Incidence of Hypothyroidism After Breast Cancer Treatment: A Retrospective Cohort Study in State of Kuwait
by Athary Saleem1,2*, Heba Shaddad3, Sami Aldaoud2,4, Khaled Alshammari1,2, Muneera Ben Nakhi1,5, Ous Alozairi1,2
1Department of General Surgery, Al-Adan Hospital, Kuwait
2Kuwait Institution of Medical Specialization (KIMS), Kuwait
3Ministry of Health, Kuwait
4Department of Histopathology, Al-Adan Hospital, Kuwait
5Department of Plastic Surgery, Al-Adan Hospital, Kuwait
*Corresponding author: Athary Saleem, Department of General Surgery, Al-Adan Hospital, Kuwait
Received Date: 27 January 2026
Accepted Date: 02 February 2026
Published Date: 04 February 2026
Citation: Saleem A, Shaddad H, Aldaoud S, Alshammari K, Nakhi MB, et al. (2026) Incidence of Hypothyroidism After Breast Cancer Treatment; A Retrospective Cohort Study in State of Kuwait. J Surg 11: 11555 https://doi.org/10.29011/2575-9760.011555
Abstract
Background: Breast malignancy is the most common cancer in females and the second death-leading etiology. Among the newly reported cancer cases in Kuwait in 2019, breast cancer ranks as the most common malignant neoplasm in females, accounting for 35.4% of Kuwaitis and 38.4 % of non-Kuwaitis.
Methods: A retrospective cohort study analyzed data from medical documents of governate and private hospitals in Kuwait during 2010 and 2019 period. Inclusion and exclusion criteria were applied while conducting data to evaluate the pattern of hypothyroidism among patients in Kuwait undergoing cancer surgery for various types of breast cancer. The study aimed to determine the risk of hypothyroidism in breast cancer patients who were either untreated or treated with surgery, radiotherapy, and chemotherapy.
Results: A total of 5860 patients were included. The results of hypothyroidism distribution in different types of breast carcinoma were identified. In patients with hypothyroidism, invasive ductal carcinoma (IDC) was highly documented. A positive association between the risk of invasiveness and hypothyroidism in breast carcinoma cases was reported. The risk of invasive breast malignancies in patients with hypothyroid disease accounts for 96% with a risk ratio of 2. Among risk estimation, the odds ratio indicated an elevated risk of invasive breast malignancies among hypothyroid cases. The statistical significance was identified between hypothyroidism and the risk of invasive breast cancer by Pearson Chi-Square.
Conclusion: Our retrospective cohort study showed a statistically significant association between breast carcinoma risk and hypothyroidism in the female population in Kuwait.
Keywords: Breast Cancer; Chemotherapy; Hypothyroidism; Invasive Carcinoma; Neoplasm; Retrospective Study
Introduction
Breast malignancy is the most common cancer in females and the second death-leading etiology [1]. In 2022, there were 2.3 million women diagnosed with breast cancer and 670,000 deaths globally [2]. An understanding of possible associated causes or correlations could provide a better understanding of dealing with this highly concerning disease. In Kuwait, cancer is one of the most prevalent health concerns [3-5]. As per the latest epidemiological data in Kuwait, cardiovascular diseases are identified as the first cause of mortality (61.4. per 100,000 population), followed by cancer (20.8 per 100,000 population) [3-5]. Among the newly reported cancer cases in 2019, breast cancer ranks as the most common malignant neoplasm in females, accounting for 35.4% of Kuwaitis and 38.4 % of non-Kuwaitis [5]. Between 2010 and 2019, 26609 new cancer cases were documented in Kuwait. Of these, 5719 cases were breast cancer [5]. In 1896, Beatson utilized thyroid agent as a highly effective treatment agent for breast cancer metastasis, documenting the correlation between breast malignancy and thyroid gland activity [6,7]. According to Loeser and Ellerker’s 1950 study, breast cancer was observed to develop more commonly in hypothyroid females than in ones with hyperthyroidism [6]. Moreover, Kapdi et al. reported the causal relationship between hypothyroidism and elevated risk of breast carcinoma in 1976 [6]. Numerous studies documented a significant association between the probability of breast neoplasms and antithyroid peroxidase autoantibodies [6]. Both mammary and thyroid glandular cells had similar pathological and physiological mechanisms that involved iodine cellular storage, transport by natrium-iodide symporter, and oxidization [6,7]. At a molecular level, the expression of thyroid hormone receptors on mammary glands and the plasma membrane of breast cancer cells can interpret the association between thyroid and breast tissue pathology [6]. Multiple studies reported the association between breast cancer cases and thyroid gland activity, especially hypothyroidism [6-8].
