Handheld Electrospinning Technology for Wound Care: An Evaluation of Performance Against Current Therapies A Systematic Review
by Nihad Kadi
Medical Student, Swansea University, Wales, UK
*Corresponding Author: Nihad Kadi, Medical Student, Swansea University, Wales, UK.
Received Date: 15 August 2025
Accepted Date: 22 August 2025
Published Date: 25 August 2025
Citation: Kadi N (2025) Handheld Electrospinning Technology for Wound Care: An Evaluation of Performance Against Current Therapies A Systematic Review. Clin Exp Dermatol Ther 10: 239. https://doi.org/10.29011/2575-8268.100239
Abstract
Electrospinning (ES) uses a high-voltage electric field to draw viscous solutions through a fine needle, producing ultrathin fibres. In the last two decades, interest has grown in ES for wound dressings, leading to developments such as handheld ES devices. Wound care remains a significant challenge in the NHS, costing an estimated £8.3 billion annually. Around £2.7 billion is spent on wounds that heal, but £5.6 billion goes towards those that fail to do so. Slow or non-healing wounds are therefore a key target for innovation. Nanofibres produced by ES have a high surface area-to-volume ratio, closely resembling the extracellular matrix (ECM). This structure supports cell adhesion, migration, and proliferation, while the mechanical strength of synthetic ES fibres addresses weaknesses in current NHS dressings such as hydrogels or hydrocolloids. These traditional dressings are effective but can lack structural integrity. ES fibres can also be loaded with drugs or growth factors, offering controlled and predictable release to aid tissue repair. Handheld ES devices have the advantage of applying these fibres directly to the wound bed, potentially improving healing outcomes, particularly in complex or chronic wounds. However, while the technology is promising, the costeffectiveness of implementing handheld ES on a large scale within the NHS is still uncertain. This review explores the ideal features of wound dressings and evaluates whether handheld ES can outperform existing treatments. Early evidence suggests ES could offer superior mechanical, biological, and drug delivery benefits, but economic feasibility remains a key barrier.
Keywords: Electrospinning; Wound care; Current Therapies;
Abbreviations:
- PLGA: Poly(lacto-co-glycolic acid)
- ES: Electrospinning
- BMSCs: Bone-marrow derived stem cells
- Kv: Kilovolts
- PVA: Polyvinyl alcohol
- Bn: Billion
- Mpa: Megapascals
- g: Gram
- PUSH: Pressure ulcer scale for healing
- CMC: Carboxymethyl cellulose
- DFUs: Diabetic foot ulcers
- PCL: Polycaprolactone
- PDLLA: Poly(DL-lactic acid) Hydrogels
- PVP: Polyvinylpyrrolidone
- VLUs: venous leg ulcers
- RCT: Randomized control trial
- NHS: National health service
- SEM: Scanning electron microscopy
Introduction
he first decades of the 20th century saw the development of electrospinning (ES) as a practical method for spinning fibres. In 1902, J. F. Cooley submitted a patent for the concept and technology of producing powders or fibres by utilising a high-voltage equipment (Figure 1) [1].
The evolution of electrospinning technology
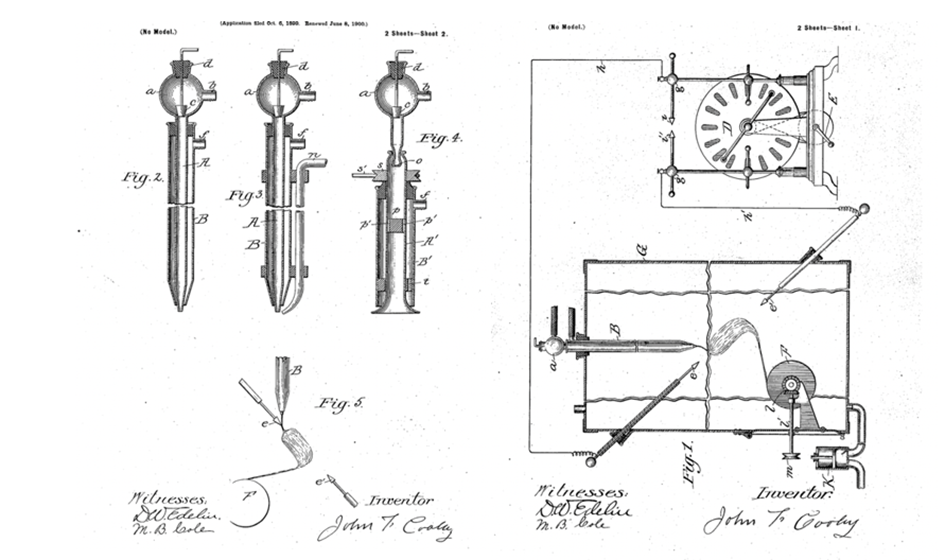
Figure 1: The first patent submitted for producing fibres using high voltage equipment [2].
The study of ES technology remained relatively untouched until the early 1990s, when Jayesh Doshi and Darrell Reneker discovered that the diameter of the fibres is inversely proportional to the distance from the needle tip to the collector, which uncovered the modifiable nature of ES fibres. This marked the beginning of the modern era of electrospinning (Figure 2) [3]. Modern day electrospinning is a highly adaptable technique that produces fibrous material in the nanometric range with a controllable surface morphology. The variability in porosity of the nanofiber-based meshes can be adjusted and regulated through modifications made to the experimental technique.
