Immune Thrombocytopenia: Dexamethasone versus Prednisolone-Systemic Review and Meta-Analysis
by Algariri Najla1*, Almoutairi Nour2, Alnawfal Bedah3
1Pediatric Hematology-Oncology fellow, King Saud Medical City, Riyadh, Saudi Arabia
2FRCPC Jaber Al-Ahmad Hospital, Kuwait
3Pediatric Hematology Oncology Consultant, King Saud Medical City, Riyadh, Saudi Arabia
*Corresponding author: Algariri Najla, Pediatric Hematology-Oncology Fellow, King Saud Medical City, Riyadh, Saudi Arabia.
Received Date: 24 January 2025
Accepted Date: 31 January 2025
Published Date: 04 February 2025
Citation: Najla A, Nour A, Bedah A (2025) Immune Thrombocytopenia: Dexamethasone versus Prednisolone-Systemic Review and Meta-Analysis. Hem Disease Therapies 9: 136. https://doi.org/10.29011/2577-1418.000136
Abstract
Background: Immune thrombocytopenia (ITP) is an acquired disorder marked by destruction of platelets and suppression of their production, which may cause a range of bleeding symptoms. The use of dexamethasone versus prednisolone in managing immune thrombocytopenic purpura is still debatable. Consistent patterns and achievements can be demonstrated by systematic review. Objectives: To evaluate the effectiveness and safety of Dexamethasone in compare with prednisolone in treatment of ITP. Search method: We searched Embase database searched through Ovid interface from 1946 to 24/September/2024. The main search concepts are covering diagnosis (immune thrombocytopenia), intervention (steroids), with relevant synonyms for both concepts, and the RCTs filters. There were no restrictions for language, date of publication, or any other restrictions. Millions of hits (for intervention and RCTs filters) were identified, so one filter was added to involve non animals’ studies, number of hits of RCTs filters was decreased, however still in millions. Then the search combined filters together ran again and 4611 hits were identified.
Additional Resources: The Last 15 years of the following conferences and meetings will be searched manually to capture eligible articles:
The European Hematology Association congress EHA.
The American Society of Hematology Annual Meeting and Exposition ASH.
Selection criteria: All published randomized clinical trials comparing patients (pediatrics and adults) with ITP receiving dexamethasone versus prednisolone/ prednisone. Studies that prematurely terminated were excluded, as well as studies that have combination of steroids with the second or third line of ITP treatment (rituximab, thrombopoietin receptor agonists, or splenectomy).
Data management: Data collection and risk of bias assessment were done by two independent reviewers. The risk of bias across all outcomes was conducted in accordance with the guidelines outlined in the Cochrane Handbook for Systematic Reviews of Interventions, using ROB 2 tool. Any disagreements that arose during this process were resolved through consensus. And data were pooled using the random-effects model.
Results: Rate of response at 6 month: Total participants from three studies are 422, the response rate for platelets at 6 months found in 145 of treatment group (67%), and 135 in control group (65.5 %), with RR 1.07, CI 95% ( 0.72- 1.6 ).
Hypertension: only two studies measured hypertension in their results, in intervention group 19 out of 140 participants developed hypertension(13.6%), while in control group 18 out of 131 participants (13.7%), RR 0.98 with CI 95% ( 0.54 to 1.78).
Hyperglycemia/glucose intolerance: only two studies measured glucose in their results, in intervention group 7 out of 106 participants developed hyperglycemia/ glucose intolerance (6.6%), while in control group 10 out of 105 participants (9.5%), RR 0.66 with CI 95% (0.11 to 3.35).
Background
Description of the condition: Immune thrombocytopenia (ITP) is an acquired disorder marked by destruction of platelets and suppression of their production, which may cause a range of bleeding symptoms. ITP usually has an acute onset, follows a viral illness that occurred in the weeks prior to presentation. The peak pediatric age is 2–6 years, but it can occur at any age. Primary ITP is defined as immune-mediated thrombocytopenia without identified causative factors. While Secondary ITP is associated with underlying cause, approximately 20% of ITP is secondary. The focus in the treatment of secondary ITP is on the underlying disease. ITP is characterized by the presence of autoantibodies against platelet glycoproteins. These autoantibodies facilitate the opsonization of platelets, subsequently leading to their phagocytosis and destruction by the reticuloendothelial system. However, in some cases, the immune system may also target and suppress megakaryocytes[1], as well as imbalance of cytokines levels can inhibit megakaryocyte function and platelet production[2].
Description of Intervention: Steroids are suppressing antibody production and enhancing platelet survival. Since 1960s steroids have become a mainstay in therapeutic regimens, often providing a confirmatory diagnosis through the observed rise in platelet counts following administration.
