Implant-Associated Amyloid Tumor: A Case Report
by Veh J1, Köpke M1,2, Reindl S3, Raab S3, Schneider M1, Dannecker C1,2, Ditsch N1,2*
1Department of Gynecology, Obstetrics and Senology, Faculty of Medicine, University of Augsburg, University Hospital Augsburg, Germany
2Bavarian Cancer Research Center (BZKF), study group breast cancer, 91054 Erlangen, Germany
3Department of Heart and Thorax Surgery, Faculty of Medicine, University of Augsburg, University Hospital Augsburg, Germany
*Corresponding author: Nina Ditsch, Department of Gynecology, Obstetrics and Senology, Faculty of Medicine, University of Augsburg, University Hospital Augsburg, Germany
Received Date: 28 February 2025
Accepted Date: 06 March 2025
Published Date: 08 March 2025
Citation: Veh J, Köpke M, Reindl S, Raab S, Schneider M, et al. (2025) Implant-Associated Amyloid Tumor: A Case Report. J Surg 10: 11273 https://doi.org/10.29011/2575-9760.011273
Introduction
Amyloid refers to misfolded proteins that have the ability to deposit in various tissues and organs in the form of fibrillar aggregates. Local and systemic forms can be distinguished. The causes of local or systemic amyloidosis may include rheumatic, inflammatory, genetic, and neoplastic factors. Amyloidosis of the breast can occur as organ specific nodular amyloidosis or as part of systemic amyloidosis. Organ specific nodular amyloidosis has been reported in skin, trachea, bronchi, and the urogenital tract, with breast being the lowest in frequency. [2] Amyloid tumors of the breast are a very rare disease. Only few cases are found in the literature. [1] Most of the reported cases were elderly women who had mammographically suspicious lesions suspicious of carcinoma. The following article presents a case of a breast/thoracic implant-associated benign amyloid tumor.
Case Report
This case concerns a patient who was 85 years old when presented at our department for the first time with an amyloid tumor after a long period of suffering and presentations in external hospitals. Regarding the patient's medical history, an invasive breast carcinoma (hormone receptor-positive, HER2-negative) was diagnosed parasternal on the left side in 2007, together with a ductal carcinoma in situ on the right side. No genetic cause was found. A bilateral incomplete mastectomy was performed, including partial resection of the pectoralis muscle on the left and axillary lymph node dissection on the left. Subsequently, breast augmentation with silicone implants and lifting was conducted bilaterally. Over the years, clinically relevant capsular fibrosis developed on the left side, leading to the indication for exchange of the left breast implant. The patient had no other relevant pre-existing diseases. Becuase of an increasing chest pain, a CT scan conducted 15 years later revealed an extensive tumor of the left thoracic wall with partial erosion of the anterior 5th and 6th ribs, prompting the patient to initially present for internal medicine evaluation. Imaging also suggested the possibility of intrapulmonary metastases. Given the suspicion of a malignant process, a core biopsy of the thoracic wall was performed. Histological examination revealed chronic fibrosing sclerosis with extensive amyloid deposits (confirmed by a reference review), leading to the decision to initially refrain from surgical intervention. A subsequent PET-CT did not show any local recurrence or lymphatic or hematogenous metastases.
To exclude systemic amyloidosis, a comprehensive internal medicine evaluation was done, including transthoracic echocardiography (showing no significant hypertrophy or restrictive filling pattern) and renal ultrasound. A skeletal scintigraphy and a fat tissue biopsy were performed, both yielding unremarkable results. Systemic amyloidosis could not be demonstrated. Further examinations were carried out to rule out other rheumatic and leukemic diseases. The patient was subsequently presented at the tumor conference, where further evaluation through paraprotein diagnostics and bone marrow puncture was recommended. A serological investigation revealed elevated levels of immunoglobulins (M-protein: 1.3 g/dl, light chains: within normal range, IgG: 3730 mg/dl) without any evidence of monoclonal gammopathy. In the bone marrow examination, the percentage of plasma cells was found to be 5 %. Flow cytometric analyses were negative for monoclonal plasma cells. Histologically, there was no indication of light chain restriction or amyloid. Collectively, the conducted investigations did not reveal any evidence of a lymphoproliferative disorder as the cause of amyloidosis. Molecular pathological analysis identified TET2 and IKZF1 mutations, which have been previously described in the context of multiple myeloma. FISH analysis showed no del(13q), del(17p), t(14;16), or t(4;14).