Electrospinning involves the application of a high electric field (kV range) to liquid substances, thereby facilitating the formation of highly charged jets with fine characteristics. Typically, the liquids consist of polymeric solutions, emulsions, polymer melts, or suspensions that include one or more active medicinal components. It has been utilised in various fields including biomedical studies, tissue engineering, environmental, biochemical, drug delivery, protective clothing, and energy storage [4].
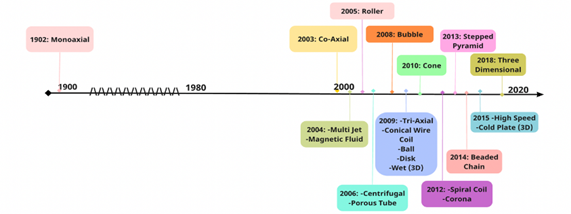
Figure 2: A diagram depicting all the evolutions of electrospinning. All the iterations from past to present [3].
Monoaxial electrospinning
The first and most prominent iteration of ES, mono-axial ES, uses a single capillary nozzle and high voltage. In monoaxial electrospinning, a polymer liquid (often a solution, suspension, or emulsion) can be used as a drugs delivery system. During the process, a greater electrical potential (approximately 5-20 kV) is supplied between the needle and the metal collector [4].
The polymer liquid is introduced into the system by the nozzle and is intermittently propelled by the pump. Subsequently, the substance is exposed to a difference in electrical potential existing between the nozzle and the counter electrode. The electrical voltage produced by the source induces a conical deformation in the polymer solution droplet. The solvent within the solution evaporates as it travels towards the counter electrode. Ultimately, this process results in the formation of solid, uninterrupted filaments [5,6]. Depending on arrangement of the equipment setup, the monoaxial device can be made to spin horizontally or vertically onto a rotating drum or conduction plate (Figure 3).
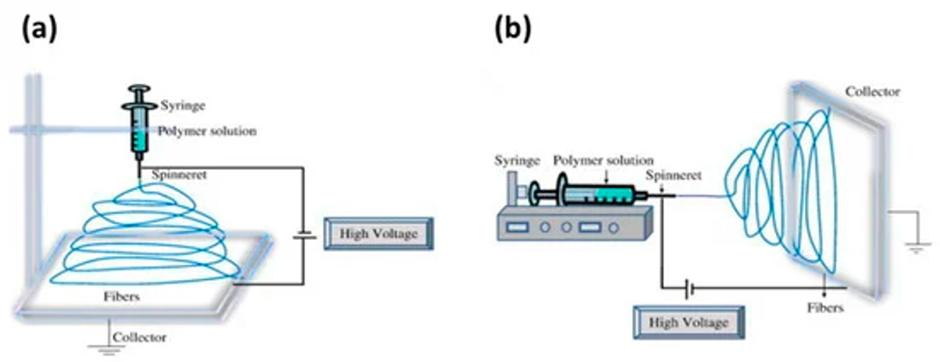
Figure 3: A diagram depicting a monoaxial ES device: (a) vertical setup and (b) horizontal setup [4].
The simplicity of monoaxial ES single needle configuration means that setup and operation of the device is straightforward. It is undeniably the most well-researched ES method, meaning it can be used to create fibres of distinct micro-morphologies by adjusting solution parameters. The basic nature of monoaxial ES does mean that production rate is limited (0.01-0.03 g h-1), so it is only effective in tasks requiring low fibre outputs (laboratory) [3].
Co-axial electrospinning
Coaxial electrospinning involves the use of a two-needle spinneret to produce nanofibers. Invented by Sun et al., 2003, this method consists of one needle inserted concentrically inside the other. During the coaxial spinning process, a single needle port is used to pump polymeric solutions with differing or immiscible properties through two spinnerets at various rates. The shell solutions circulate around the core spinneret, which houses the core solution, until they reach the end of the spinneret. At this point, the two solutions make contact and are drawn towards the collector because of the electrostatic potential difference that has been applied. Throughout the course of this process, the core-shell structure remains intact, resulting in the creation of elongated fibrous mats with a core-shell configuration (Figure 4) [4].
The utilisation of coaxial electrospinning to fabricate core-shell nanofibers has the potential to address the limitations associated with the burst release of monoaxial. In general, the polymeric core is drug embedded, while the shell functions as a physical barrier separating the core from the surrounding fluid. The inclusion of a barrier within coaxial fibres enables the medicine to be released over an extended period, hence enhancing its protection against environmental deterioration [5].
Coaxial ES also enables production of materials that are inherently non-electro-spinnable due to their chemical composition, such as oligomers. This is achieved by including these components within the core of the resulting fibres, provided that the core and shell solutions exhibit appropriate compatibility [3].
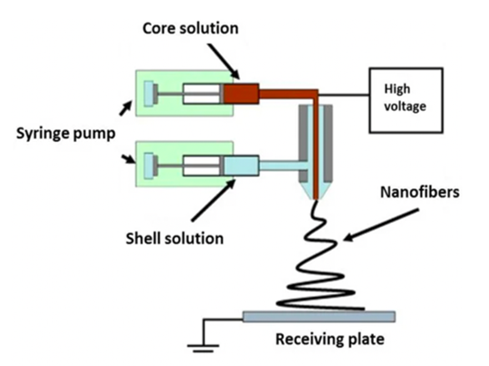
Figure 4: Illustration of a two-pump coaxial electrospinning device. 4
However, coaxial ES is a more complex process compared to monoaxial ES, as uses specialised apparatus or, at the very least, a coaxial needle and two syringe pumps. The identification and determination of suitable polymers and process parameters may necessitate a greater amount of time in comparison to simpler methodologies [5].
