In Vitro Evaluation of Apical Debris Extrusion After the Root Canal Preparation with Gel Irrigants
by Evaldo de Aguiar Braga1, Adriana de Jesus Soares2, Ana Grasiela Limoeiro3*, Wayne Martins Nascimento1, Leonardo Pinto Fontes1, Marilia Fagury Videira Marceliano-Alves4-6, Cristiane Ferreira Alfenas7, Cesar Augusto Perini Rosas8, Monique Aparecida de Lima Rios Pitzschk1,9, Marcos Frozoni1
1Department of Endodontics, São Leopoldo Mandic College, São Leopoldo Mandic Research Institute, Campinas, São Paulo, Brazil
2Division of Endodontics, Department of Restorative Dentistry, Piracicaba Dental School, State University of Campinas, Piracicaba, São Paulo, Brazil
3Department of Dentistry, Endodontics, and Dental Materials, School of Dentistry of Bauru, USP, Bauru, São Paulo, Brazil
4Postgraduate Program in Dentistry, Nova Iguaçu, RJ, Brazil
5Department of Endodontics, Maurício de Nassau University Centre (UNINASSAU), Rio de Janeiro, Brazil
6Department of Dental Research Cell, Dr. D. Y. Patil Dental College and Hospital, Dr. D. Y. Patil Vidyapeeth, Pune 411018, India
7Department of Endodontics, FAMINAS University Center, Muriaé, Minas Gerais, Brazil
8Department of Endodontics, State University of Northern Paraná, Jacarezinho Campus, Paraná, Brazil
9Endodontics Department, Educational Society University of Santa Catarina, Joinville, Brazil
*Corresponding author: Ana Grasiela Limoeiro, Rua Nestor Ribeiro, 812, Centro, Jequié-BA, Brazil
Received Date: 19 September 2025
Accepted Date: 24 September 2025
Published Date: 26 September 2025
Citation: Braga EDA, Soares ADS, Limoeiro AG, Nascimento WM, Fontes LP, et al. (2025) In Vitro Evaluation of Apical Debris Extrusion After the Root Canal Preparation with Gel Irrigants J Surg 10: 11452 https://doi.org/10.29011/2575-9760.011452
Abstract
During root canal instrumentation, debris can extrude through the apical foramen, causing postoperative pain and periapical inflammation. To investigate the influence of different auxiliary solutions in gel on the extrusion of debris through the apical foramen during root canal instrumentation. Thirty extracted mandibular premolars were randomly divided into three groups (n=10) based on the irrigant used: CHXg group (chlorhexidine 2% gel), NaOClg group (NaOCl 3% gel) and control group (NG: Natrosol gel). The teeth were placed in Eppendorf tubes containing 1.5% agar gel, the tube-agar sets were weighed before and after root canal instrumentation. Debris extrusion was quantified by the weight difference. Data were analyzed using analysis of variance (ANOVA), with P<0.05 considered significant. Apical debris extrusion was significantly influenced by the irrigant (P = 0.009), with lower extrusion in the CHXg and NG groups, which did not differ from each other, compared to the NaOClg group. All groups showed extrusion of debris through the apical foramen; however, the NaOClg group exhibited a greater amount of extruded material.
Keywords: Chlorhexidine; Debris; Extrusion; Hypochlorite Gel; Instrumentation
Introduction
During root canal instrumentation, apical extrusion of debris such as pulp tissue, microorganisms, irrigant and dentin remants can occur potentially leading to complications like postoperative pain and delayed periapical healing [1]. Debris extrusion is an inevitable phenomenon [2] and is influenced by several factors [3]. The use of effective irrigants for cleaning and neutralizing necrotic contents during root canal preparation is essential for successful endodontic treatment [4]. Sodium Hypochlorite (NaOCl) is widely used due its antimicrobial efficacy and ability to dissolve organic material [5]. However, due to its cytotoxicity, it should be used cautiously, especially at high concentrations, as it can cause acute pain and edema if it contacts vital tissue [6]. NaOCl in gel form (NaOClg) has been proposed as an alternative to NaOCl solution, retaining its antimicrobial effect [7] but with a lower risk of apical extrusion, which may enhance procedural safety [8,9]. One study found significantly less extrusion of NaOCl in gel form compared to solution, especially in small-diameter apical foramina [10].