How the intervention might work: Steroids result in increased platelet counts in two-thirds of patients [3]; however, these effects are transient. Also, Steroids may contribute to increased platelet production. Despite their benefits, the use of steroids is associated with adverse effects, including weight gain, hypertension, hyperglycemia, mood alterations, and an increased risk of osteoporosis. These side effects necessitate careful monitoring during treatment. Since many years, the standard therapy is prednisone at 1 mg/kg/day for 2-4 weeks, followed by tapering it; this approach was based on results from a randomized controlled trial conducted in ITP patients in 2002 and showing that longer steroid treatment improved platelet counts [4]. Some experts propose using higher-dose prednisone for shorter duration to minimize side effects, while others prefer dexamethasone, which is more potent, for longer duration. It is reported that high-dose dexamethasone (HDD) modifies T-cell function effectively [5]. HDD has been used as daily dose of 40 mg/kg/day (24 mg/m2) orally for four days given every 14 days for three cycles; this approach has been shown to have an initial response rate of 85% in adults and children but the durable response is not nearly as good [6].
One study showed comparable long-term effects of HDD and standard prednisone. However, HDD may result in quicker increases in platelet counts, particularly in acute cases [7].To determine whether HDD is more likely to result in a sustained response, a trial involved 192 adults with newly diagnosed ITP who were randomly assigned to receive 1–2 cycles of HDD or four weeks of prednisone. Individuals who received two cycles of HDD experienced a faster rise in platelet counts, but the 6-month sustained response rates were similar in both groups.8 Mithoowani et al. expanded on this information with a systematic review and meta-analysis that encompassed nine randomized trials comparing different steroid regimens. Among these, five studies (n = 533) compared one to three cycles of HDD to standard prednisone dosing. Results showed that 14 days after treatment initiation, the platelet count was higher in adults treated with HDD regimens, but 6-month durable response rates were found to be similar across all groups.9
Why it is important to do this review: The use of dexamethasone versus other steroids in managing ITP is still debatable. Dexamethasone has a more potent anti-inflammatory effect than all other steroids and has a longer half-life. Understanding the implications of these pharmacokinetic differences on treatment effectiveness and safety will help clinicians to make decisions, it is important to evaluate the sustainability of such responses. Moreover, comparing side effects associated with each medication is a crucial factor in any patient. Consistent patterns can be demonstrated by systematic review and aggregating safety data will form a clearer picture regarding risks associated with each treatment. The results of this review will be disseminated among clinicians through local conferences to help them in making informed decisions.
PICO:
P (Population):People of all age groups whose diagnosed with primary ITP, regardless of severity levels of thrombocytopenia and bleeding symptoms.
I (Intervention):Administration of dexamethasone.
C (Comparison): prednisolone
O (Outcomes):1. Response Rate: the percentage of patients who achieve a significant increase in platelet count (as each study defined this significance) recorded at 6 months. 2. Side Effects: hyperglycemia or glucose intolerance, as well as hypertension.
Methods
Protocol and registration:
This review’s protocol was registered in 19th January 2025. INPLASY registration number: INPLASY202510072.
Criteria for selecting studies for this review:
A. Study types:1. Study Design: Randomized controlled trials that compare dexamethasone to other steroids in the treatment of primary ITP were included.
3. publication status: Only published studies were included to ensure the data is complete.
B. Intervention and control types: All forms of dexamethasone versus prednisolone/ prednisone, (intervenous, oral) were used as a single agent, or in combination with other first-line pharmaceutical agents like intravenous immunoglobulin, or anti-D immunoglobulin were included.
C. Participants: Adults and pediatric patients with primary ITP.
Exclusion Criteria:
A.Study types: 1. Premature Termination: Trials that were stopped prematurely will be excluded to ensure the integrity of the data. 2. No enough data for response rate. 3. Pilot studies
B. Intervention types:Combination of assigned intervention with the second or third line of ITP treatment (rituximab, thrombopoietin receptor agonists, or splenectomy) was excluded, to avoid bias in the outcome.
Search methods for identifications of studies:
Bibliographic databases and additional resources to be searched:
Embase database searched through Ovid interface from 1946 to 24/September/2024
Search strategy and restrictions:
The main search concepts are covering diagnosis (immune thrombocytopenia), intervention (steroids), with relevant synonyms for both concepts, and the RCTs filters. There were no restrictions on language, date of publication, or any other restrictions.
Millions of hits (for intervention and RCTs filters) were identified, so one filter was added to involve non animals’ studies, the number of hits of RCTs filters was decreased, however still in millions. Then the search combined filters together ran again, and 4611 hits were identified.