Tri-axial electrospinning
Triaxial electrospinning can generate adjustable drug release kinetics and transport mechanisms, including drug delivery systems that involve multistep diffusion. The morphology of the fibres provides capability to integrate multiple single-substance drug release profiles or the potential to load variant substances in each compartment, which could be used to deliver specific drugs at various locations in the body. Research carried out by Han & Steckl, 2013 [7-10] confirmed the feasibility of a triaxial drug delivery system, reporting that releasing 80% of encapsulated substance from the core of triaxial woven fibres was approximately 24x slower than that of coaxial fibres. Additionally, the hydroscopic layer of the triaxial fibres provide an initial burst release as fast as that of conventional mono-axial techniques, thus presenting the different release kinetics that can be achieved by one fibre. They concluded that this method was greatly beneficial for biomedical applications, but manufacturing costs and usability for untrained individuals will be the limiting factor of this technique (Figure 5) [5].
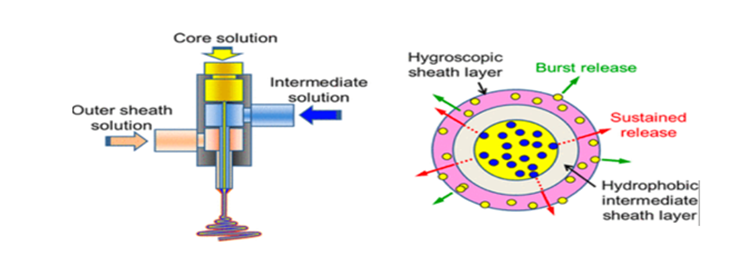
Figure 5: Transmission electron microscopy (TEM) cross-section of tri-axial fibre consisting of a PVP core loaded with Keyacid Blue (blue particles), a PCL intermediate layer, and a PCL outer layer [10].
Handheld electrospinning device: What’s the need?
The aforementioned ES devices clearly demonstrate the extent to which fibre morphology can be modified to suit a specific biomedical application. Whilst the development and evolution of ES has been almost exponential, almost all devices fail to address two key considerations: the reliance on a constant electric supply and the lack of portability and usability [11]. This means that practical application of ES is limited as not all healthcare providers will have the funding to buy and maintain ES devices, whilst hiring individuals with specialist training to carry out the tedious processes.
Handheld/portable ES devices address the shortcomings of earlier ES equipment. To develop a compact and portable iteration of ES the design approach must focus on minimising costs and maximising functionality. Revia et al., 2019 achieved this by including readily accessible electronic components from the commercial market and utilising 3D printed components for structural purposes [12] (Figures 6 and 7).
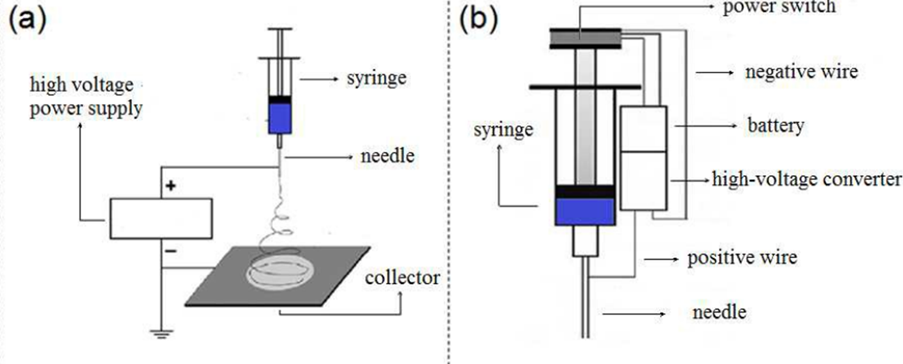
Figure 6: A diagram illustrating (a) the layout of conventional monoaxial ES compared to (b) the layout of a handheld ES device [11].
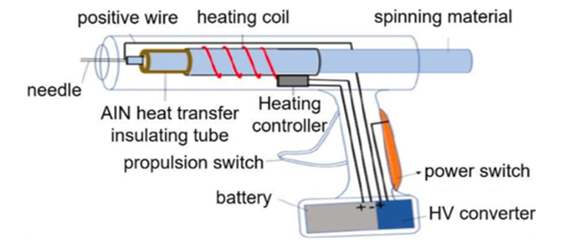
Figure 7: A schematic of the proposed layout of a handheld ES device [13].
One of the main advantages associated with handheld ES devices is that there is no requirement for a collector plate, meaning electrospun fibres can be applied directly to skin [12]. The high surface area to volume ratio of these fibres can be used to mimic characteristics of the extra-cellular matrix (ECM) [7]. For these reasons, ongoing research is taking place to explore the use of portable ES devices in wound care and regenerative medicine. The ability to produce fibres on-site coupled with the exceptional usability of the device makes it an ideal candidate for this application [3].