In this study, a 3% NaOCl gel (Chlorcid V, Ultradent, South Jordan, USA) with a pH between 11 and 13 was used, containing sodium hydroxide for stabilizing the NaOCl and polyacrylic acid as a thickening agent. Chlorhexidine Gel (CHXg) is another alternative to NaOCl and is characterized by broad antimicrobial activity, lower cytotoxicity, lubricating properties, and clinical efficacy [11]. The CHXg used in this study, with a pH between 6 and 7, contains Natrosol gel as a thickening agent. Additionally, CHXg has a rheological effect that keeps debris in suspension [11], reducing the risk of debris extrusion [12,13] and consequently minimizing postoperative complications. To date, no studies have compared CHXg and NaOClg regarding debris extrusion. Therefore, this study aims to evaluate the extrusion of debris and irrigants using these auxiliary chemicals in gel form during endodontic treatment, with NG serving as a control. The null hypothesis is that there will be no significant difference in debris extrusion between the tested substances.
Methods
Sample Selection and Preparation
This study was approved by the local ethics committee (CAAE: 42304521.9.0000.5374). The manuscript was written according to the Preferred Reporting Items For Laboratory Studies In Endodontology (PRILE) 2021 guidelines (Figure 1). Thirty single-rooted human mandibular premolars, recently extracted for periodontal or orthodontic reasons, were selected. Digital periapical radiographs (Spectro 70X X-ray unit, Dabi Atlante, Ribeirão Preto, Brazil) were taken to determine the degree of root curvature and to observe the root canal’s anatomy. Exclusion criteria included teeth with atresia, resorption, calcification, previous endodontic treatment and fractures or cracks. Teeth with single canals with a moderate curvature between 10-20° [14] and class I canals [15] were selected, ensuring a foraminal diameter compatible with any Kerr #15 file. Surgical access was achieved using 1014 HL spherical drills (KG Sorensen, Serra, Brazil), at high speed and cooled with an air/water spray. Subsequently, the tooth crowns were ground with a diamond disk (KG Sorensen) under an optical microscope at 40x magnification, standardizing the length of the roots to 17 mm (from apical to coronal), considering 4 mm apical, 4 mm middle, 4 mm cervical and 5 mm coronal rest (Figure 2). Root canal length determintion was performed using the direct visual method, inserting a #10/.02 C-Pilot file (VDW, Munich, Germany) until its tip was visible at the apical foramen.