Consultation for librarian was done through emails, to figure out the problems in this search strategy as the famous articles that are eligible for the project did not show within the search strategy attempts which were done before. Although, the previous similar systemic review’s terms were applied. Advice from the librarian was followed, particularly, in Cochrane RCTs filters, and Mesh terms were applied, and formal errors were corrected. Famous articles appeared after that.
Additional Resources: The Last 10 years of the following conferences and meetings will be searched manually to capture eligible articles:
The European Hematology Association congress EHA.
The American Society of Hematology Annual Meeting and Exposition ASH.
Since unpublished studies was excluded, YODA project and clinical trail.gov were not searched.
List possible search terms for the major concepts:
Diagnosis: Idiopathic thrombocytopenic purpura, thrombocytopenia, ITP. (Initially immune thrombocytopenia was used as a filter as well, however, the results were ther same as idiopathic thrombocytopenic purpura, so in the next attempts this term was excluded). Intervention: corticostéroids, glucocorticosteroids, steroids, dexamethasone, prednisolone, prednisone, adrenal cortex hormones. RCTs filters: we followed Cochrane RCTs filters (see the below search strategy appendix (1).
Data collection and analysis:
The titles and abstracts of the retrieved studies were screened mainly through chaton AI software initially then by two independent reviewers, and Abstract and full text of the studies that passed the screening were also assessed by the same two reviewers independently considering the inclusion criteria. Any disagreement between the reviewers was resolved through discussion. See figure (1) and table (1).
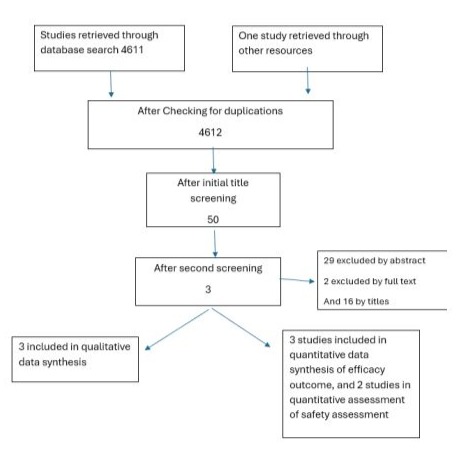
Figure 1: Flow diagram of selecting articles
|
Actual number of study in search strategy |
Name of author / publication date |
screening level |
Cause of exclusion from our review |
|
|
1 |
71 |
Wang JD et al. 2024. May |
Title and abstract |
Observational cohort study |
|
2 |
154 |
Huang QS et al. 2021 Oct. |
Title and abstract |
The intervention is All-tans retinoic plus dexamethasone acid versus dexamethasone alone |
|
3 |
184 |
Sadeghi et al. 2020 |
Full text |
Short follow up period only 1 month for rate of response outcome |
|
4 |
445 |
Marcello Candelli et al. 2021 |
Title and Abstract |
Case report |
|
5 |
504 |
Willian EL. Et al. 2021 |
Title and Abstract |
Not RCT |
|
6 |
580 |
Ma.J et al. 2020 |
Title and Abstract |
Comparing dexamethasone with prednisone (not prednisolone) |
|
7 |
623 |
Li.Ju et al. 2020 |
Title and Abstract |
Not RCT |
|
8 |
712 |
Wang X. et al.2019 |
Title and Abstract |
Not RCT |
|
9 |
856 |
Takase K. et al . 2019 |
Title and Abstract |
Not RCT |
|
10 |
873 |
Shao.X.et al 2018 |
Title and Abstract |
Not RCT |
|
11 |
911 |
Arai.Y. et al .2018 |
Title |
Not RCT |
|
12 |
1056 |
Arai.Y.et al . 2017 |
Title |
Not RCT |
|
13 |
1090 |
Mithoowani S. et al. 2016 |
Title |
Not RCT |
|
14 |
1123 |
Matschke J. et al 2016 |
Title and Abstract |
Comparing dexamethasone with prednisone (not prednisolone) |
|
15 |
1185 |
Wei Y. et al. 2016 |
Full text |
Comparing dexamethasone with prednisone (not prednisolone) |
|
16 |
1503 |
Zhan Y et al. 2013 |
Title and Abstract |
Not RCT |
|
17 |
1564 |
Cao J. et al. 2012 |
Title |
Not RCT |
|
18 |
1604 |
Hua F. et al 2012 |
Title |
Not RCT |
|
19 |
1747 |
Bilgir O. et al. 2011 |
Title and Abstract |
Not RCT |
|
20 |
1780 |
Liu XG. Et al. 2011 |
Title and Abstract |
Not RCT |
|
21 |
1886 |
Yadav D. et al. 2010 |
Title and Abstract |
Not RCT |
|
22 |
1902 |
Naithani R. et al. 2010 |
Title and Abstract |
Not RCT |
|
23 |
1973 |
Shan NN.et al 2009 |
Title and Abstract |
Not RCT |
|
24 |
1964 |
Su Y, et al .2009 |
Title |
Observational study |
|
25 |
2303 |
Mazzucconi MG. et al. 2007 |
Title and Abstract |
Not RCT |
|
26 |
2503 |
Jubinsky PT. et al. 2005 |
Title |
Not ITP study |
|
27 |
2571 |
Borst F. et al. 2004 |
Title and Abstract |
Not RCT |
|
28 |
2660 |
Cheng Y. et al. 2003 |
Title and Abstract |
Not RCT |
|
29 |
2712 |
Gutierrez-Espindola et al. 2003 |
Title and Abstract |