The NHS spend approximately £8.3bn annually on wound care. Of this, £2.7bn is spent to treat 70% of wounds that heal, whilst £5.6bn is spent on the remaining 30% of wounds that fail to heal [26]. Since handheld ES is arelatively new concept, it is too early to say whether it could present an effective solution to the financial burden of wound care in the NHS. Currently, there are various invitro, in-vivo and, pre-clinical trials taking place to investigate the true potential of handheld ES in wound care. This review aims to identify characteristics of an optimal wound dressing and use this information to evaluate whether it would be justified to use this iteration of ES over current wound care alternatives, subsequently providing a prediction of the potential use of handheld ES in NHS establishments.
Methods
Initial literature search
To begin with, an initial literature search was completed to become familiar with the topic of ES and understand how this technology had been utilised in medicine, more specifically, drug delivery and tissue regeneration. This was done by using broad search terms through google and reading through supporting literature. It was quickly understood that while there was plenty of literature on ES nanofibers in tissue regeneration, very few investigated the potential of a handheld device in this format. This made my research question justifiable.
Key search terms
After gaining a grasp of the concept of handheld ES, key search terms (Table 1) were identified and grouped into concepts. These terms were inputted into the relevant databases and laid the foundations for the search strategy.

Table 1: Key search terms grouped into concepts, used for the literature search.
Search strategy
Key search terms were inputted into the following the databases: PubMed, Cochrane, Web of Science and Wiley online library. The search strategy for PubMed and Cochrane can be found in the appendix. An additional technique, citation chasing, was also applied. When I found high quality, on topic pieces of literature, I manually searched through the reference section to find related articles to include in my literature review.
Study selection
To begin with, all duplicate studies were removed from selection. Studies included in the review had to be on either NHS wound dressings or ES produced nanofiber dressings. For ES studies, ideally, they should be completed using a handheld device. However, other ES iterations could also be included if the literature was on topic and high quality. The exclusion criteria for ES nanofiber dressing studies was studies published earlier than 2015. The inclusion criteria were in vivo studies on animal models or clinical studies on humans. For NHS dressings, there was no exclusions criteria, however the inclusion criteria was that the study must be on the same dressing and manufacture used by the NHS. Out of 112 articles found, 25 were downloaded for having information useful for the review. The eligible studies were read in full and 7 articles that met the inclusion and exclusion criteria were included. All results obtained were qualitative.
Grey literature
To support the structure of the review grey literature was used in places. An NHS wound care formulary was included in the review to aid in identification of dressings used by the NHS [31]. Additionally, literature regarding the classification of wounds and different types of wounds were also included to aid in the understanding of studies.
Results Classification of wounds
Wounds are defined as a deterioration of skin, mucous membrane, or tissue integrity, leading to a disruption in the normal anatomical structure and function of the affected area [14]. The variability of wounds and their unpredictable causes means that no two wounds are completely alike. As a result of this, wounds can be categorised in various ways [15-24]:
1. By cause:
- Traumatic wounds: caused by physical injuries, including cuts, abrasions, punctures, and fractures.
- Burn wounds: result from exposure to heat, chemicals, electricity, or radiation.
- Ulcers: typically develop due to poor blood circulation, pressure, or underlying medical conditions, like diabetic ulcers and pressure ulcers.
- Surgical wounds: incisions made during surgical procedures, which can be categorised based on their purpose: elective, emergency, exploratory.
2. By depth:
- Superficial wounds: only affect the top layers of the skin (above sub-dermis), like abrasions and first-degree burns.
- Partial-thickness wounds: extend through the skin and possibly into the underlying tissue, such as second-degree burns.
- Full-thickness wounds: affect all layers of the skin and underlying tissue, like deep lacerations and third-degree burns.
3. By appearance:
- Open wounds: have a break in the skin, making the wound visible, such as lacerations, punctures, and abrasions.
- Closed wounds: the skin remains intact, but there may be internal injuries, like contusions or crush injuries.
4. By infection status:
- Clean wounds: occur in a controlled environment, with minimal risk of infection. For example, surgical wounds made under sterile conditions.
- Contaminated wounds: have a higher risk of infection due to exposure to microorganisms, but no active infection is present.
- Infected wounds: microorganisms have already entered the wound, leading to an active infection.
5. By chronicity:
- Acute wounds: are recent injuries that typically follow a predictable healing process (<6 weeks).
- Chronic wounds: persist for an extended period and often do not heal as expected, often due to underlying medical conditions, poor circulation, or infection (>6 weeks).
6. By location:
- Midline laparotomy: incision down the middle of the abdomen.
- Lanz: oblique incision made along Langer’s lines.
- Suprapubic: surgical incision of lower abdomen, near hips.
- Thoracotomy: cut between rib bones, typically on the left side of the chest.
7. By special considerations:
- Bite wounds: the result of either animal or human bites and may pose a potential risk of infection due to the presence of germs in the oral cavity.
- Radiation wound: a consequence of being exposed to ionising radiation, such as radiation therapy utilised for the treatment of cancer.
- Chemical wounds: the result of contact with corrosive substances, such as acids or alkalis, leading to the occurrence of chemical burns.