A surgical microscope (Alliance Microscopia, São Carlos, Brazil) with 20x magnification was used for this purpose. The working lenght (WL) was determined to be 17 mm. The teeth were placed in Eppendorf tubes (Eppendorf do Brasil, Sumarezinho, Brazil) containing 1.5% agar. Debris extrusion was measured using a modified method [16]. Tubes were drilled centrally to create a hole compatible with the cervical diameter of the root. Roots of the teeth were wrapped with Teflon tape, leaving 1 mm of the apical third exposed. They were then inserted into the caps of the Eppendorf tubes up to the level of the cervical, with 12 mm of the root positioned inside the tube and 5 mm of the coronal remnant outside the tube, as shown in Figure 3. The space between the tube cap and the tooth was sealed with Cyanocrylate and the interface was protected with Top Dam Gingival Barrier (FGM, Joinville, Brazil) to prevent liquid ingress. Each cap/tooth set was numbered and weighed in triplicate on a high-precision electronic scale (NOVAK, São José do Rio Preto, Brazil), for initial weight (P1). Next, 2 mL of 1.5% agar gel (Kasvi, São José dos Pinhais, Brazil) was injected into the tubes, which were inverted to immerse the roots in the agar solution (Figure 2). After 24 hours, when the agar had completely gelled, the tubes were weighed again in triplicate and the weight obtained was designated as P2. The weight of agar alone (P3) was calculated as the difference between P2 and P1. Finally, the tubes were placed in an opaque glass container so that the operator could not see the samples during the endodontic procedure (Figure 4). Finally, once the endodontic preparation was complete, the samples were weighed. Extreme care was taken to remove the cap/tooth set from the Eppendorf tube so as not to dislodge the agar gel. The samples were weighed in triplicate on a high-precision electronic scale (NOVAK, São José do Rio Preto, Brazil), and the average of the three values was recorded as weight 4 (P4). The weight difference, representing debris and rinsing liquid extrusion, was calculated by subtracting P3 (weight of the tube with agar) from P4, resulting in value P5. The samples were
randomly divided into three groups (n=10) according to the chemical substance used in the canal preparation as follows
- Group CHXg: Chlorhexidine gel 2%
- NaOClg group: Sodium hypochlorite 3% gel (ChlorCid V)
- Group NG: Natrosol gel (control group)
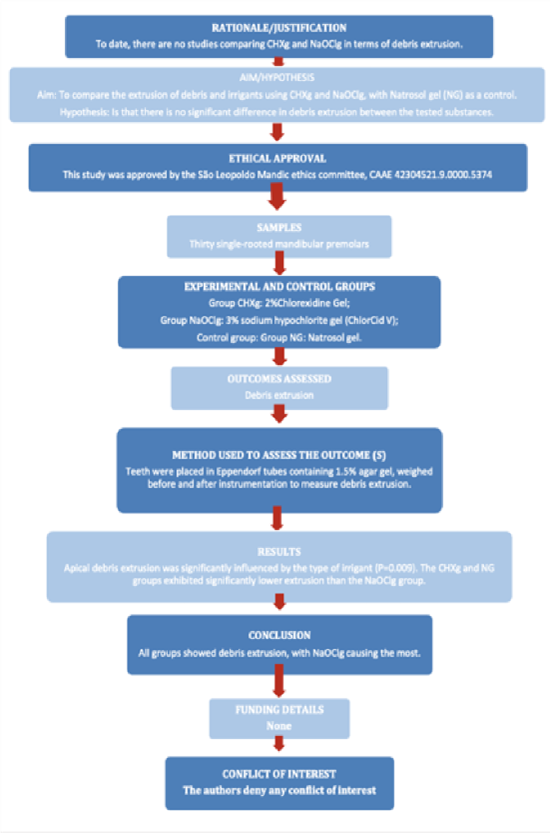
Figure 1: PRILE flowchart.
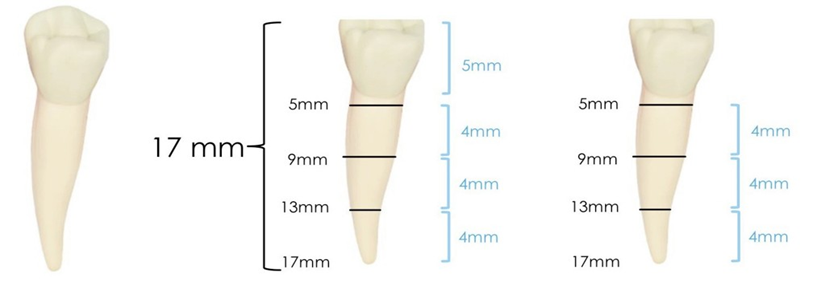
Figure 2: Standardization of samples and division into thirds.
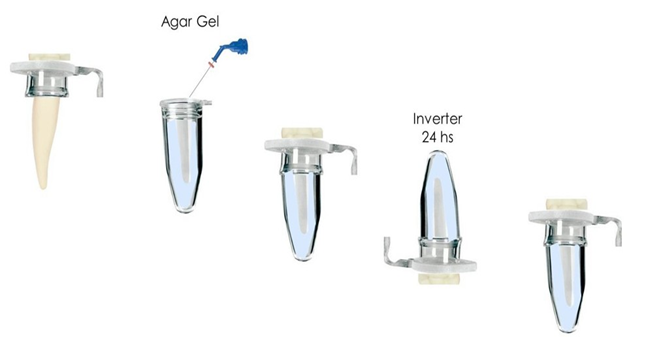
Figure 3: Sample preparation.