Our study aimed to evaluate the pattern of hypothyroidism among patients in Kuwait undergoing cancer surgery for various types of breast cancer. We planned to study and determine the risk of hypothyroidism in breast cancer patients who were either untreated or treated with surgery, radiotherapy, and chemotherapy.
Materials and Methods
This study was approved by the Standing Committee for Coordination of Health and Medical in the Ministry of Health of Kuwait (Research official number 2199/2022). The database was searched for English literature that assessed the association between breast malignancies and hypothyroidism.
Study Design
This study is a retrospective, cohort analysis using data from medical documents of governate and private hospitals in Kuwait. The governate hospitals included the Kuwait Cancer Control Centre (KCCC) as well as all major General hospitals in Kuwait (Adan, Mubarak, Amiri, Jahra, Farwaniya, Jaber Al-Ahmad, Ahmadi Kuwait Oil Company (KOC), and Armed Forces
Hospital). Moreover, private hospitals were involved in the study. These compromise Al-Salam, Hadi, Royal Hayat, Taiba, New Mowasat, Al Seef, and Dar Al Shifa hospitals.
Study Population
The study population included patients who underwent breast cancer surgery and their level of hypothyroidism. The extracted data was from 10 years, from January 2010 to December 2019. A total of 5860 patient’s information was included in the study. Our retrospective research was based on inclusion and exclusion criteria. The inclusion criteria include females, aged 35 years or older, operated on for breast cancer, and diagnosed between January 2010 and December 2019. All these patients were operated in governate and private hospitals. On the other hand, we exclude females with hyperthyroidism at the time of breast cancer and females aged less than 35 years. The retrieved information included menopausal status, age at diagnosis, thyroid hormone level, recurrence of breast malignancy, follow-up with treating physician, surgery type, exposure to chemotherapy and radiotherapy, HER-2 status, and histologic grade. So, our exclusion criteria involved hyperthyroidism, unknown tumor stage, and unknown primary surgery or radiotherapy. Statistical analysis and study outcome assessment were done by SPSS software.
Results
A total of 5860 patients were included. The results of hypothyroidism distribution in different types of breast carcinoma are shown in Table 1. In patients with hypothyroidism, Invasive Ductal Carcinoma (IDC) was highly documented (93% of total cases represented IDC with a p-value of 0.181).
|
Total n (%) |
Hypothyroid n (%) |
No thyroid n (%) |
p-value |
|
|
5860 (100.0) |
271 (4.6) |
5589 (95.4) |
0.181 |
|
|
DCIS |
148 (2.5) |
2 (0.7) |
146 (2.6) |
|
|
IDC |
5218 (89.0) |
252 (93.0) |
4966 (88.9) |
|
|
IMC |
211 (3.6) |
5 (1.8) |
206 (3.7) |
|
|
ILC |
49 (0.8) |
3 (1.1) |
46 (0.8) |
|
|
LCIS |
202 (3.4) |
7 (2.6) |
195 (3.5) |
|
|
Other type |
32 (0.5) |
2 (0.7) |
30 (0.5) |
Table 1: Distribution of hypothyroidism in different cancer types.