Common wound types
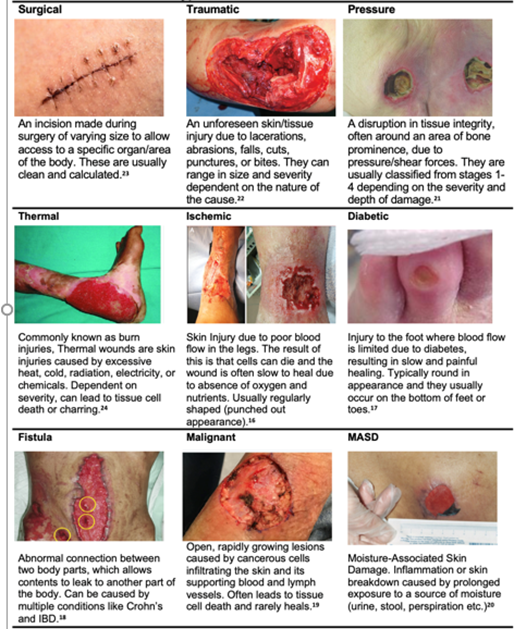
Table 2: Common Wound Types.
Characteristics of an optimal wound dressing
A wound dressing is selected based on several parameters, such as: location, depth, type and the possibilities of discharge and infection, meaning each type of wound will require slightly different characteristics for optimal wound healing. However, over time, several characteristics have been recognised as ideal for general wound care (Figure 8) [25-38].
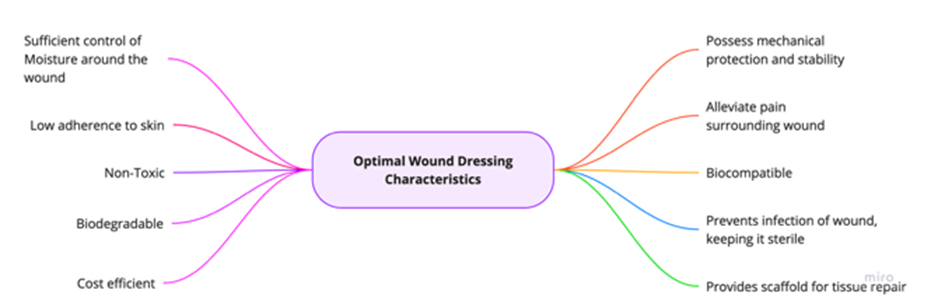
- Sufficient control of moisture around the wound: moist/ wet dressings can accelerate the process of wound healing when compared to dry dressings as repair of skin without inflammation can only take place in a moist environment [34].
- Low adherence to skin: Allows exudate to pass into a secondary dressing while maintaining a moist environment [35].
- Non-toxic
- Biodegradable: A biodegradable dressing could decrease morbidity by reducing contact during wound care, eliminating need for frequent dressing changes, reducing the risk of infection [36].
- Cost efficient.
- Possess mechanical protection and stability.
- Alleviate pain surrounding wound: less pain can lead to better patient satisfaction and better outcomes.
- Biocompatible: natural polymers with excellent biocompatibility have been shown to promote wound healing and restoration of the skin [37].
- Prevent infection of wound keeping it sterile
- Provide scaffold for tissue repair: dressings with scaffolds provide the structural support for cell attachment and subsequent tissue development [38].
Traditional wound dressings like cotton bandages or gauzes absorb most of the moisture within the environment of the wound, leading to a dried surface and decreased healing rate, as well as pain in separating the dressing from the skin. By comparison, a wide variety of polymers have been created which can come in the form of films, foams, and gels [25]. These polymers can provide many of the ideal dressing characteristics explained above. An NHS wound dressing formulary, recognises the potential of polymerbased dressings and utilises them in the form of hydrogels and hydrocolloids [31].
Hydrogel (Activheal)
Hydrogels are a modern wound dressing alternative derived from natural/synthetic polymers [27]. These three-dimensional polymeric chains usually contain hydrophilic moieties, allowing them to absorb large volumes of water. The hydrophilic attributes of hydrogel dressings can be used to create a moist environment at the wound site which is favourable when considering tissue regeneration and allows for autolytic debridement of necrotic tissue [25]. This is preferable to dry dressings, which generally only offer physical protection and do not help heal the wound. Regarding this attribute, it must also be noted that the fluid accumulation can lead to skin infection or bacterial growth if not properly managed [28] (Figure 9).
Figure 9: Schematic of the structure of hydrogel dressings.
Hydrogels have been a common practice in modern wound care as they combine the biocompatibility of natural polymers with the elastic/protective properties of synthetic polymers. This dressing can be used either in an amorphous gel form or as an elastic sheet dependent on the morphology of the polymers used. Their versatility makes them suitable for several wounds, including pressure ulcers, skin tears, diabetic foot ulcers, surgical wounds and burns [28].