After an initial watchful waiting approach and a few PET-CT scans (Figures 1&2), the patient presented for the first time to our department due to tumor size progression, increasing osteolysis, intrathoracic infiltration, and associated significant clinical symptoms. The patient reported fatigue, dyspnea, and dizziness, along with intermittent pain in the left thoracic wall, described as electrifying which led to the indication for mammography and breast ultrasound. Results of the cardiac echocardiography most likely attributed the fatigue to compression of the right ventricle by the unclear tumor with amyloid deposits. Imaging revealed a hypermetabolic mass along the left thoracic wall with progressive destruction of the rips and left thoracic wall, and a suspected residual implant located cranio-laterally on the left side. Differentiation between liquid/necrotic and vital areas, as well as delineation from an internal capsule rupture, was not possible. The imaging confirmed the clinical suspicion of a residual implant on the left side. Consequently, a prompt indication for surgical intervention an removal of the tumor and rips was established of the gynaecologic team in collaboration with the collegues of the thorax surgery.
Intraoperatively, the first step involved an ablatio simplex on the right side through a skin incision, which included the removal of the entire residual glandular tissue along with the fascia of the pectoralis muscle. On the left side, a more differentiated interdisciplinary approach was necessary. Initially, the remaining and glandular tissue were excised and the implant remnant including the tumor were visualised (Figures 3a,3b). Upon opening the tumor or implant capsule, a significant amount of old hematoma and organized material was observed. For optimal access to the intrathoracic area, the capsule was uncovered and completely evacuated. The ribs C4 to C6 were found to be completely osteolytically destroyed, resulting in an impression of the thoracic wall towards the mediastinum, particularly affecting the right ventricle. Within the next step, the pleura was opened laterally with sufficient distance from the mediastinum using an electrosurgical device, and the intrathoracic portion of the capsule was resected (Figure 3c). For this, ribs C4 to C6 were partially resected. Ultimately, the capsule was completely removed. An open, partially blunt-digital, and partially sharp pleurolysis of the lung was performed in this area, revealing multiple adhesions. A superficial laceration of the lung parenchyma was continuously reconstructed with 4.0 PDS sutures. A resection of the rib stumps C4 to C6 was performed laterally using a Luer clamp or rib scissors, and the ribs were also resected medially up to the parasternal area. Finally, the mass was completely resected. A 24 French thoracic drain was placed in a dorso-apical direction. Subsequently, the reconstruction of the chest wall was performed. Initially, a Bard mesh was sutured in using a periosteal technique to prevent herniation of lung parenchyma. Following this, reconstruction of the bony chest wall was carried out using the StraTos system. A straight, golden titanium plate was vertically anchored between the intact ribs C3 and C7 with two golden three-segment clamps. The resected ribs were then reconstructed and fixed laterally with flexible golden rib clamps and a connecting bridge of approximately 6 cm in length (Figure 3d).
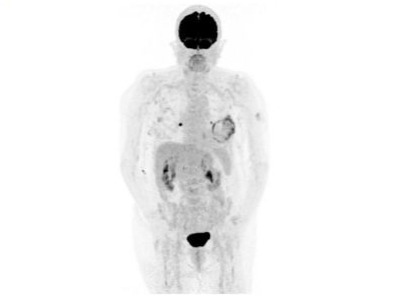
Figure 1: Skeletal scintigraphy with the described amyloid tumor on the left side.
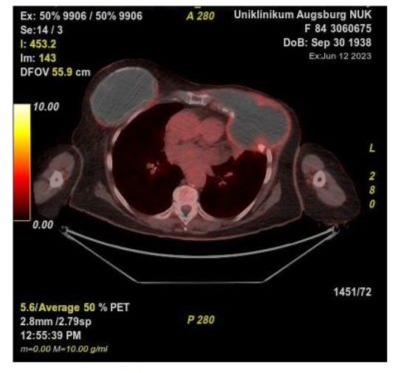
Figure 2: PET-CT with a right-sided breast implant and a left-sided amyloid tumor with accompanying osteodestruction, intrathoracic invasion.
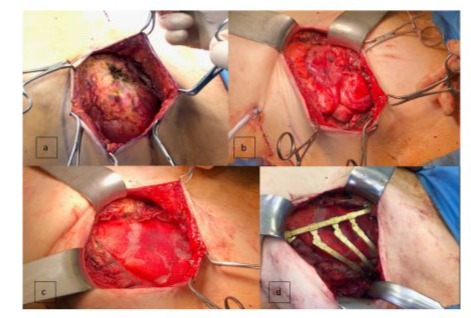
Figure 3: Surgical tumor resection followed by reconstruction of lung parenchyma and thorax. 3a: View of the fully prepared amyoid tumor on the bottom of a silicone implant remnant. 3b: left lung; 3c: Insert of Meh; 3d: flexible golden rib clamps and a connecting bridge.