Figure 4: Eppendorf tubes placed in a dark bottle.
Root Canal Preparation
Instrumentation was performed using the WaveOne® Gold Primary #25/.07 instrument (Dentsply Sirona, Charlotte, USA), driven by the X-Smart Plus endodontic motor (Dentsply Sirona) in the Wave One Gold reciprocating function. Three movements with an amplitude of 3 mm in pecking motion. After each sequence of three movements, the file was removed and disinfected with sterile gauze soaked in 70% alcohol. The Working Length (WL) was set to the WL. The entire process was performed by an endodontic specialist. A peristaltic pump (LAP-101-3 Tecnopon, Piracicaba, Brazil) with a flow rate of 5 mL/minute was used for irrigation.
The tubing was attached to a 1-mL injection syringe (BD Plastipak, Curitiba, Brazil) and an extra-short 29-G needle (21 mm) (Ultradent, Indaiatuba, Brazil). The preparation was carried out with the same protocol for all tested groups with the root canal preparation of the cervical and middle thirs.
It was started with a initial irrigation with 1 mL of CHXg 2% (Formula & Action, Brazil) in the canal until the needle reached 9 mm from the crown to the apex. Patency was then confirmed with the #10/.02 C-Pilot file (VDW, Munich, Germany) up to the apex. The cervical and middle third were instrumented with the #25/.07 WaveOne® Gold Primary, which penetrated 13 mm of the root length (end of the middle third). Subsequently, 1 mL of 0.9% saline solution (Formula & Action) was irrigated up to 13 mm into the canal. Patency was then confirmed with a #10/.02 C-Pilot file. After, a new irrigation with 1 mL of CHXg in the canal up to 13 mm. The apical third was instrumented until the WaveOne® Gold Primary #25/.07 reached 17 mm (end of apical third - WL). The canal was irrigated with 1 mL SF 0.9% until it reached 15 mm (2 mm below the apical third). Final patency was achieved with the #10/.02 C-Pilot file, followed by a final irrigation with 1 mL SF 0.9% inside the canal up to 15 mm. At the end of the preparation, 3 mL of SF was used as a irrigation solution and 2 mL of CHXg, 2 mL 3% NaOClg (ChlorCid V), and Natrosol gel was used as an auxiliary chemical solution. The distribution of the auxiliary substances per third of the canal is shown in Table 1.
Table 1: Volume of irrigant solution used per group.
|
Cervical and middle |
Apical |
Total |
|
|
CHXg |
1 mL CHXg |
1 mL CHXg |
5mL |
|
1 mL SF |
2 mL SF |
||
|
NaOClg |
1 mL NaOClg |
1 mL NaOClg |
5 mL |
|
1 mL SF |
2 mL SF |
||
|
NG |
1 mL NG |
1 mL NG |
5 mL |
|
1 mL SF |
2 mL SF |
CHXg: chlorhexidine gel; NaOClg: sodium hypochlorite gel; SF: saline solution; NG: natrosol gel
Statistical Analysis
A one-way ANOVA was performed as the data conformed to normal distribution and homoscedasticity. Tukey’s test was used for multiple comparisons. Statistical calculations were performed using the SPSS 23 program (SPSS INC., Chicago, IL, USA) at a significance level of 5%.
Results
Apical debris extrusion was significantly influenced by the irrigant (p = 0.009), with high extrusion in the NaOClg group than in the CHXg and NG groups, which did not differ significantly (Table 2).
Table 2: Means and standard deviations of apical debris extrusion (in grams), according to irrigant substance.
|
Irrigant |
Mean |
Standard deviation |
|
Clorexidine 2% gel |
0.044 A |
0.009 |
|
NaOCl 3% gel |
0.059 B |
0.017 |
|
Natrosol gel |
0.040 A |
0.012 |
Means followed by different letters indicate a significant difference between irrigants.