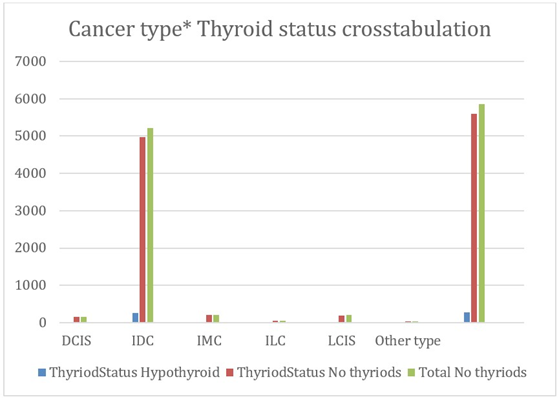
According to the Kuwait Cancer Registry, Invasive ductal carcinoma was the most frequent cancer morphology accounting for about 84% [5].
A positive association between the risk of invasiveness and hypothyroidism in breast carcinoma cases was reported in Table 2. The risk of invasive breast malignancies in patients with hypothyroid disease accounts for 96% with a risk ratio of 2 (95% CI 1.0-3.9, p-value 0.031).
|
Carcinoma Type |
Total n (%) |
Hypothyroid n (%) |
No thyroid n (%) |
Risk ratio (95% CI) |
P-value |
|
Invasive |
5480 (93.5) |
262 (96.7) |
5218 (93.4) |
2.0 (1.0, 3.9) |
0.031 |
|
Non-invasive |
380 (6.5) |
9 (3.3) |
371 (6.6) |
Table 2: Risk ratio of hypothyroid in invasive carcinoma.
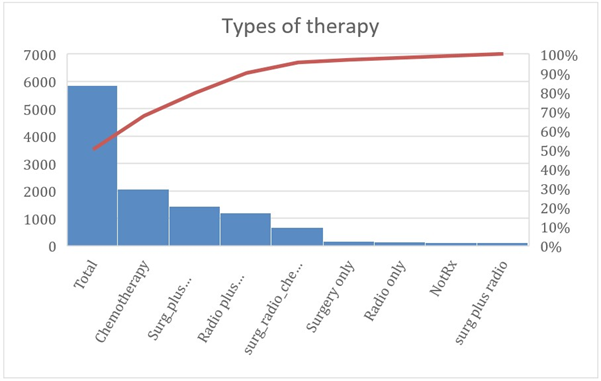
As a result of the high invasiveness status, the relationship between the hypothyroid breast cancer patients and multiple therapeutic modalities is reported. It is noticed that breast surgery was the primary treatment method to be performed in these cases. The various treatment methods in hypothyroid patients with breast carcinomas are statistically presented in Table 3. The combination of surgery and chemotherapy agents is the most utilized treatment method (25.5%, p-value of 0.04).
Among risk estimation, the odds ratio indicated an elevated risk of invasive breast malignancies among hypothyroid cases (OR 2.019, 95% CI 1.047-3.891).
|
Risk Estimate |
Value |
95% Confidence Interval |
|||||||
|
Lower |
Upper |
||||||||
|
Odds Ratio for invasive (Invasive / Non-invasive) |
2.07 |
1.056 |
4.056 |
||||||
|
For cohort Thyroid Status = Hypothyroid |
2.019 |
1.047 |
3.891 |
||||||
|
For cohort Thyroid Status = No thyroid |
0.975 |
0.959 |
0.992 |
||||||
|
N of Valid Cases |
5860 |
||||||||
|
Therapeutics |
Total n (%) |
Hypothyroid n (%) |
No thyroid n (%) |
P-value |
|||||
|
Chemotherapy |
2068 (35.3) |
110 (40.6) |
1958 (35.0) |
0.04 |
|||||
|
Surgery only |
149 (2.5) |
11 (4.1) |
138 (2.5) |
||||||
|
Radiotherapy only |
138 (2.4) |
5 (1.8) |
133 (2.4) |
||||||
|
Surgery & chemotherapy |
1427 (24.4) |
69 (25.5) |
1358 (24.3) |
||||||
|
Surgery, radiotherapy, & chemotherapy |
661 (11.3) |
25 (9.2) |
636 (11.4) |
||||||
|
Surgery & radiotherapy |
99 (1.7) |
2 (0.7) |
97 (1.7) |
||||||
|
Radiotherapy & chemotherapy |
1199 (20.5) |
40 (14.8) |
1159 (20.7) |
||||||
|
Data not available |
119 (2.0) |
9 (3.3) |
110 (2.0) |
||||||
Table 3: Distribution of hypothyroidism in different therapeutic.