Palpation revealed a stable chest wall. With sufficient mobility in the thoracic wall area, a tension-free wound closure was achieved through double fascial, continuous subcutaneous, and intradermal sutures. The histological examination of the specimen revealed an extensive hematoma with progressive organization on the left side, along with the previously described amyloid deposits, without any indication of malignancy. In the further postoperative course, the patient developed a wound healing disorder on the right side due to nocturnal dehiscence and an infected seroma, which was punctured and treated with antibiotics. A seroma was also developed on the left side. A drain was inserted by the colleagues in the thoracic surgery department. As a result, the seroma regressed and the patient improved clinically. Overall, the patient's recovery was delayed, but she was ultimately discharged home in good general condition. The pre-existing symptoms (dyspnoea, pain, fatigue) were no longer detectable. The follow-up two months later showed no irritation on either side of the wound. The patient was fully mobile and in a very good general condition for her age. When contacted by telephone one year later, the patient refused further follow-up examinations due to her age.
Discussion
Whether the amyloid tumor had grown on the base of an silicone breast implant or independently of it cannot be conclusively clarified, as this is a special case that has not yet been described in the literature. For other lymphoma-related diseases, a clear connection with implant placement in the breast is known. BIA-ALCL, Breast implant-associated anaplastic large cell lymphoma, is a distinct type of T-cell lymphoma, developing around implants, that can occur 2 to 25 years (median 8 years) post-implantation.
The genesis of the described localized amyloid tumor, which developed following implant placement performed many years before, remains a subject of discussion. It is a chronic inflammatory response maybe triggered by the presence of the implant, leading to an increased deposition of misfolded acute-phase proteins, as well as a potential genetic predisposition, represent plausible contributing factors. In the literature, benign amyloid tumors have been described in various anatomical locations, including the lungs, colon, and lacrimal gland. Unlike the present case, these previously reported amyloid tumors did not exhibit malignant characteristics. Their growth remained localized and self-limiting, whereas the implant-associated amyloid tumor in this case demonstrated an aggressive behavior, leading to rib destruction and severe functional impairment for the patient and therefore should be viewed in a differentiated manner [3-6]. Notably, osteodestruction resulting from a benign amyloid tumor, as observed in this case, has not been previously documented. In contrast, systemic amyloidosis has been reported to cause bone destruction in certain cases [7]. Furthermore, the association between amyloid tumors and breast implants represents a novel finding.
Summary
The amyloid tumor in this case maybe originated from residual implant material. While this is the first documented case of an implant-associated amyloid tumor, the existence of malignant implant-associated diseases, such as implant-Associated Anaplastic Large Cell Lymphoma (ALCL) and breast implant capsule-associated squamous cell carcinoma, is well established. These malignancies necessitate oncological treatment, including chemotherapy, upon confirmation of malignancy. Ultimately, implants remain foreign bodies, and their long-term effects are not yet fully understood. Implant-associated masses should be thoroughly evaluated preoperatively to ensure accurate diagnosis and the selection of an appropriate therapeutic strategy.
References
- Walker AN, Fechner RE, Callicott JH Jr (1982) Amyloid tumor of the breast. Diagn Gynecol Obstet 4: 339-341.
- Deolekar MV, Larsen J, Morris JA (2002) Primary amyloid tumour of the breast: a case report. J Clin Pathol 55: 634-635.
- Yoshimura A, Gondo N, Adachi Y, Kataoka A, Sugino K, et al. (2019) Amyloid tumor of the breast. Surg Case Rep 5: 31.
- Massry GG, Harrison W, Hornblass A (1996) Clinical and computed tomographic characteristics of amyloid tumor of the lacrimal gland. Ophthalmology 103: 1233-1236.
- Heiders J M, Koryllos A (2012) Pulmonaler Amyloidtumor unter dem Bild eines linksseitigen Pancoast-Tumors. Pneumologie 2012: P66 - P187.
- Schlomm T, Lenkewitz B, Müller D, Zierott G, Füzesi L (2002) Amyloidtumor im Kolon: Differenzialdiagnose stenosierender Darmprozesse. Viszeralchirurgie 37: 365-368.
- Mendeleeva LP, Rekhtina IG, Kovrigina AM, Kostina IE, Khyshova VA, et al. (2020) Bone disease as the first manifestation of systemic AL-amyloidosis. Ter Arkh 92: 85-89.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.