Discussion
This study evaluated debris extrusion during endodontic instrumentation using CHXg and NaOClg, with NG as a control. Results showed that CHXg and NG had less debris extrusion compared to NaOClg, rejecting the null hypothesis. Teeth were selected based on type, size, canal curvature, number of canals, and working length to ensure the influence of irrigants rather than tooth morphology. The working length was defined by the actual length of the canal (0.0), based on a previous study that showed that instrumentation to the apical foramen or 1 mm below did not affect debris extrusion [17]. The size of the apical preparation was standardized with WaveOne Gold 25.07 after it was shown that the size of the preparation had no effect on debris extrusion [17]. The irrigants, CHX and NaOCl gel, are recognized in endodontics, while NG was used as a control [18]. NG, also known as hydroxyethyl cellulose, is a water-soluble non-ionic polymer that acts as a thickening agent and rheology modifier [19]. It was chosen as a control because it is the basis of CHXg and because it is similar to the gel used in NaOClg. Polyacrylic acid is used as a thickening agent in NaOCl gel because it can form stable and viscous gels that not only facilitate the precise application of irrigation, but also increase the antimicrobial efficacy of NaOCl, thus improving the cleaning and disinfection of root canals during endodontic treatment [20]. The use of NaOCl gel aims to reduce the risk of apical extrusion and avoid accidents related to NaOCl extrusion [8] Studies have shown that NaOClg has comparable antibacterial efficacy to the solution [5,8].
The WaveOne Gold instrument was selected for canal preparation because it emits less debris [21]. To simulate the density of the periodontal ligament, a 1.5% agar gel was used [16] with a density of 1045 kg/m³, which is similar to that of periapical tissue (1000-
1100 kg/m³) and allows the measurement of fluid and leaked debris [22]. In addition, there is no interaction between NaOCl and the agarose gel, making the system ideal for measuring extruded debris in the apical region [18]. Standardization of the experimental protocols is essential for the internal validity of the study. Irrigation was performed using a Tecnopon LAP-1013 peristaltic pump with a flow rate of 5 mL/min to control the volume of irrigation fluid [23]. High irrigation flow and pressure is associated with greater debris [24] highlighting the importance of standardization. In this study, CHXg and Natrosol showed less extrusion of debris compared to NaOClg. The greater extrusion observed with NaOClg may be attributed to the effect of residual oxygen in the dentin, which can potentially reduce the cleaning capacity of NaOCl gel [25,26]. Another explanation could be due to the different surface tensions of the thickening agents, which may affect the stability of the products [25]. Polyacrylic acid has the same surface tension as water, while the hydroxyethyl cellulose solution has a lower surface tension than the values observed for water [24]. Due to the lower surface tension, the CHX gel can penetrate more anatomical irregularities and trap a greater amount of debris than the NaOCl gel, thereby removing a greater amount of debris from within the canal. The additives contained in commercial formulations of NaOCl may alter the physical properties of the gel, affecting penetration into the root canal and apical extrusion [26]. The lower extrusion observed with CHXg can be attributed to its higher viscosity compared to NaOClg, which keeps the debris in suspension during chemical-mechanical preparation [23] and to its thixotropic effect. This property allows the gel to liquefy under the endodontic instrumentation, enclosing more debris and keeping it in suspension [25], which facilitates debris removal. This can lead to less apical extrusion as the debris is contained in the gel and removed with it [24].
