Overweight/obese, n (%) |
6 (50%) |
|
8 |
Diet, n (%) |
Non-vegetarian: 10 (83%); Vegetarian: 2 (17%) |
|
9 |
Hypertension, n (%) |
2 (17%) |
|
10 |
Constipation history, n (%) |
12 (100%) |
|
11 |
Cardiovascular comorbidities, n (%) |
0 (0%) |
|
12 |
Haemorrhoid grade, n (%) |
Grade I: 6 (50%); Grade II: 6 (50%) |
SD: Standard Deviation, BMI: Body Mass Index
Table 1: Baseline demographic and clinical characteristics.
Proctoscopic Findings
At baseline, all patients presented with tenderness, discharge, and prolapse (100%) ( Video 1 ). Inflammatory changes were prominent: redness was moderate in 58% and severe in 25%, while swelling was moderate in 83% and severe in 17%.From Day 3 onward, a progressive reduction in redness and swelling was observed ( Video 2 ). Mild redness increased from 17% at baseline to 33% at Day 3, with severe redness persisting in 25%. By Day 7, the first cases of complete resolution were seen (8% with no redness or swelling), and moderate redness still affected over half the cohort (58%) ( Video 3 ). Day 10 marked a substantial shift, with 25% having no redness, 67% showing mild redness, and only one patient (8%) with moderate redness. By Day 14, half of the participants were free from redness, and the remainder exhibited only mild changes. On Day 21, almost all patients (91%) had no visible redness or swelling; the remaining 9% exhibited only mild findings. Swelling followed a similar course, with marked reductions from Day 7 and a rapid decline after Day 10. Tenderness, discharge, and prolapse persisted throughout follow-up in all participants (Tables 2,3). (Figure 1) shows the proctoscopic images obtained during the study, demonstrating the gradual reduction in mucosal redness and swelling over the course of treatment.
|
Baseline (n=12) |
||||
|
Severity of inflammation |
None |
Mild |
Moderate |
Severe |
|
Redness |
- |
2 (17%) |
7(58%) |
3(25%) |
|
Swelling |
- |
- |
10 (83%) |
2(17%) |
|
Tenderness |
12(100%) |
- |
- |
- |
|
Discharge |
12(100%) |
- |
- |
- |
|
Prolapse |
12(100%) |
- |
- |
- |
|
FU-Day 3 (n=12) |
||||
|
Severity of inflammation |
None |
Mild |
Moderate |
Severe |
|
Redness |
- |
4(33%) |
5(42%) |
3(25%) |
|
Swelling |
- |
3(25%) |
7(58%) |
2(17%) |
|
Tenderness |
12(100%) |
- |
- |
- |
|
Discharge |
12(100%) |
- |
- |
- |
|
Prolapse |
12(100%) |
- |
- |
- |
|
FU-Day 7 (n=12) |
||||
|
Severity of inflammation |
None |
Mild |
Moderate |
Severe |
|
Redness |
1(8%) |
4(33%) |
7(58%) |
- |
|
Swelling |
1(8%) |
3(25%) |
7(58%) |
1(8%) |
|
Tenderness |
12(100%) |
- |
- |
- |
|
Discharge |
12(100%) |
- |
- |
- |
|
Prolapse |
12(100%) |
- |
- |
- |
|
FU-Day 10 (n=12) |
||||
|
Severity of inflammation |
None |
Mild |
Moderate |
Severe |
|
Redness |
3(25%) |
8(67%) |
1(8%) |
- |
|
Swelling |
1(8%) |
9(75%) |
2(17%) |
- |
|
Tenderness |
12(100%) |
- |
- |
- |
|
Discharge |
12(100%) |
- |
- |
- |
|
Prolapse |
12(100%) |
- |
- |
- |
|
FU-Day 14 (n=12) |
||||
|
Severity of inflammation |
None |
Mild |
Moderate |
Severe |
|
Redness |
6(50%) |
6(50%) |
- |
- |
|
Swelling |
3(25%) |
8(67%) |
1(8%) |
- |
|
Tenderness |
12(100%) |
- |
- |
- |
Table 2: Findings of Proctoscopy Examination.
|
Completed Treatment Day |
Redness (n=11) |
Swelling (n=11) |
||
|
Median |
P-value |
Median |
P-value |
|
|
DAY 3 Q |
3 |
0.3173 |
3 |
0.1573 |
|
DAY 7 |
3 |
0.0196 |
3 |
0.0588 |
|
DAY 10 |
2 |
0.0021 |
2 |
0.0057 |
|
DAY 14 |
1 |
0.0024 |
2 |
0.0024 |
|
DAY 21 |
1 |
0.0025 |
1 |
0.0013 |
P-value <0.05 is considered as statistically significant; None=1, Mild=2, Moderate=3, Severe=4
Table 3: Severity of inflammation for redness and swelling compared to baseline.
