Inflammatory Cell Response Evaluation After Total Temporomandibular Joint Replacement in an Animal Experiment
by Maurice Y. Mommaerts1*, Louise Cras2, Denis Verwilghen3, Michael P. Ward3, Nikolas De Meurechy1,4
1European Face Centre, Universitair Ziekenhuis Brussel, Brussels, Belgium
2Department of Pathology, Universitair Ziekenhuis Brussel, Brussels, Belgium
3Sydney School of Veterinary Science, Faculty of Science, University of Sydney, Sydney, Australia
4Doctoral School of Life Sciences and Medicine, Vrije Universiteit Brussel, Brussels, Belgium
*Corresponding author: Maurice Mommaerts, Face Ahead Surgicentre, Van Putlei 35a - 2018 Antwerp, Belgium
Received Date: 16 June 2025
Accepted Date: 20 June 2025
Published Date: 23 June 2025
Citation: Mommaerts MY, Cras L, Verwilghen D, Ward MP, Meurechy ND, et al. (2025) Inflammatory Cell Response Evaluation After Total Temporomandibular Joint Replacement in an Animal Experiment. J Surg 10: 11356 https://doi.org/10.29011/2575-9760.011356
Abstract
Introduction: A sheep animal experiment was conducted to study mastication features, wear of fossa and condylar components and local tissue reaction to a novel patient-specific total Temporomandibular Joint Replacement (TMJR). This paper aims to evaluate the inflammatory response in the peri-articular tissues, as well as the amount of wear debris found in said tissues.
Materials and Methods: Thirteen TMJRs were implanted in Swifter crossbreed sheep. Postoperatively, clinical and inflammatory parameters were monitored daily and recorded weekly. After 288 days, the sheep were sacrificed, after which the peri-articular soft tissues were collected, embedded in paraffin and hematoxylin-eosin and Picrosirius Red stained. Five 0.2mm² grids per sample were applied on all samples and five randomly selected grids were analyzed for both acute and chronic inflammation, as well as the presence of macrophages. A cell count for all inflammatory cells was performed. A Kruskal-Wallis non-parametric ANOVA was used to assess differences in macrophage and lymphocyte counts between a group of coated (n= 6 sheep, 12 tissue samples and 60 grids) and uncoated (n= 7 sheep, 14 tissue samples and 70 grids) condyles. The peri-articular tissues obtained from a sham surgery served as a control group (n = 1 sheep, 2 tissues, 10 samples). A qualitative analysis of the amount of wear debris present (Grade 0-IV) was performed.
Results: A significant increase in lymphocytes was found in the DLC-coated (p = 0.018) and uncoated tissue samples (p = 0.001), compared to the control sample. A significant increase in macrophages was found in the coated samples (p = 0.019). A macrophage-to-surface count revealed that none of the animals developed a synovitis and in neither experimental group, the concentration of macrophages, nor lymphocytes exceeded the threshold as used in orthopedic literature.
Conclusion: None of samples that were examined showed any sign of ‘neo-synovitis’ caused by either wear or infection. No adverse local tissue reactions were identified. We can conclude that this TMJR can be applied for human implantation, as we do not expect any adverse reactions based on our results.