The statistical significance was identified between hypothyroidism and the risk of invasive breast cancer by Pearson Chi-Square (value of 4.690, Sig. 0.030). Furthermore, the Chi-Square test demonstrated a strong correlation between hypothyroid status and breast malignancies (value 7.580, Sig. 0.181).
|
Chi-Square Tests |
|||
|
Value |
df |
Asymptotic Significance (2-sided) |
|
|
Pearson Chi-Square |
7.580a |
5 |
0.181 |
|
Likelihood Ratio |
9.435 |
5 |
0.093 |
|
Linear-by-Linear Association |
0.239 |
1 |
0.625 |
|
N of Valid Cases |
5860 |
||
|
a. 2 cells (16.7%) have expected count less than 5. The minimum expected count is 1.48. |
|||
Discussion
In the current situation, there is conflicting evidence concerning the causal connection between hypothyroidism and breast cancer risk in different targeted populations [7,9,10]. Despite some studies that reported no association between breast carcinoma and hypothyroidism in the literature, others document evidence of this relationship [6,7,9-11]. Currently, this research is the largest population-based study in Kuwait, and other Gulf Cooperation Council (GCC) countries, documenting the association between hypothyroidism and breast carcinoma in the region. Considering the discrepancy in the prior research findings, we conducted a retrospective cohort study. Our retrospective cohort study showed a statistically significant association between breast carcinoma risk and hypothyroidism in the female population in Kuwait. In neoplasms of the head and neck region, hypothyroidism is a documented late consequence following radiotherapy [8,12]. In 2019, Falstie-Jensen et al. reported insignificant results while assessing the correlation between breast carcinoma and hypothyroidism, and mortality in the Danish female population [8]. On the other hand, studies reported a significant association between breast malignancy and thyroid conditions either autoimmune or non-autoimmune [9,13]. Further animal studies examined hypothyroidism’s effects on tumor multiplication, invasiveness, and metastasis formation in both breast cancer and hepatocarcinoma [14]. It reported its noticeable role in tumor progression and metastasis [14]. Angelousi et al. observed a considerable correlation between reduced levels of lymph node metastasis in breast cancer patients having a history of thyroid dysfunction [15]. Despite the efforts of numerous researchers in examining the association between hypothyroidism and breast malignancies, Bolf et al, in 2018, documented the co-occurrence of both, breast and thyroid, neoplasms with unknown underlying etiology [12]. Additional studies on drug-induced hypothyroidism indicated that the functional activity of the thyroid gland might protect against progressive breast carcinoma [12]. In a Danish publication, comprising 203,306 matched controls and 44,574 breast cancer cases, the risk of hypothyroidism was greater in cases than in controls [8,16]. Moreover, in 2022, the incidence of hypothyroidism in breast cancer patients after radiotherapy exposure was significant in the Korean population [16]. The study results can be explained by the cellular and vascular injuries of the thyroid gland that are induced by radiation therapy [16]. A Canadian study demonstrated the effective role of thyroxine administration on mortality reduction in geriatric breast carcinoma individuals [17]. Epidemiologically, international studies investigate the clinical effect of hypothyroidism on long-term outcomes of breast malignancies [17]. Although the positive relation between elevated free thyroid hormone levels and good prognosis of breast tumors was identified in the study, hypothyroidism was observed to be associated with a high risk of breast cancer metastasis and invasiveness [18].
Conclusion
Our retrospective cohort study showed a statistically significant association between breast carcinoma risk and hypothyroidism in the female population in Kuwait. The underlying cause of these research findings is believed to be multifactorial, including our population’s exceptional genetic characteristics, geodemographic factors, and dietary features. Our study had some limitations and provided crucial recommendations. The limitations involve the absence of screening guidelines for thyroid hormone levels post-breast cancer treatment modalities, the majority of medical files being manually prepared and stored, and minimal usage of computer software and electronics. Furthermore, missing patient data besides the limited publications and literature regarding the hypothyroid and breast cancer association represented an extensive challenge. We recommend additional studies to identify this association, suggest an annual thyroid hormone test as a follow-up protocol, and assess mortality and morbidity post-treatment.