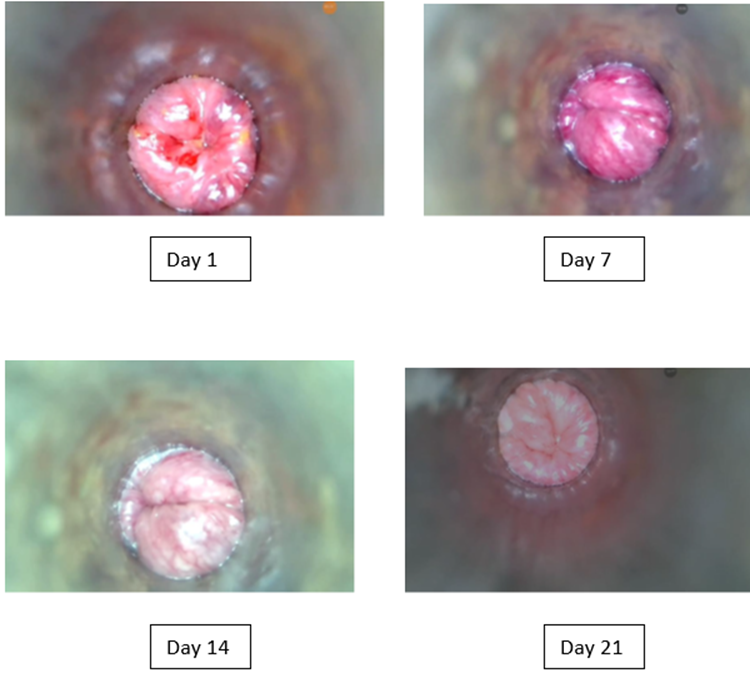
Figure 1: Serial proctoscopic images of a patient showing progressive therapeutic response to Micronized Purified Flavonoid Fraction (MPFF). At Day 1 (baseline), marked redness, swelling, and prolapse were evident. By Day 7, mucosal inflammation and swelling had partially regressed, with further improvement and only minimal residual changes by Day 14. At Day 21, near-complete resolution was observed, with restoration of normal mucosal appearance.
HEMO-FISS-Qol Questionnaire
Domain-specific analysis showed statistically significant improvements across all areas by Day 21 compared with baseline (p < 0.05 for all). The largest absolute change was observed in the defaecation domain (mean change: –83.34 ± 10.54), followed by sexuality (mean CI range: –62.50 to –50.00). Improvements in physical disorder (–47.43 ± 10.52) and psychology (–14.94 ± 7.17) were also substantial. The overall QoL score decreased from a mean of 45.51 ± 8.01 at baseline to 2.80 ± 1.84 at Day 21, corresponding to a mean change of –42.70 ± 7.57 (p < 0.05) (Table 4, Figure 2).
|
Hemo-Fiss Dimensions |
Mean (SD) |
95% CI |
P-Value |
|
Physical Disorder |
-47.4273 (10.52) |
(-54.4812, -40.3725) |
<0.05a |
|
Psychology |
-14.9364 (7.17) |
(-19.7510, -10.1217) |
<0.05a |
|
Defaecation |
-83.3364 (10.54) |
(-90.4177, -76.2551) |
<0.05a |
|
Sexuality |
- |
(-62.5000, -50.0000) |
<0.05b |
|
Overall |
-42.7018 (7.57) |
(-47.7872, -37.6165) |
<0.05a |
P < 0.05 is considered statistically significant; SD: Standard deviation; a Paired t-test; b Wilcoxon test
Table 4: Comparison of HEMO-FISS Questionnaire in baseline and Day 21 (n=11).
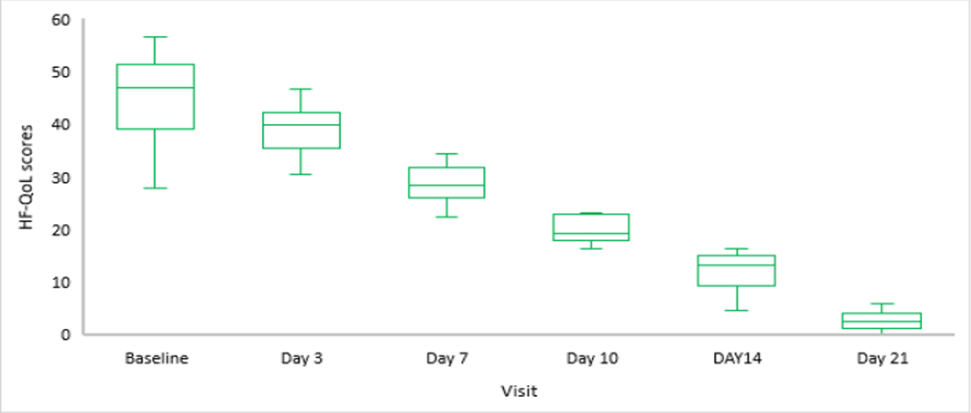
Figure 2: Box-Plot for Total HF-QoL scores at each visit (n=11).