Keywords: Animal Research; Arthroplasty; Inflammation; Joint Prosthesis; Pathology; Temporomandibular Joint
Introduction
Since total Temporomandibular Joint Replacement (TMJR) was first introduced in modern medicine, many different systems have been developed [1,2]. Whilst the indications for the prosthetic replacement have become well-defined [3,4]; the quality standards these systems need to meet, remain poorly regulated. Often proper in vivo and in vitro testing of TMJR prostheses is lacking [5]. with implantation of improper materials leading to significant detrimental patient side effects [6,7].The Vitek-Kent TMJR, for instance set back the further development and use of TMJR following its poorly tested introduction. A 2mm thick articulating Teflon® coating was found to be an unsuitable articulating surface and resulted in the accumulation of wear debris several years after implantation [1]. This caused severe inflammatory reactions such as synovitis, Foreign Body Giant Cell Reactions (FBGCR), bone resorption and implant failure [1,8,9].The US Food and Drug Administration (FDA) and the American Association of Oral and Maxillofacial Surgeons (AAOMS) recommended the removal of the implant system in any patients with inflammatory symptoms. The latter illustrates the absolute importance of proper TJR evaluation, before human use [1]. Thus, in order to evaluate the novel patient-specific additively Titanium (Ti) alloy TMJ replacement system developed by CADskills BV (Ghent, Belgium) [10], three focal points were selected: wear evaluation [5]; Lateral Pterygoid Muscle (LPM) enthesis integration [11];and adverse tissue reactions. Here for, an in vivo experiment was designed. With different animal models for consideration, such as the primate, with a TMJ very similar to that of a human, ethical as well as cost and time restraint issues directed to the use of a sheep model. The latter considered as a gold standard in large animals orthopedic models [12,13]. With 128 mastication cycles per minute for 4 hours per day whilst eating and 100 cycles per minute for 8 to 9 hours per day whilst ruminating, the daily mastication rate of sheep makes it a very attractive model of TMJ research [14]. As such, 288 days of sheep mastication, equals 22 years of masticatory function of humans [15]. Previously published papers evaluated the proper implant integration and LMP enthesis reconstruction [11], as well as the linear and volumetric wear rate of the fossa and condylar components [5]. The present project aims to evaluate the amount of inflammation of the peri-articular tissues, whilst also comparing the inflammatory response in TMJR with and without a condylar Diamond-Like Carbon (DLC) coating [6]. In addition, an interpretation of foreign bodies in the peri-articular tissues was made as well.
Materials and Methods
After obtaining approval by the ethical committee at Medanex Clinic (license number LA 1210576 - code of approval EC MxCl 2018-090), fourteen ewes (Swifter crossbreed) aged 2–5 years, with an average weight of 73.4 kg (range: 52–86 kg) and without any missing teeth were enrolled in the study. Two sheep were selected for a pilot surgery. The TMJ in the first ewe was surgically approached, opened and then closed, as would be done in all other animals. However, no condylectomy or prosthesis implantation was performed, thus acting as a control group (Sham). The second sheep had its TMJ approached in similar fashion, but in this case, a TMJR was performed. By means of this pilot surgery, the surgical approach was further optimized and a standard surgical protocol was established, as elaborately described in our previously published research [5,11]. In total, thirteen sheep were implanted with a TMJR system.
Post-operative evaluation
During the first week, all sheep were kept in solitary confinement. Vital parameters (heart rate, respiratory rate, and body temperature) were evaluated daily. During the daily renewal of the compressive bandaging, the surgery site was evaluated for possible signs of infection. A blood sample was taken daily, allowing for a white cell count and formula, during the first post-operative week. An ionogram and evaluation of inflammatory parameters was performed twice during this week. After one week, all sheep were put together in a large indoor confinement, where they stayed during the remainder of the study. A weekly blood sample was taken, simultaneously allowing for a clinical evaluation as described above. 288 days after implantation, all 14 animals were euthanized. A final clinical evaluation and blood sampling was performed, prior to the euthanasia. Next xylazine 0.1mg/kg (Xyl M, V.M.D. nv, Arendonk, Belgium) was administered for induction, as well as heparine 300 IU/kg to prevent coagulation. Induction was performed by a combination of ketamine 4mg/kg (Nimatek, Dechra Pharmaceuticals PLC, Northwich, United Kingdom) and midazolam 0.2 mg/kg (Dormazolam, Le Vet Pharma BV, Oudewater, Netherlands). The product that was administered to achieve euthanasia remains undisclosed per agreement with Medanex Clinic (Diest, Belgium).