Acknowledgment
We acknowledge the Kuwait Cancer Control Center (KCCC) and the Kuwait Cancer Registry department for contributing to data collection and providing cancer information and annual reports in Kuwait from 2010 to 2019. Further acknowledgment to Dr. Ali Alenezi, Dr. Saqer Alenezi, and Dr. Rashed Alazmi for their help in data collection. This research has a research committee number 2199/2022.
References
- American Cancer Society (n.d) About breast cancer.
- (2024) World Health Organization: WHO & World Health Organization: WHO. (2024, March 13). Breast cancer.
- Al Ramadhan MA (2017) Eradicating breast cancer: longevity impact on Kuwaiti women. Asian Pacific journal of cancer prevention: APJCP 18: 803.
- Al-Shaibani H, Bu-Alayyan S, Habiba S, Sorkhou E, Al-Shamali N, et al. (2006) Risk factors of breast cancer in Kuwait: case-control study. Iranian Journal of Medical Sciences 31: 61-64.
- Kuwait Cancer Control Center (KCCC). Kuwait Cancer Registry (20102019). Kuwait, Ministry of Health. For years from 2010 and 2019.
- Angelousi AG, Anagnostou VK, Stamatakos MK, Georgiopoulos GA, Kontzoglou KC (2012) Mechanisms in endocrinology: primary HT and risk for breast cancer: a systematic review and meta-analysis. European journal of endocrinology 166: 373-381.
- Bolin Wang ZL, Huang Y, Li R, Lin T (2020) Does hypothyroidism increase the risk of breast cancer: evidence from a meta-analysis 20: 733.
- Falstie-Jensen AM, Kjærsgaard A, Lorenzen EL, Jensen JD, Reinertsen KV, et al. (2019) Hypothyroidism and the risk of breast cancer recurrence and all-cause mortality-a Danish population-based study. Breast cancer research 21: 1-11.
- Turken O, NarIn Y, DemIrbas S, Onde ME, Sayan O, et al. (2003) Breast cancer in association with thyroid disorders. Breast Cancer Research 5: 1-4.
- Smyth PP (1997) The thyroid and breast cancer: a significant association?. Annals of medicine 29: 189-191.
- Søgaard M, Farkas DK, Ehrenstein V, Jørgensen JOL, Dekkers OM, et al. (2016) Hypothyroidism and hyperthyroidism and breast cancer risk: a nationwide cohort study. European journal of endocrinology 174: 409-414.
- Bolf EL, Sprague BL, Carr FE (2019) A linkage between thyroid and breast cancer: a common etiology?. Cancer Epidemiology, Biomarkers & Prevention 28: 643-649.
- Fiore E, Giustarini E, Mammoli C, Fragomeni F, Campani D, et al. (2007) Favorable predictive value of thyroid autoimmunity in high aggressive breast cancer. Journal of endocrinological investigation 30: 734-738.
- Martínez-Iglesias O, García-Silva S, Regadera J, Aranda A (2009) Hypothyroidism enhances tumor invasiveness and metastasis development. PloS one 4: e6428.
- Angelousi A, Diamanti-Kandarakis E, Zapanti E, Nonni A, Ktenas E, et al. (2016) Is there an association between thyroid function abnormalities and breast cancer?. Archives of Endocrinology and Metabolism, 61: 54-61.
- Park J, Kim C, Ki Y, Kim W, Nam J, et al. (2022) Incidence of hypothyroidism after treatment for breast cancer: A Korean populationbased study. Plos one 17: e0269893.
- McVicker L, Cardwell CR, McIntosh SA, McMenamin ÚC (2022) Cancer-specific mortality in breast cancer patients with hypothyroidism: a UK population-based study. Breast Cancer Research and Treatment 195: 209-221.
- Brandt J, Borgquist S, Almquist M, Manjer J (2016) Thyroid function and survival following breast cancer. Journal of British Surgery 103: 1649-1657.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.