Not RCT |
|
30 |
2718 |
Hedlund-Treutiger et al. 2003 |
Title and Abstract |
Chronic ITP. Comparison between dexamethasone and IVIG. |
|
31 |
2742 |
Olcay L. et al. 2002 |
Title |
Pilot study, not RCT |
|
32 |
2784 |
Wali YA. et al. 2002 |
Title and Abstract |
Not RCT |
|
33 |
2811 |
Adams JR. et al. 2002 |
Title and Abstract |
Not RCT |
|
34 |
2947 |
Stasi R. et al. 2000 |
Title and Abstract |
Not RCT |
|
35 |
3021 |
Ohmine K. et al 2000 |
Title and Abstract |
It Compared prednisolone with different doses |
|
36 |
3109 |
Baronci, C. et al. 1998 |
Title |
Observational study |
|
37 |
3189 |
Arruda VR. et al. 1997 |
Title |
Case report |
|
38 |
3214 |
Demiroglu et al. 1997 |
Title and Abstract |
Not RCT |
|
39 |
3208 |
Byrne JD. et al. 1997 |
Title and Abstract |
Case report |
|
40 |
3650 |
Vermylen C et al. 1990 |
Title and Abstract |
Not RCT, retrospective comparison between steroids and IVIG |
|
41 |
4052 |
Ozhegov, A M.. et al. 1981 |
Title |
No abstract , no full text available |
|
42 |
4028 |
Weinblatt, M E. et al 1982 |
Title and Abstract |
Rétrospective study |
|
43 |
4087 |
Harry W. Carloss et al. 1980 |
Title and Abstract |
No full text available |
|
44 |
4116 |
F Romero-García et al. 1979 |
Title |
No abstract, nor full text available |
|
45 |
4302 |
Ojeda Perez E. et al. 1973 |
Title |
No abstract, nor full text available |
|
46 |
4601 |
Clement F. et al. 1966 |
Title |
No abstract, nor full text available |
|
47 |
4604 |
Pizzi F. et al. 1966 |
Title |
No abstract, nor full text available |
Table 1: Studies excluded after second screening
Data extraction and management: excel sheet was prepared for extracting data from the included articles, and two reviewers independently extracted data into her own sheet for the listed outcomes. Differences between the reviewers regarding the data were resolved by discussion. The gathered data were described in the attached excel sheet (see tables 2 and 3).
|
Study/year of publication |
Design of study |
eligibility criteria |
Duration of the study (months) |
Groups |
Patient (N) |
Age |
Male % |
|
Sung Hwa Bae et al/ 2010 10 |
randomized multicentre trial |
treatment-naïve patients, 16 years or older, with a diagnosis of ITP according to the practice guidelines of the American Society of Hematology and a platelet count of 30×109/L or less. |
48 |
Group A DEX 40 mg/day on day 1–4 (If the platelet count dropped below 3×109/L within the first six months, another four-day course of DEX was given) Group B Prednisolone 1mg/kg/day for 4 weeks |
151 |
Median age 44 years in both groups |
30.5% |
|
Mashaddi et al./ 2012 11 |
single-blind randomized prospective single center study |
Newly diagnosed primary ITP with platelet count less than 20,000/microliter. Exclusion criteria: Patients with previously treated ITP, diabetes, liver and kidney dysfunction, infections including HIV, HCV, HBV, pregnancy and SLE or any acute illness |
12 |
Group A received dexamethasone 40mg/day/IV(10mg/q6h) for four days and then prednisolone 1mg/kg/day/PO with 10 mg/week tapering and discontinuation after 6weeks. Group B treated with prednisolone 1mg/kg/day for four weeks and then tapering 10mg/week. |
60 |
Mean group A 24.9(17.5–44) Group B 27.2(18–48.4) |
21.6% |
|
Jie Ma. et al. 2020 12 |
prospective, single-center, non-inferiority, randomized controlled clinical trial |
1) ITP diagnosed based on the recently released international consensus guidelines , (at least two blood tests with platelet counts below 100 × 109/L); normal blood cell morphology, bleed ing of the skin, ecchymosis and/or mucous membranes, organ bleeding or other clinical manifestations; no sple nomegaly, exclusion of other secondary thrombocytopenia types, such as aplastic anemia or inherited thrombocytope nia secondary to other immune diseases, infection or drug factors; (2) age between 1 month and 18 years; (3) platelet count ≤ 30 × 109/L; (4) no previous targeted platelet eleva tion treatment; (5) follow up possible in our hospital. Exclusion criteria were: (1) non-primary ITP, including inherited thrombocytopenia and acquired thrombocytopenia; (2) a history of previous targeted platelet elevation treatment; (3) life-threatening severe bleeding risk (Buchanan bleeding score of five) |