Sample processing and coloring
After euthanasia, all sheep were decapitated, and the skull was cut in half midsagitally. All the bodies and right sides of the skulls were properly disposed of. The left half was then further trimmed down by systematically removing the neurocranium, the anterior half of the mandible and maxilla, the upper half of the orbit and the orbital contents. The remaining tissue was then fixated by immersion in formaldehyde 4% for three months. Once properly fixated, all samples were rinsed for 3 days as to remove the excess formalin. The peri-articular and synovial tissues were then resected, with attention as to not contain scar tissue from the implantation surgery. The tissues were infiltrated through an increasing ethanol series and xylene process at Morphisto GmbH (Frankfurt am Main, Germany). Lastly these samples were stained in either hematoxylin-eosin, for an inflammatory cell count, or in Picosirius-Red (PSR), for debris analysis, after which they were embedded in paraffin.While Hematoxylin is a nuclear stain, resulting in a purple to blue color after processing, Eosin is a cytoplasmic stain. It results in a bright pinkish-red color in red blood cells; muscle fibers; collagen fibers and was applied to evaluate the tissue, including the inflammatory cells present. To achieve a proper Hematoxylin-Eosin (HE) stain, the following process was applied: Tissues were dipped in Hematoxylin 7211 (Richard-Allan Scientific, Kalamazoo, MI) for 1.5 minutes, followed by washing with running tap water to remove excess stain. Next, tissues were put into Clarifier I solution (Richard-Allan Scientific) for 30 seconds to help remove background staining caused by excessive use of tissue adhesive and to increase cell transparency. Tissues were then rinsed in running tap water for 30 seconds, followed by bluing reagent for 1 minute to ensure proper alkalinity (pH, 8) and crisp nuclear detail. Next, tissues were rinsed for 1 minute in running tap water and then rinsed again with 95% alcohol. Tissues were then dipped in Eosin-Y (Richard-Allan Scientific) for 1.5 minutes, followed by three 1-minute washes in 100% alcohol and three 1-minute washes in Xylene. After staining, coverslips were applied to tissues by use of Permount mounting medium (Biomeda, Foster City, CA). Analysis of the slides was performed using a light microscope (BX40 (Olympus Belgium N.V., Antwerp, Belgium)) at a magnification of 4 times, 10 times, 20 times and 100 times. A PSR stain is most often used for evaluation of collagen fibers, which is more difficult using a HE-stain. By applying PSR, dissolved in a saturated picric acid solution, to a tissue sample, collagen fibers will become bright yellow to orange of color. To achieve a proper PSR stain, the following process was applied: First deparaffinization and hydration in distilled water was done of a selected sample. Next, sections were incubated in 0.1% (w/v) Direct Red 80/Sirius Red (C.I- 365548- 5G, Sigma-Aldrich, Switzerland) with saturated Picric acid solution (Qualigens, Mumbai, India), during 1 hour at room temperature. Afterwards, the sample was rinsed with distilled water and stained with Mayer’s Haematoxylin (HI-media Labs. Mumbai, India) and differentiated in 1% HCl, alkalinization with tap water followed by the steps of dehydration and mounting. Observation of the samples was done using a polarizing microscopy (BX40 (Olympus Belgium N.V., Antwerp, Belgium)) at a magnification of 4 times, 10 times, 20 times and 100 times.
Histological analysis
To obtain an unbiased tissue evaluation, like a Lovins Field Finder grid, a 0.20mm² digital grid was projected on five random locations per tissue samples. (Figure 1) The location of the grid projection onto the tissue was randomized by first obscuring the view of the sample itself. In case a grid was either only partially filled out with tissue, or if a proper sample grid view was obscured due to for instance the presence of a blood vessel, a new random grid projection was generated. By means of light microscopic evaluation with a manual cell count being performed, a differentiation between acute and chronical inflammatory response was made. Lymphocytes were identified based on cell morphology. To be counted as a lymphocyte, cells had to be mononuclear with a solitary round and dark blue nucleus (no multiple lobes as in neutrophils) and have minimal surrounding cytoplasm. Macrophages had to be round to oval (10-30 µm in diameter), with an eccentrically located, oval or indented nucleus. While the cytoplasm is usually "foamy", this does not have to be the case and was not used as an exclusion criterium. A qualitative evaluation of foreign bodies within the grids was undertaken using a polarizing microscope.
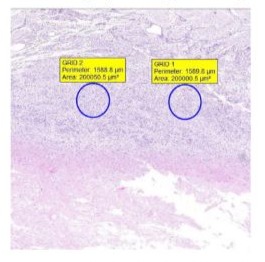
Figure 1: A tissue sample, HE colored, with two 0.20mm² digital grids applied.