General Outcomes
Over the 21-day observation, MPFF administration was associated with a progressive reduction in proctoscopic inflammation and significant improvements in all QoL domains. Redness and swelling showed the most visible regression by Day 14, while tenderness, discharge, and prolapse persisted but with subjective symptomatic relief reflected in QoL scores.
Discussion
The present study evaluated the therapeutic role of micronized purified flavonoid fraction in patients with hemorrhoidal disease, focusing on symptom relief, reduction in inflammation, and overall patient-reported outcomes. Our findings demonstrated a marked improvement in pain, bleeding, and perianal discomfort among patients receiving MPFF (Daflon 1000 mg), with benefits apparent within 7 days. These improvements were accompanied by a favourable tolerability profile, reinforcing the clinical utility of MPFF as an adjunct to conventional management strategies. We implemented the recommended step-down dosing of Daflon 1000mg for 7 days. We started with a higher initial dose of 3 tablets per day for 4 days for rapid symptom control. It was followed by a tapering dose of 2 tablets per day for 3 days for improved tolerability and patient adherence. It was continued optionally for 21 days at a dose of 1 tablet per day based on their response. The magnitude and timeline of symptom relief observed in this study parallels outcomes from study by Kang SI (2025), which reported significant reduction in bleeding and pain within the first week of MPFF initiation [17]. Similarly, a randomized, placebo-controlled trial by Misra et al (2000) documented complete cessation of bleeding in over 80% of patients by day 7, a pattern mirrored in our results [18]. This rapid improvement can be attributed to MPFF’s multifaceted pharmacological action, which includes reduction of capillary hyperpermeability, inhibition of inflammatory mediators (e.g., prostaglandins and free radicals), and enhancement of venous tone. Our findings regarding quality-of-life improvement align closely with the observations of Sheikh P et al. (2020), who noted that patients on MPFF scored significantly better on the HEMO-FISS-QoL index compared with standard care alone [14]. In our study, these benefits were evident not only in physical symptom relief but also in reduced discomfort during defecation and improved daily functioning, underscoring the broader functional impact of effective haemorrhoid treatment.Physician-assessed improvements in perianal inflammation, prolapse severity, and tenderness provide objective confirmation of the patient-reported gains. Comparable physician-based assessments in the French multicentre trial by Godeberge et al., (2021) revealed similar rates of edema reduction and normalization of mucosal appearance within 7–10 days of MPFF therapy [13]. This congruence between patient and physician ratings reinforces the robustness of the observed therapeutic effect.
In our study, MPFF use was associated with a rapid and sustained improvement in pain, edema, and prolapse severity, even among patients with moderate to advanced disease. These findings reinforce its role as a fast-acting adjunct to conventional management. However, a trial by Poskus T et al., (2022) observed a slower resolution of pain and edema in postpartum women, a subgroup with unique hormonal and vascular physiology that may influence treatment response [19]. Similarly, Schulten SFM et al., (2022) documented less pronounced improvement in prolapse severity, potentially attributable to the inclusion of a higher proportion of patients with advanced hemorrhoidal grades [20]. Such differences suggest that while MPFF demonstrates consistent efficacy across diverse populations, the speed and extent of clinical benefit may be modulated by patient demographics, disease stage, and associated comorbidities. The clinical implications of these findings are significant. By accelerating symptom resolution, MPFF may shorten the need for analgesics, improve adherence to dietary and lifestyle recommendations, and potentially reduce progression to surgical intervention in acute cases. The micronization process increases bioavailability, ensuring more rapid tissue penetration and sustained therapeutic levels, an aspect that may partly explain the swift improvements noted here. While the strengths of our study include real-world applicability, validated outcome measures, and alignment with prior randomized evidence, certain limitations warrant acknowledgment. The observational design precludes causal inference, and the lack of a comparator arm limits direct attribution of effects solely to MPFF. Moreover, the follow-up period was relatively short, preventing evaluation of recurrence rates or long-term prophylactic benefits. Overall, the present findings, viewed in the context of corroborative and contrasting literature, reinforce the role of MPFF as a safe and effective agent for the rapid relief of symptoms in acute hemorrhoidal disease, while also illustrating the need for further work to delineate optimal dosing regimens and population-specific outcomes.