12 |
The HDD group received 0.6 mg/kg/day dexamethasone (maximum 40 mg/day) by intravenous infusion for 4 consec utive days. Patients who did reach a response after 2 weeks received a second cycle of HDD. The PDN group received the standard PDN 2 mg/kg dose (maximum 60 mg/day) for 2 consecutive weeks. In respond ers, the prednisone was gradually tapered over the 4–8 con secutive weeks. If patients were not able to reach a complete response (CR), a low dose was used, maintaining a platelet count above 30×109/L with an absence of bleeding symp toms; taper schedule by physicians. |
211 |
Median age, m (range) In dexamethsone group 30(2–159) And in predinsolone group 29(1–147) |
62% |
Table 2: Baseline characteristics for included studies
|
Dexamethasone group |
Prednisolone group |
RR |
N of participants |
GARDE |
Comments |
|
|
Plat response rate at 6 months |
145/216 |
135/206 |
1.07(0.72 to 1.6) |
422 |
v.low |
That means 7% higher response in dexamethasone group compared with prednisolone group at 6 months however, CI crossin1 which mean statistically insignificant As well as 7% much less than predetermined MID 30% |
|
Mean Platelets count at 6 months |
Not mentioned plat counts at 6 months in all studies |
|||||
|
Hypertension |
19/140 |
18/131 |
0.98 (054 to 1.78) |
271 |
Study Sung Hwa Bae et al. 2010 did not document hypertension as a side effect Study Jie Ma et al 2020 showed dexamethasone group has 1.5% higher risk in developing hypertension than prednisolone group While Meshaddi et al. 2016 showed 6.7% lower risk in developing hypertension in dexamethasone group Jia Ma et al study contributes more in pooled estimate, however the conflicting results drom individual studies contributed in the overall uncertainty CI is statistically insignificant |
|
|
Hyperglycemia/glucose intolerance |
7/106 |
10/105 |
0.66 (0.11 to 3.35) |
Study number 3 Jie Ma et al. 2020 did not document hyperglycemia or glucose intolerance as a side effect. RR is 0.66 which indicates lower risk of hyperglycemia in dexamethasone group by 60% which meet MID 30% or more, however, CI is wide and crossing 1 which indicates statistical insignificant |
||
Table 3: Summary of the findings.
The collected variables were studies’ baseline characteristics (see table 2 for more details), platelet count /mean at 6 months, people response rate number and percentage at 6 months, hyperglycemia/ glucose intolerance percentage, hypertension percentage. (see table (2, 3)). Appendix (3).
Assessment of risk of bias: To assess the risk of bias across all outcomes, two authors conducted an independent evaluation of the studies in accordance with the guidelines outlined in the Cochrane Handbook for Systematic Reviews of Interventions. Any disagreements that arose during this process were resolved through consensus. The evaluation employed the RoB2 tool developed by Cochrane, concentrating on specific domains to identify potential biases: biases stemming from the randomization process (including sequence generation and allocation concealment), biases resulting from deviations from the intended intervention (such as participant and personnel blinding), biases associated with missing outcome data, biases in outcome measurement, and biases related to selective reporting. Each item and subitem was assessed using signaling questions from the tool, facilitating responses categorized as “Yes,” “No,” “Probably Yes,” “Probably No,” and “No Information.” Subsequently, an overall bias assessment was reported. " The overall trial was classified as follows: • “Low risk of bias” if all domains were assessed as low risk; • “Some concerns of bias” if there were concerns in one domain but no high risk in any domain; and • “High risk of bias” if at least one domain was rated as high risk or if multiple domains presented concerns. Figure 1 illustrates the summary of risk of bias judgements for each of the five domains, along with the overall risk of bias assessment for the Response Rate, defined as the percentage of patients achieving a significant increase in platelet count (as defined by the study) recorded at 6 months post-treatment. (see table (4) and appendices (2).