Statistical analysis
Two HE- and PSR-stained samples were evaluated per TMJR, resulting in a total of 60 grids analyzed for those sheep who received a DLC-coated condyle (n=6) and 70 grids for those with an uncoated condyle (n=7). A total of ten randomly selected grids were examined for the ewe that underwent sham surgery. For each grid, the number of macrophages and lymphocytes was counted, as well as polymorphonuclear leukocytes if present. Normality of the counts of macrophages and lymphocytes was assessed with a Kolmogorov-Smirnov and Shapiro-Wilk test, which revealed non-normal distributions (P < 0.001). Thus, a Kruskal-Wallis non-parametric ANOVA test was used to test for differences in counts of macrophages and lymphocytes between treatment groups, with a Bonferroni correction. The mean cell count per group was calculated. All data are expressed as mean ± SD.
Results
No signs of acute infection, marked by the presence of neutrophiles were found in any of the samples. There were signs of chronic inflammation and presence of macrophages in all samples. (Figure 2,3) Analyzing the distribution plot for lymphocytes (Figure 4) in the DLC-coated and uncoated samples, reveals higher outliers in the uncoated (140), compared to the coated group (91), as well as a larger mean and distribution in the uncoated group (34.51 ± 28.58) compared to the coated samples (24.6 ± 18.45). In comparison, a relatively low number of lymphocytes was found in the peri-articular sham tissue (9.5 ± 5.2). Furthermore, statistical analysis using the Bonferroni correction found a statistically significant difference in both groups, compared to the sham-group. This significantly higher number of lymphocytes was more outspoken in the non-coated tissues (p = 0.001), compared to the coated samples (p = 0.02). No significant difference was found between the uncoated and coated peri-articular tissue regarding the concentration of lymphocytes. Evaluating the presence of macrophages in the samples (Figure 5), the sham tissues once again show the least number of macrophages (7.4 ± 10.36). The coated system’s tissues have a higher macrophage count (22.15 ± 25.31) as compared to the uncoated samples (17.76 ± 21.16), yet this difference was not significant (p = 0.405). However, the coated group did show a significantly higher number of macrophages compared to the sham-group (p = 0.019), whereas the uncoated group did not (p = 0.141). The higher macrophage count in the coated system was influenced by one sheep which showed a significantly higher macrophage count compared to the other samples. When the data that was recorded for this sheep was excluded, both the mean (17) and SD (16.08) severely decreased. Even more so, there was no longer a significant difference (p = 0.71) in the number of macrophages found between the coated and uncoated system.
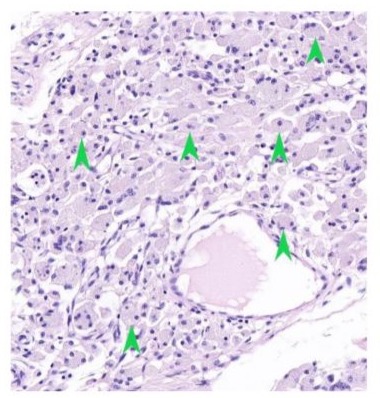
Figure 2: Peri-articular tissue of a DLC-coated condyle, showing lymphocytes and macrophages present (hematoxylin-eosin stain, original magnification X 100). Green arrow: Lymphocytes; Black arrow: Macrophages
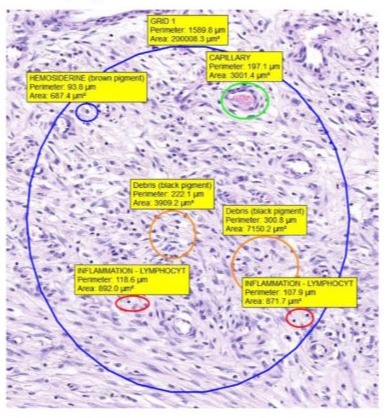
Figure 3: Peri-articular tissue of an uncoated condyle, showing lymphocytes and macrophages present (hematoxylin-eosin stain, original magnification X 100). Green arrow: Lymphocytes; Black arrow: Macrophages.