On the other hand, the increased viscosity limits the penetration of NaOClg into the dentinal tubules. This can be considered a limitation of the gel form of NaOCl, along with other problems such as questionable penetration into inaccessible areas and the inability to completely remove debris from the root canals [23]. The highly alkaline pH of NaOCl gel may affect the viscosity and thixotropic properties of the gel. Although NaOClg also has a thixotropic effect, its higher reactivity and lower viscosity compared to CHX can lead to greater extrusion of debris. The alkaline environment can also lead to greater dissolution of the organic content, potentially increasing the amount of debris extruded. At a more neutral pH (6-7), the CHX gel has a higher viscosity, which can help keep debris in suspension. The difference in pH between chlorhexidine gel and NaOCl gel can significantly affect the extrusion of debris through the apical foramen, mainly due to its thixotropic properties, viscosity, interaction with dentin and effect on periapical tissues. The more neutral pH of CHXg appears to reduce debris extrusion and minimize postoperative complications, while the highly alkaline pH of NaOClg, although effective in disinfection, may increase the risk of extrusion and irritation of periapical tissues [22,23].
Another study concluded that PUI could not prevent debris extrusion. However, when 2% CHX gel was used, the amount of extruded debris was lower [12]. The reason for this result could be related to the higher viscosity of CHX gel, which helps to keep the debris suspended during irrigation [25]. CHXg showed less extruded debris than CHX solution and NaOCl solution [12] These results confirm the results of this study, in which there was minimal leakage of irrigation fluid when CHXg and NG were used. Microcomputed tomography could further quantify debris extrusion, offering an alternative to weight-based measurements, though it cannot specify irrigant amount [22,25]. Limitations of this study include not analyzing debris content or evaluating exact amounts required to trigger inflammatory responses. Further studies are needed to evaluate the extrusion conditions and to compare different formulations and viscosities of the auxiliary chemicals.
Conclusion
In summary, the gel formulation did not prevent debris extrusion, but NaOClg showed a greater amount of extruded material.
Acknowledgments: This study was supported by grants from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ - E26/200.184/2023) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - 200280/2022-8), Brazilian Governmental Institutions.
Ethical Considerations: The authors deny any conflict of interests.
References
- Mendonça de Moura JD, Bueno CE da S, Fontana CE, Pelegrine RA (2019) Extrusion of Debris from Curved Root Canals Instrumented up to Different Working Lengths Using Different Reciprocating Systems. J Endod 45: 930-934.
- Zand V, Lotfi M, Soroush MH, Abdollahi AA, Sadeghi M, et al. (2016) Antibacterial Efficacy of Different Concentrations of Sodium Hypochlorite Gel and Solution on Enterococcus faecalis Biofilm. Iran Endod J 11: 315-319.
- Abu Hasna A, Pereira Da Silva L, Pelegrini FC, Ferreira CLR, de Oliveira LDet al. (2020) Effect of sodium hypochlorite solution and gel with/without passive ultrasonic irrigation on Enterococcus faecalis, Escherichia coli and their endotoxins. F1000Res 9: 642.
- Nesser SF Al, Bshara NG (2019) Evaluation of the apical extrusion of sodium hypochlorite gel in immature permanent teeth: An in vitro study. Dent Med Probl 56: 149-153.
- Yavagal CM, Subramani SK, Patil VC, Yavagal PC, Talwar RP, et al. (2023) Disinfection Efficacy of Laser Activation on Different Forms and Concentrations of Sodium Hypochlorite Root Canal Irrigant against Enterococcus faecalis in Primary Teeth. Children 10.
- Ozlek E, Neelakantan P, Khan K, Cheung GSP, Rossi-Fedele G (2021) Debris extrusion during root canal preparation with nickel-titanium instruments using liquid and gel formulations of sodium hypochlorite in vitro. Aust Endod J 47: 130-136.
- Mehra D, Sinha DJ, Singh S, Verma N, Rani P, et al. (2023) Comparison of single and multiple file rotary endodontic instruments for debris and irrigant extrusion: An in vitro study. J Conserv Dent 26: 288-291.
- Schneider SW (1971) A comparison of canal preparations in straight and curved root canals. Oral Surg Oral Med Oral Pathol 32: 271-275.
- Vertucci FJ (1984) Root canal anatomy of the human permanent teeth. Oral Surg Oral Med Oral Pathol 58: 589-599.
- Parepalli PS, Raju TBVG, Prasad PK, Dondapati GD, Kintada VS, et al. (2023) An in vitro comparison of alterations in surface topographies of three different rotary files after root canal preparation with different irrigating solutions: Atomic force microscopic study. J Conserv Dent 26: 299-304.