A clinical study by Ousey, 2011 evaluated Activ Heal hydrogel, the hydrogel of choice for NHS wound care [29]. During each
dressing change, the attending nurse and patient were asked to assess the wound dressing on several parameters:
- Comfortability
- Maintaining moist wound environment
- Ease of use o Overall rating
Eleven patients were included in this study:
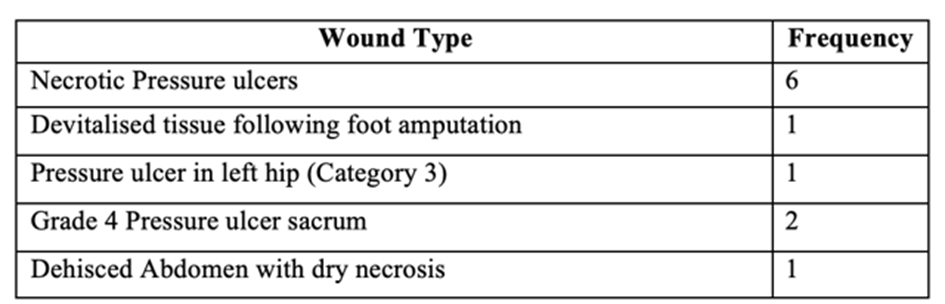
Table 3: All wound types included in the hydrogel study.
Results from this study were generally positive (Figure 10), with the only recording of dissatisfaction being the dressing’s ability to maintain a moist environment, which 4% of participants were dissatisfied with. Overall, the dressing performed well with a 79% satisfaction rate. It was also noted by nurses that the hydrogel dressing possessed high viscosity meaning application was straightforward and did not cause any extra underlying trauma to the wound. Additionally, minimal residue remained prior to reapplication, suggesting that a degree of rehydration had been achieved.
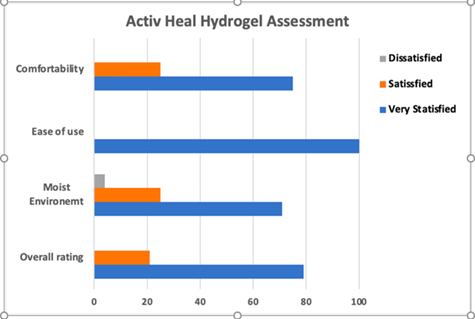
Figure 10: Bar chart conveying the results of the Activheal hydrogel assessment [29].
When NHS first switched to ActivHeal wound care alternatives in 2011, it was recorded that approximately £35,500 could be saved annually by an acute NHS trust from the next cheapest foam alternative (Figure 11). However, there is no recent literature to suggest there is currently a more cost-effective dressing which aligns with the versatility of ActivHeal hydrogel.

Figure 11: Average savings of an acute NHS trust with use of Activheal [29].
While generally, this clinical trial proved the efficacy or Activheal’s hydrogel, there are several issues that surround it. To begin with, the trial was funded and published on behalf of Advanced Medical Solutions, the company which owns the Activheal dressing range. This may have induced bias in the study for reasons surrounding financial gain. Additionally, the study had a total of 11 participants, all of which are presumed to be from the same clinical centre. This lack of diversity as-well as the lack of participants also make it challenging to draw any real conclusions from the study. The clinical trial also seemed to take place in an open or ‘unblinded’ format meaning both patients and investigators were aware of the dressing they were using, this again could increase the likelihood of bias.
A more comprehensive clinical study completed by Zoellner et al., 2007 mitigates some of the issues encountered with the previous study [39]. The study took place across 15 German medical centres with a total 81 participants meaning the results are diverse and representative. The hydrogel dressing used was Hydrosorb Comfort which has almost identical characteristics to those of Activheal hydrogel (Paul Hartman). The wound aetiologies of the patients were as followed (Table. 4)
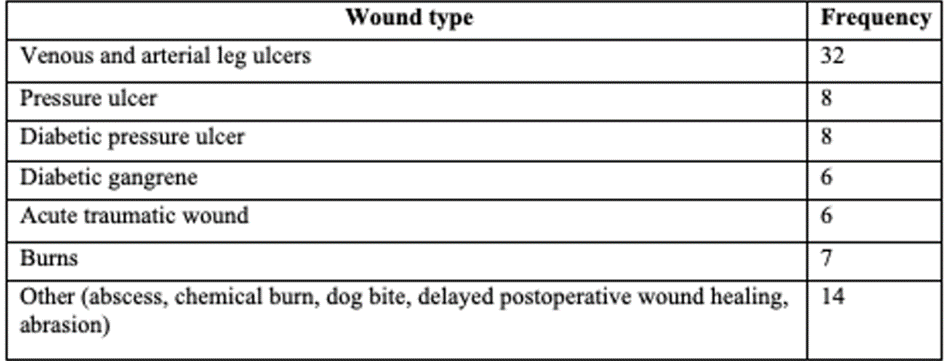
Table 4: Wound types used in study [39].
The proportion of wound slough, granulation tissue, epithelial tissue and amount of exudate were documented (Figure 12). Both patients have clinicians were asked to assess the dressing on several characteristics.
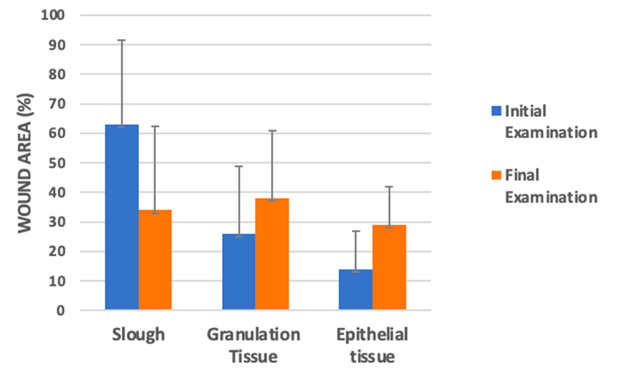
Figure 12: Bar chart representing the amount of slough, granulation tissue and epithelial tissue relative to the wound area. Measurements taken at initial examination and final examination [39-41].