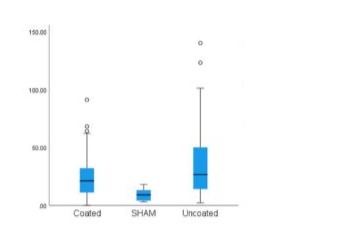
Figure 4:Box plot for lymphocyte distribution.
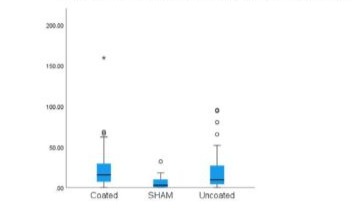
Figure 5: Box plot for macrophage distribution.
Discussion
Considering the severe adverse inflammatory reaction that was seen in patients treated with the Vitek-Kent replacement system [1,6,8] , we set out to provide a quantitative analysis of the inflammatory cell types found in sheep treated with a novel TMJR system, developed by CADskills BV (Ghent, Belgium), as to determine its suitability for human implantation. To properly evaluate periprosthetic tissue reactions, the ‘Synovial-Like Interface Membrane’ (SLIM) consensus classification (Table 1) has proven to be an extremely useful system. For a ‘neo-synovitis’ to be classified as being wear-induced (Type I), more than 20% of the sample must be filled in with macrophages. In addition, multinucleated foreign-body giant cells should be found as well. In addition to these inflammatory cells, wear particles are present within the macrophages [16-18]. Evaluation of peri-articular tissues of both the DLC-coated and uncoated systems revealed an increase in macrophagic cells compared to the tissues from the control group. Upon further analysis, none of the samples were infiltrated for 20% or more with macrophages. As such we could conclude that no wear induced ‘neo-synovitis’ was found in any of our samples. (Table 2, 3) Evaluating the results for the sheep treated with a coated condyle, developing a significantly increased macrophage count compared to the other samples, still had a total surface infiltration of less than 10%. Attempting to find an explanation as to why this one animal showed a more significant microphagous response, compared to the rest of the sheep, a previously performed radiological [11] and wear-analysis [5] was revisited. However, no significant radiological findings were seen, nor was this found to have had a severely increase amount of volumetric, or linear wear-amount, compared to other samples.
|
Type I |
Wear-induced synovitis/SLIM |
|
Type II |
Infection-induced synovitis/SLIM |
|
Type III |
Mixed synovitis/SLIM |
|
Type IV |
Indifferent (not wear-induced, not infection-induced) synovitis/SLIM |
|
Type V |
Prosthesis-associated arthrofibrosis |
|
Type VI |
Adverse local tissue reactions to implant wear particles |
|
Type VII |
Local osseous pathologies |
Table 1: SLIM consensus classification.
|
Sheep number |
Sample 1 |
|
Sheep 1 |
0.059 |
|
Sheep 2 |
0.021 |
|
Sheep 3 |
0.013 |
|
Sheep 4 |
0.02 |
|
Sheep 5 |
0.059 |
|
Sheep 6 |
0.032 |
Table 2: Surface area (in mm²) covered per 1mm² with macrophages, in the uncoated tissue samples.
|
Sheep number |
Sample 1 |
|
Sheep 1 |
0,028 |
|
Sheep 2 |
0.052 |
|
Sheep 3 |
0.072 |
|
Sheep 4 |
0.019 |
|
Sheep 5 |
0.042 |
|
Sheep 6 |
0.017 |
Table 3: Surface area (in mm²) covered per 1mm² with macrophages, in the DLC-coated tissue samples.