Conclusion
MPFF (Daflon 1000 mg) administration over 07days (three tablets/day for 4 days followed by two tablets/day for 3 days, and optionally one tablet/day until Day 21) was associated with progressive resolution of proctoscopic inflammation and meaningful improvements in quality-of-life parameters in patients with hemorrhoids. Redness and swelling showed the earliest and most prominent regression, followed by gradual symptomatic relief in tenderness, discharge, and prolapse. These findings suggest that MPFF may be a valuable adjunct in conservative management, offering both objective mucosal healing and subjective symptomatic benefits within a short treatment window.
References
- Johanson JF, Sonnenberg A (1990) The prevalence of hemorrhoids and chronic constipation: an epidemiologic study. Gastroenterology 98: 380-386.
- Tournu G, Abramowitz L, Couffignal C, Juguet F, Sénéjoux A, et al (2017) Prevalence of anal symptoms in general practice: a prospective study. BMC Family Practice 18: 78.
- Asakura K, Nakano M, Omae K (2018) Relationship between bidet toilet use and hemorrhoids and urogenital infections: a 3-year follow-up web survey. Epidemiology & Infection 146: 763-770.
- Balmiki P, Jain A, Kushwah G (2025) A study on risk factors of hemorrhoidal disease among adults in a tertiary care centre of Vidisha, India. Journal of Population Therapeutics and Clinical Pharmacology 32:1686-1692.
- Bhoyate A, Priya LR, Moirangthem GS (2024) Clinicopathological profile of hemorrhoids in north east India. International Journal of Pharmaceutical and Clinical Research 16: 544-549.
- Lohsiriwat V (2012) Hemorrhoids: from basic pathophysiology to clinical management. World journal of gastroenterology: WJG 18: 2009.
- Kibret AA, Oumer M, Moges AM (2021) Prevalence and associated factors of hemorrhoids among adult patients visiting the surgical outpatient department in the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. Plos one 16: e0249736.
- Marco SD, Tiso D (2021) Lifestyle and Risk Factors in Hemorrhoidal Disease. Front Surg 8: 729166.
- Sun Z, Migaly J (2016) Review of hemorrhoid disease: presentation and management. Clinics in colon and rectal surgery 29: 22-29.
- Katsenis K (2005) Micronized Purified Flavonoid Fraction (MPFF): a review of its pharmacological effects, therapeutic efficacy and benefits in the management of chronic venous insufficiency. Curr Vasc Pharmacol 3:1-9.
- Ulloa JH (2019) Micronized Purified Flavonoid Fraction (MPFF) for Patients Suffering from Chronic Venous Disease: A Review of New Evidence. Adv Ther 36: 20-25.
- Lurie F, Branisteanu DE (2023) Improving Chronic Venous Disease Management with Micronised Purified Flavonoid Fraction: New Evidence from Clinical Trials to Real Life. Clin Drug Investig 43: 9-13.
- Godeberge P, Sheikh P, Lohsiriwat V, Jalife A, Shelygin Y (2021) Micronized purified flavonoid fraction in the treatment of hemorrhoidal disease. Journal of Comparative Effectiveness Research 10: 801-813.
- Sheikh P, Lohsiriwat V, Shelygin Y (2020) Micronized purified flavonoid fraction in hemorrhoid disease: a systematic review and meta-analysis. Advances in therapy 37: 2792-2812.
- Davis BR, Lee-Kong SA, Migaly J, Feingold DL, Steele SR (2018) The American Society of Colon and Rectal Surgeons clinical practice guidelines for the management of hemorrhoids. Diseases of the Colon & Rectum 61: 284-292.
- Rubbini M, Ascanelli S (2019) Classification and guidelines of hemorrhoidal disease: Present and future. World J Gastrointest Surg 11:117-121.
- Kang SI (2025) Latest Research Trends on the Management of Hemorrhoids. J Anus Rectum Colon 9:179-191.
- Misra MC, Parshad R (2000) Randomized clinical trial of micronized flavonoids in the early control of bleeding from acute internal hemorrhoids. British Journal of Surgery 87: 868-872.
- Poskus T, Sabonyte-Balsaitiene Z, Jakubauskiene L, Jakubauskas M, Stundiene I, et al (2022) Preventing hemorrhoids during pregnancy: a multicenter, randomized clinical trial. BMC Pregnancy Childbirth 22: 374.
- Schulten SFM, Claas-Quax MJ, Weemhoff M, van Eijndhoven HW, van Leijsen SA, et al (2022) Risk factors for primary pelvic organ prolapse and prolapse recurrence: an updated systematic review and meta-analysis. Am J Obstet Gynecol 227:192-208.
Video 1
Video 2
Video 3
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.