- Mittal N, Baranwal HC, Gupta S, Shankari T, Gupta S, et al. (2023) Comparative analysis of reduction in pain scores after single visit root canal treatment using endodontic irrigation protocols, namely, Conventional needle irrigation, PUI, PIPS and SWEEPS: A randomized control trial. J Conserv Dent 26: 143-149.
- Bakhrushina E, Anurova M, Demina N, Kashperko A, Rastopchina O, et al. (2020) Comparative study of the mucoadhesive properties of polymers for pharmaceutical use. Open Access Maced J Med Sci 8: 639-645.
- Noreen A, Zia KM, Tabasum S, Khalid S, Shareef R (2020) A review on grafting of hydroxyethylcellulose for versatile applications. Int J Biol Macromol 150: 289-303.
- Hemalatha S, Srinivasan A, Srirekha A, Santhosh L, Champa C, et al. (2022) An in vitro radiological evaluation of irrigant penetration in the root canals using three different irrigation systems: Waterpik WP-100 device, passive irrigation, and manual dynamic irrigation systems. J Conserv Dent 25: 403-408.
- Alves FRFF, Paiva PL, Marceliano-Alves MF, Cabreira LJ, et al. (2018) Bacteria and Hard Tissue Debris Extrusion and Intracanal Bacterial Reduction Promoted by XP-endo Shaper and Reciproc Instruments. J Endod 44: 1173-1178.
- Machado AG, Guilherme BPSS, Provenzano JC, Marceliano-Alves MF, Gonçalves LS, et al. (2019) Effects of preparation with the Self-Adjusting File, TRUShape and XP-endo Shaper systems, and a supplementary step with XP-endo Finisher R on filling material removal during retreatment of mandibular molar canals. Int Endod J 52: 709-716.
- Costa JL de SG, Barros APO, Manzoli TM, Escalante-Otárola WG, Alencar C de M, et al. (2024) Formulations of NaOCl-based in liquid, gel form or with surfactants on dentin deproteinization before fiber post cementation. Dent Mater J 43: 126-135.
- Costa JL de SG, Manzoli TM, Besegato JF, Zaniboni JF, Oliveira ECG DE, et al. (2023) Effect of sodium hypochlorite-based formulations on the adhesion interface after fiber post cementation. Dent Mater J 42: 878-885.
- Hu RYZ, Wang ATA, Hartnett JP (1991) Surface tension measurement of aqueous polymer solutions. Exp Therm Fluid Sci 4: 723-729.
- Rossi-Fedele G, Prichard JW, Steier L, de Figueiredo JAP (2013) The effect of surface tension reduction on the clinical performance of sodium hypochlorite in endodontics. Int Endod J 46: 492-498.
- Kumar K, Teoh YY, Walsh LJ (2023) Root canal cleaning in roots with complex canals using agitated irrigation fluids. Aust Endod J 49: 5665.
- Ferraz CC, Gomes NV, Gomes BP, Zaia AA, Teixeira FB, et al. (2001) Apical extrusion of debris and irrigants using two hand and three engine-driven instrumentation techniques. Int Endod J 34: 354-358.
- Kotecha N, Shah NC, Doshi RJ, Kishan KV, Luke AM, et al. (2023) Microbiological Effectiveness of Sodium Hypochlorite Gel and Aqueous Solution When Implemented for Root Canal Disinfection in Multirooted Teeth: A Randomized Clinical Study. J Funct Biomater 14.
- Thirunarayanan S, Hegde MN (2022) Value addition property of a cationic surfactant on endodontic irrigant: A confocal laser scanning microscope study. J Conserv Dent 25: 380-384.
- Monteiro TM, Cortes-Cid VO, Marceliano-Alves MFV, Campello AF, Bastos LF, et al. (2024) Intracanal removal and apical extrusion of filling material after retreatment using rotary or reciprocating instruments: A new approach using human cadavers. Int Endod J 57: 100-107.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.