In all samples the total wear volume and linear amount of wear that was well acceptable compared to the golden standard and well below the 1mm/year linear wear and 80mm³ volumetric wear at which osteolysis can occur [5,19]. This was also reflected in the low macrophagous response, which averaged at 3.8% of the total surface area in case of the DLC-coated system and 3.1% in case of the uncoated system. Even with these very respectable results in mind, if the outlier in the DLC-coated samples was removed, this average infiltration rate even dropped further down to 2.9%.In addition to this Type I-reaction, wear particles can also lead to local toxicity, resulting in adverse local tissue reactions (Type VI-reaction), as was seen in the Vitek-Kent replacement system [6,7]. This Type VI-reaction can be divided into three different groups. Firstly, mainly macrophagic pattern with absent or minimal lymphocytic response can be seen (2); a mixed inflammatory pattern, with both macrophagic and lymphocytic cells, with variable presence of plasma cells, eosinophils, and mast cells and (3) a granulomatous pattern, predominant or associated with the mixed inflammatory pattern [16,17]. Again, none of our samples met these criteria.While one sheep developed a peri-articular swelling 2 months prior to euthanasia, potentially indicative for an infection-induced ‘neo-synovitis’ (Type II-reaction) , drainage revealed the swelling was of a hemorrhagic nature. A blood sample showed no leukocytosis, nor did a bacterial culture of the drained fluid yield any results. Histopathological evaluation revealed the presence of both macrophages and a lymphocytic response (limited to less than 20% of the sample surface), yet no polymorphonuclear leukocytes were found. Considering the negative microbiological diagnosis, together with the absence of polymorphonuclear leukocytes, as well as the absence of abscess formation were seen, we concluded no prosthetic joint infection or Type II-reaction occurred [16,20]. Instead, post-mortem radiological evaluation revealed that the fossa component had been luxated. This was probably caused because of the screw size diameter that was chosen. This was like that in humans, whilst a larger diameter would have provided better fixation. The event of the fossa component luxation might well have led to the hematoma that was formed. While no wear-induced ‘neo-synovitis’ was found, we did find a significantly increased number of lymphocytes in the tissue samples of the uncoated TMJR compared to both the coated TMJR and the control tissues. This suggests that a chronic inflammatory response, or at least more chronical inflammation, was present in the uncoated TMJR group. This is of importance, as research by both Hobza et al [21]. as well as Lohmann et al [22]. found that higher tissue concentrations of metals, resulted in a higher lymphocytic infiltration. Their findings are consistent with those of our own, as less wear was found in the coated system compared to the uncoated system. Somewhat unexpectedly, several samples revealed a brownish intra-capsular material during dissection, which was found to be amorphous. This material revealed to contain high amounts of hemosiderin and clusters of erythrocytes, as were found by Van Loon et al [23]. during their sheep experiment. While they hypothesized this were clusters of degenerated erythrocytes, we hypothesized that this brown material was a remnant of the hemostatic gelatin sponge (Spongostan, Ethicon, New Jersey, USA) that was applied into each of the intra-capsular spaces during implantation.Foreign bodies were found interspersed between the lymphocytes. It could not be determined if this debris was formed during the implantation process, due to the drilling and screw fixation, during implant functioning or due to the post-euthanasia tissue cutting and preparation. Whereas we did not focus on the implant integration and thus implant-bone interface in this paper, previously published studies showed good histological results with concern to the bony ingrowth into the implant surface. Thus, no type V or VI-reactions were seen.
Conclusion
A significant increase in lymphocytes was seen in both samples treated with the DLC-coated and the uncoated condyle, although this increase was more significant in the uncoated system. A significant increase in macrophages was also seen in the tissue samples of the coated system, yet none of samples that were examined showed any sign of ‘neo-synovitis’ caused by either wear or infection. No adverse local tissue reactions were seen either. We can conclude that this TMJR can be applied for human implantation, as we do not expect any adverse reactions based on our results.
Funding: This work was supported by VLAIO (Grant number HBC.2018.0093); the internal funds Catholic University Louvain by the starting grant of Annabel Braem (Grant number STG/17/024).
References
- De Meurechy N, Mommaerts MY (2018) Alloplastic temporomandibular joint replacement systems: A systematic review of their history. Int J Oral Maxillofac Surg 47: 743-754.
- Elledge R, Mercuri LG, Attard A, Green J, Speculand B (2019) Review of emerging temporomandibular joint total joint replacement systems. Br J Oral Maxillofac Surg 57: 722-728.
- Koslin M, Indresano AT, Mercuri LG (2012) Temporomandibular joint surgery. J Oral Maxillofac Surg 70: 204-210.