|
Study |
randomization process |
deviations from the intended interventions |
Missing outcome data |
measurement of the outcome |
selection of the reported result |
Overall risk of bias |
|
Mashaddi et al. 2016 |
Low |
Some concern |
Low |
Low |
Low |
Some concern |
|
Jie Ma et al. 2020 |
Low |
Some concern |
Low |
Low |
Low |
Some concern |
|
Sung Hwa Bae et al. 2010 |
Low |
Some concern |
High |
Low |
Low |
High risk |
Table 4: Summary of risk of bias (for 6 month response rate) of included studies
Measure of treatment effect:
We used data-party website to perform the data analysis. For dichotomous outcomes, we expressed the difference as risk ratios (RRs) with 95% confidence intervals (CIs). For platelets count increase as continuous data, we planned to express results as mean differences (MD) in percent of changes with 95% CI however, no study mentioned platelets count at 6 months.
Dealing with missing data:
We planned to contact the authors of studies with missing data. For any calculation of measurement, we did by ourselves, we planned to report such data in the result of the analysis of the related outcome.
Assessment of heterogeneity:
We assessed the heterogeneity between pooled trials using a combination of Chi-squared test (P<0.10 was considered significant) and the I² statistic. The I² value was assessed as “low or unimportant” (more than 30% and below 40%), “moderate” (30% to 60%), or “high” (>60% to 100%). We also considered the qualitative evaluation of heterogeneity.
Assessment of reporting biases:
We planned to generate and assess funnel plots (if there were more than 10 RCTs in meta- analysis) to assess the effects of small studies. However, we got fewer than 10 RCTs in the meta-analysis. Although limitation of funnel plots in providing good assessment for publication bias with this little number of studies that we included, we did it for sake of exploring the technique. (See figure 4)
Figure 4: Funnel plot for included studies
Data synthesis: We pooled the results of trials using a random-effects model.
Subgroup analysis and investigation of heterogeneity:
We planned to perform subgroup analyses of studies that were done on adults versus children with ITP. In case of detecting any heterogeneity, we planned to perform sensitivity analysis for studies with a high risk of bias, however we ended up with few studies and all have same level of bias risk.
Assessment of the certainty of the evidence:
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework was employed to evaluate the certainty of evidence for each of the predefined outcomes in our review. A duplicate GRADE assessment was conducted for each outcome, and We utilized the five GRADE criteria—risk of bias, inconsistency, indirectness, imprecision, and publication bias—to assess the quality of the evidence contributing to this meta-analysis, it was categorized as high, moderate, low. Given that all included studies were randomized controlled trials (RCTs), the evidence was initially rated as high. The rationale for any downgrading of the evidence is detailed in table (5). For interpreting the significance of differences between dexamethasone and prednisolone groups, a minimal important difference of 30% was adopted for outcome of response rate at 6 months, this was based on clinical experience.
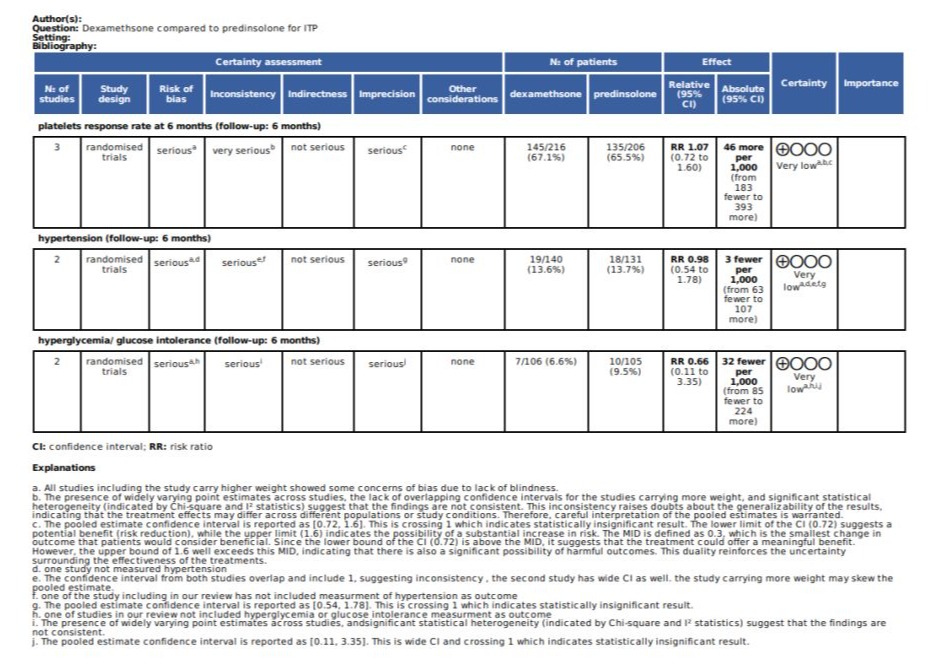
Table 5: Grade Assessment.