The mean proportion of wound area covered with slow fell significantly (63% → 34%) (Figure 12) and significant increases were documented in the mean area of granulation and epithelial tissue. Additionally, 5 wounds completely re-epithelialized. Since epithelialisation is the defining parameter of a successful wound closure, it clearly proves the hydrogels wound healing capabilities, though, only 5 out of the 81 wounds achieved this [42].
The percentage of wounds not exudating increased from 0% at baseline evaluation to 22%, and the percentage of moderately and heavily exudating wounds fell from 37% to 23% [39]. This is beneficial as when high levels of exudate are not properly managed, the wound bed will become over-hydrated, leaving it more prone to damage [41].
Regarding the more subjective assessments, the ratings aligned with those of the Activheal hydrogel study. Hydrating characteristics and ease of removal were generally regarded as ‘good’ or ‘very good’. The investigating physicians also had a ‘good’ overall impression in 83% of cases [41].
Hydrocolloid (Comfeel Plus)
Hydrocolloid dressings are made up of hydrophilic, self-adhesive colloid granules coated with an external polyurethane film (PU). The external film serves as a form of mechanical protection from environmental factors, while the colloid granules absorb exudate from the wound. Granules utilised in hydrocolloid dressing typically consist of gelatine, pectin and carboxymethyl cellulose (CMC). The granules and PU film can be modified to produce dressings of various size, shapes, and thicknesses [28]. The biocompatibility of hydrocolloid dressings makes them suitable for surface ulcers, such as minor burns, shock injuries, and bruises. However, they are not suitable for deeper/chronic wounds, especially those with infections, as no oxygen is supplied to the wound due to the adhesive, thus the healing process is compromised [25].
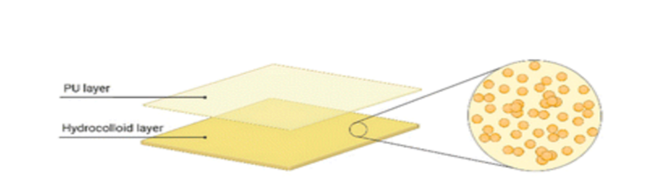
Figure 13: Schematic of the dual-layered structure of a hydrocolloid [28].
An article by Goodhead, 2002 published in the British Journal of Nursing sought to evaluate the NHS hydrocolloid dressing of choice, Comfeel Plus Transparent [30,31]. Comfeel Plus is a thin hydrocolloid dressing composed of a 25 µm outer semipermeable polyurethane membrane with a thin 300 µm absorbent and adhesive hydrocolloid interface. The study used 3 patients to evaluate the dressing, with 4 wounds in total. The healing time of each wound was also recorded:
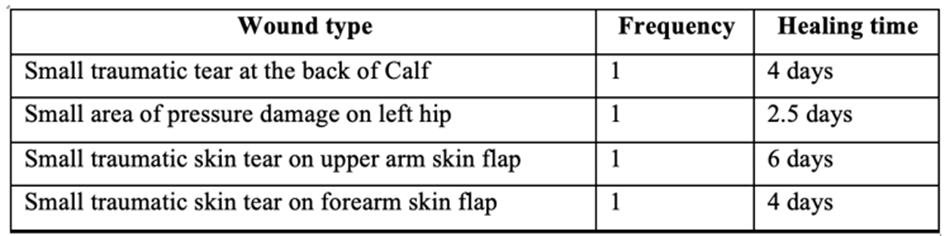
Table 5: Table of all wound types used [30].
It was reported that healthcare staff found the dressing easy to apply and remove. Additionally, another thin hydrocolloid dressing had been evaluated before comfeel, however, its use had resulted in issues like wrinkling and rolling at the edges. No such issues were reported when evaluating the Comfeel Plus transparent hydrocolloid dressing. There was also no odour reported on dressing removal and all the wounds had low exudate levels [30].
Whilst there is a very little evidence to disprove the efficacy of the Comfeel hydrocolloid, the study lacks diversity as the there was a total of 3 patients with 4 wounds total, meaning a broader analysis may produce different. In conjunction with this, a case study by Grange-Prunier et al. 2002 found that a 66-year-old women treated for a leg ulcer with the dressing developed pruriginous, erythematous, and vesiculous dermatitis under the hydrocolloid dressing [32]. The case study does present the findings that allergic contact dermatitis is a possible side-effect of Comfeel plus hydrocolloid dressings, so there may be issues surrounding biocompatibility. Regarding price, it was recorded by NHS Devon Clinical Effectiveness team, 2019 that the NHS pay approximately £2.66 10x10cm and £5.70 for, 15cm x 15cm [43-46, 33].
Handheld electrospinning for wound care
Polymers used for handheld electrospinning
Before addressing the feasibility of handheld ES devices in wound care, it is important to understand structure and characteristics of an electrospun wound dressing. Electrospun dressings are the product of ultra-fine nanofibers in a non-woven, randomly arranged structure [47]. Since ES is in an incredibly versatile technique, with capability of producing fibres with diameters anywhere between 50 nm and 5 μm [48], the diameter and morphology of electrospun fibres are dependent on several parameters, including:
- The type of polymer used.
- Polymer viscosity.