- National Institute for Health and Care Excellence (NICE) (2014) Scope. Natl Inst Heal Care Excell 1: 1-10.
- De Meurechy N, Aktan MK, Boeckmans B, Huys S, Verwilghen DR, Braem A, Mommaerts MY (2022) Surface wear in a custom manufactured temporomandibular joint prosthesis. J Biomed Mater Res B Appl Biomater 110: 1425-1433.
- De Meurechy N, Braem A, Mommaerts MY (2018) Biomaterials in temporomandibular joint replacement: current status and future perspectives—a narrative review. Int J Oral Maxillofac Surg 47: 518-526.
- Alonso A, Kaimal S, Look J, Swift J, Fricton J, Myers S, Kehl L (2009) A quantitative evaluation of inflammatory cells in human temporomandibular joint tissues from patients with and without implants. J Oral Maxillofac Surg 67: 788-796.
- Trumpy IG, Roald B, Lyberg T (1996) Morphologic and immunohistochemical observation of explanted Proplast-Teflon temporomandibular joint interpositional implants. J Oral Maxillofac Surg 54: 63-68.
- Trumpy IG, Lyberg T (1993) In vivo deterioration of Proplast-Teflon temporomandibular joint interpositional implants: a scanning electron microscopic and energy-dispersive X-ray analysis. J Oral Maxillofac Surg 51: 624-630.
- Mommaerts M (2019) On the reinsertion of the lateral pterygoid tendon in total temporomandibular joint replacement surgery. J Craniomaxillofac Surg 47: 1913-1917.
- De Meurechy N, Verwilghen D, De Brucker Y, Van Thielen B, Mommaerts MY (2021) Lateral pterygoid muscle enthesis reconstruction in total temporomandibular joint replacement: An animal experiment with radiological correlation. J Craniomaxillofac Surg 49: 256-268.
- Angelo DF, Morouço P, Alves N, Viana T, Santos F, et al (2016) Choosing sheep (Ovis aries) as animal model for temporomandibular joint research: Morphological, histological and biomechanical characterization of the joint disc. Morphologie 100: 223-232.
- Almarza AJ, Brown BN, Arzi B, Ângelo DF, Chung W, Badylak SF, Detamore M (2018) Preclinical animal models for temporomandibular joint tissue engineering. Tissue Eng Part B Rev 24: 171-178.
- Domingue BM, Dellow DW, Barry TN (1991) The efficiency of chewing during eating and ruminating in goats and sheep. Br J Nutr 65: 355-363.
- Verplancke K, De Waele W, De Bruyn H (2011) Dental implants, what should be known before starting an in vitro study. Conf Sustain Constr Des 2: 360-366.
- Krenn V, Perino G (2017) Histological Diagnosis of Implant-Associated Pathologies. Springer, Berlin, Heidelberg.
- Krenn V, Morawietz L, Perino G (2014) Revised histopathological consensus classification of joint implant-related pathology. Pathol Res Pract 210: 779-786.
- Morawietz L, Classen RA, Schroder JH (2006) Proposal for a histopathological consensus classification of the periprosthetic interface membrane. J Clin Pathol 59: 591-597.
- Dumbleton JH, Manley MT, Edidin AA (2002) A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J Arthroplasty 17: 649-661.
- Zmistowski B, Della Valle C, Bauer TW (2014) Diagnosis of periprosthetic joint infection. J Orthop Res32 Suppl 1: 98.
- Hobza M, Milde D, Slobodova Z, Gallo J (2021) The number of lymphocytes increases in the periprosthetic tissues with increasing time of implant service in non-metal-on-metal total joint arthroplasties: A role of metallic particles? Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 165: 416
- Lohmann CH, Meyer H, Nuechtern JV (2013) Periprosthetic tissue metal content but not serum metal content predicts the type of tissue response in failed small-diameter metal-on-metal total hip arthroplasties. J Bone Joint Surg Am 95: 1561.
- Van Loon JP, De Bont LG, Spijkervet FK, Verkerke GJ, Liem RS (2000)A short-term study in sheep with the Groningen temporomandibular joint prosthesis. Int J Oral Maxillofac Surg 29: 315
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.