Results:
Description of studies and results of the search:
The search was done in September 2024. Embase database searched through Ovid interface from 1946 to 24/September/2024. Considering the search keywords, a total of 4611 studies were retrieved. In addition to one study was obtained from Searching other sources. Initial screening was done for titles with one reviewer and chaton AI as second reviewer, then another screening process was done by another reviewer for titles, after that we end up with 50 studies, 47 was excluded as explained in table (1). The disagreements were resolved by consensus. Finally, considering the inclusion and exclusion criteria and after in-depth evaluation, three articles were selected for included in the analyses. Details of the process of screening and selection of articles are shown in figure (1).
Figure 1: Forest plot for platelets response at 6 months.
Included studies:
Table (2) shows the basic characteristics of the three included studies10,11,12. In total 422 patients were included in all studies consisting of 145 patients in treating arms and 135 patients in the control arms for first outcome (response rate at 6 months). The studies were published between 2010 and 2020, and the follow up period ranged from 12 months to 48 months (median of 12 months). The total doses of dexamethasone that had been used in almost all studies was about 40 mg per kg per day. Two studies were single center, and one was multicenter.
Excluded studies:
Table (2) shows the characteristics and reasons for excluding studies.
Risk of bias in included studies:
We assessed risk of bias in all included studies, which showed overall risk of bias from some concern to high risk. Table (4) shows the summarized risk of bias in various domains. Appendix (2) shows detailed judgements about the risk of bias in different studies.
Effects of interventions and assessment of the evidence:
Efficacy: Rate of response at 6 month:
Total participants from three studies are 422, the response rate for platelets at 6 months found in 145 of treatment group (67%), and 135 in control group ( 65.5 %), with RR 1.07, CI 95% ( 0.72- 1.6 ) which indicates that the treatment group has 7% positive response rate higher than control group, however, quite wide confidence interval indicates uncertainty of the results. Range of CI 0.72 to 1.6, indicating the effect size is imprecisely estimated and could range from 28% lower platelets response to 60% higher response in the treatment group compared with control group. As well as we predefined MID as 0.3 (30%), we calculated absolute risk increase (ARI) 0.031 (3.1%), CI 95% ( -3.1 to 9.2), this ARI is smaller than the threshold for clinical importance, as well as wide confidence interval indicates uncertainty and impression of the data. Heterogeneity is high (I2is 81%) which indicates substantial variability in the response rates among the three studies included in this review. This heterogeneity could be caused by different population age groups, could be related to different treatment protocols. Due to small numbers of study, it will be difficult to do subgroup analysis.
Safety:
Hypertension: only two studies measured hypertension in their results, in intervention group 19 out of 140 participants developed hypertension (13.6%), while in control group 18 out of 131 participants (13.7%), RR 0.98 with CI 95% (0.54 to 1.78), this indicates in development of hypertension is 2% lower in treatment group compared with control group, however, this is statistically insignificant as CI is crossing 1, and quite wide. (this is equal to 0.1% absolute risk lower in treatment group compared with control group 95%CI -0.27 TO 0.27).
Hyperglycemia/glucose intolerance: only two studies measured glucose in their results, in intervention group 7 out of 106 participants developed hyperglycemia/ glucose intolerance (6.6%), while in control group 10 out of 105 participants (9.5%), RR 0.66 with CI 95% ( 0.11 to 3.35), this indicates in development of hyperglycemia / glucose intolerance absolute risk is 3% lower in treatment group compared with control group, however, this is statistically insignificant as CI is crossing 1, and wide.( As well as this outcome has moderate heterogeneity (I2is 55%), which indicates some variability in effect size across the studies included.
Summary of Findings Table:
Table (3) shows the summary of findings for different outcomes.
Discussion/conclusions:
Summary of main results and certainty of the evidence: The main results indicate the treatment group had a 7% higher positive platelet response rate at 6 months compared to control (67% vs 65.5%), but the wide 95% CI (0.72 to 1.6) suggests substantial uncertainty around the precision of this effect. The predefined minimal important difference was 30%, and the 3.1% absolute risk increase was smaller, with wide CIs (-3.1% to 9.2%) again indicating uncertainty around clinical importance. High heterogeneity (I2=81%) suggests variability in response rates across studies, potentially related to population or protocol differences. Regarding safety, the review found no statistically significant differences between groups in the development of hypertension or hyperglycemia/glucose intolerance. Absolute risk differences were small, and there was moderate heterogeneity for hyperglycemia.
Overall completeness and applicability of evidence: Overall, this review provides a mixed assessment of the current evidence. While the treatment group had a slightly higher positive response rate, the uncertainty around the effect size and the lack of clinically meaningful differences limit the strength of the conclusions. Additional high-quality studies may be needed to further clarify the relative efficacy and safety of different corticosteroid regimens for the management of immune thrombocytopenia.
Potential biases in the review process:
Agreements and disagreements with other studies or reviews: The findings of this review are generally in agreement with other published systematic reviews on this topic. For example, the review by Mithoowani et al. found no difference in long-term platelet response between high-dose dexamethasone and standard-dose prednisone for previously untreated immune thrombocytopenia9. However, the current review provides additional insights by exploring the impact of different dexamethasone regimens.
Authors’ conclusions:
Implications for practice: Low level of evidence in improvement of platelets response at 6 months with using of dexamethasone, indicates uncertainty of clinical benefit of using dexamethasone in ITP, as well as low level of evidence regarding safety of this steroid in compare with standard treatment (prednisolone), indicates no clinical benefit of dexamethasone over prednisolone. However further low risk bias trials need to be done to confirm these findings.
Implications for research:
As only one trial was done including the pediatric age group, further research is needed for this age group. As well as all the trials have concern in bias assessment, further low risk bias trials needed to be conducted to provide good evidence for clinical practice.
Acknowledgments:
We acknowledged chaton AI in helping for initial screening. No funding was received for this project.
References
- Martínez-Carballeira D, Bernardo Á, Caro A, Soto I, Gutiérrez L (2024) Pathophysiology, Clinical Manifestations and Diagnosis of Immune Thrombocytopenia: Contextualization from a Historical Perspective. Hematol. Rep 16: 204-219.
- Tang F, Tang CF, Jiang X, Jia XF, Liu SC, et al. (2020) [Correlation analysis of genotypes and the enzymatic activities of glucose-6-phosphate dehydrogenase in neonates in Guangzhou]. Zhonghua Yu Fang Yi Xue Za Zhi 54:1275-1282.
- Donald M Arnold, Adam Cuker Initial-treatment-of-immune-thrombocytopenia-itp-in-adults.
- Godeau B, Chevret S, Varet B, Lefrere F, Zini JM, et al. (2002) Intravenous immunoglobulin or high-dose methylprednisolone, with or without oral prednisone, for adults with untreated severe autoimmune thrombocytopenic purpura: a randomised, multicentre trial. Lancet 359: 23–9.
- Liu Z, Wang M, Zhou S, Ma J, Shi Y, et al. (2016) Pulsed high-dose dexamethasone modulates Th1-/Th2-chemokine imbalance in immune thrombocytopenia. J Transl Med 14: 301.
- Mazzucconi MG, Rodeghiero F, Avvisati G, De Stefano V, Gugliotta L, et al. (2024) Prednisone vs high-dose dexamethasone in newly diagnosed adult primary immune thrombocytopenia: a randomized trial. Blood Adv 8: 1529-1540.
- Matschke J, Muller-Beissenhirtz H, Novotny J, Vester I, Hertenstein B, et al. (2016) A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naive adult patients with immune thrombocytopenia: EIS 2002 study. Acta Haematol 136: 101–7.
- Wei Y, Ji XB, Wang YW, Wang JX, Yang EQ, et al. (2016) High-dose dexamethasone vs prednisone for treatment of adult immune thrombocytopenia: a prospective multicenter randomized trial. Blood. 127: 296-302.
- Mithoowani S, Gregory-Miller K, Goy J, Miller MC, Wang G, et al. (2016) High-dose dexamethasone compared with prednisone for previously untreated primary immune thrombocytopenia: a systematic review and meta-analysis. Lancet Haematol 3: e489-e96.
- Sung Hwa Bae, Hun-Mo Ryoo, Won Sik Lee, Young Don Joo, Kyoo Hyung Lee, et al. (2010) High Dose Dexamethasone Vs. Conventional Dose Prednisolone for Adults with Immune Thrombocytopenia: a Prospective Multicenter Phase III Trial. Disorders of Platelet Number or Function: Poster III. 116: 3687.
- Mashhadi MA, Kaykhaei MA, Sepehri Z, Miri-Moghaddam E (2012) Single course of high dose dexamethasone is more effective than conventional prednisolone therapy in the treatment of primary newly diagnosed immune thrombocytopenia. Daru. 20: 7.
- Ma J, Fu L, Chen Z, Gu H, Ma J, et al. (2020) High-dose dexamethasone as a replacement for traditional prednisone as the first-line treatment in children with previously untreated primary immune thrombocytopenia: a prospective, randomized single-center study. Int J Hematol. 112: 773-779.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.