- Polymer elasticity.
- Polymer concentration.
- Operational conditions of the device: feeding rate, distance from the tip of the device to the wound area and the strength of the applied electrical field [47].
- Humidity and temperature of the environment.
Currently, there are hundreds of polymers which have been successfully electrospun for wound dressing purposes. This includes both natural and synthetic polymers, with each of these types presenting both advantages and drawbacks [49].
Natural polymers have excellent biocompatibility with human tissue as well as low toxicity and biodegradable properties. Some can contribute to tissue repair as they exhibit antimicrobial and anti-inflammatory properties which allow the wound to surpass the chronic inflammatory stage, a place where current dressings tend to fail [50]. Natural polymers commonly used (Figure 14) in electrospinning include collagen, gelatine, alginate, and cellulose [49,50].
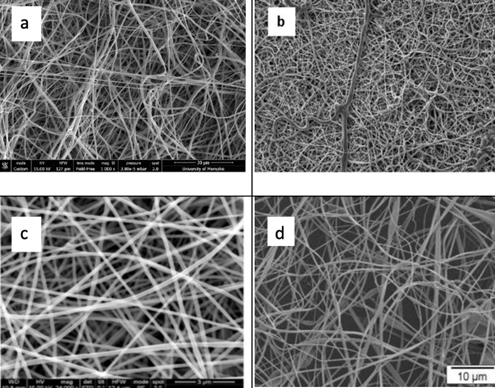
Figure 14: Scanning Electron microscope (SEM) images of electrospun: a: collagen [51], b: Fish gelatine, [52] c: Alginate, [53] d: Cellulose [54].
While natural polymers present many advantages for wound care, they are generally less cost-effective when compared to their synthetic counterparts. Additionally, they lack the mechanical strength of synthetic polymers and are more difficult to process [50]. When considering whether they would be worthy of replacing current NHS wound dressing, while they do offer great benefits in terms of biocompatibility and biodegradability, the extra cost associated with natural polymers would likely make them prohibitively expensive.
As mentioned, synthetic polymers are more cost effective than natural polymers due to the simplified processing and production [50]. As a result of the lower costs associated with synthetic polymers, there is far more extensive research on their use in wound dressings [49], making it a good starting point to evaluate what polymers would work in the handheld ES format. They also have outstanding thermal stability and mechanical properties which offer better supports for cell adhesion and proliferation of the wound [49,55]. The main advantage of synthetic polymers is they offer controllable characteristics, which include surface characteristics, hydrophilicity, permeability, and deformability which can be altered and optimised for wound care [55]. Commonly used polymers (Figure 15) include polyvinylpyrrolidone (PVP), Polycaprolactone (PCL), Poly(DL-lactic acid) (PDLLA) and poly(lacto-co-glycolic acid) (PLGA) [49].
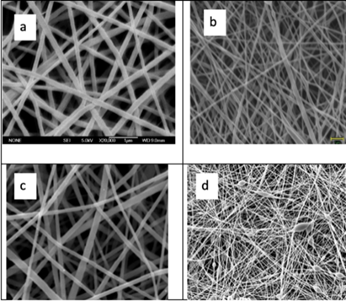
Figure 15: Scanning Electron microscope images of electrospun: a: PVP, [56], b: PCL [57], c : PDLLA, [47] d :PLGA. [58].
Performance of handheld electrospinning
Several in vivo studies have taken place to assess the efficacy of handheld ES and determine whether it is a viable solution to issues surrounding chronic wound care. A study conducted by Xu et al., 2022 sought to address the issue of full-thickness skin wounds, a type of wound that extends beyond the dermis and epidermis, using handheld ES [59]. Their approach was to use the synthetic polymer, polyvinyl alcohol (PVA) in combination with bonemarrow derived stem cells (BMSCs) on 10 rats aged 2-3 months. Three groups of mice were assessed: a PVA/BMSC group, a PVA only group, and a control group (Figure 16).
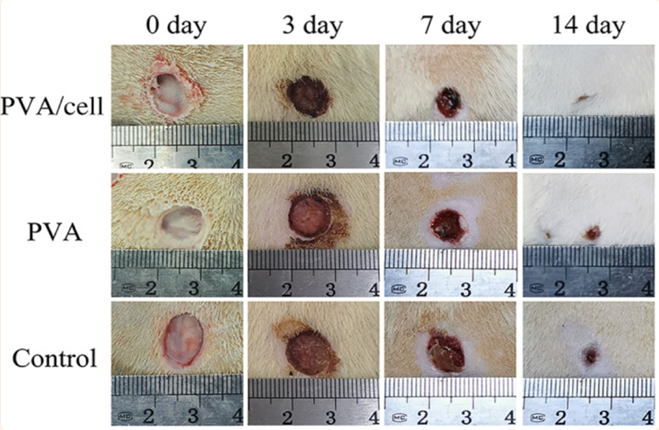
Figure 16: The progression of wound healing over 14 days in the PVA/BMSC group (top), the PVA only group (middle) and the control group (bottom) [59].
The visual results show wound closure time in the PVA/BMSC group was significantly shorter than the PVA and control group. One of handheld ES advantages over the current NHS dressings is its ability to incorporate cells into the polymeric solution. In this case, the use of BMSCs shortened repair time and promoted wound healing, while the PVA provided structural integrity and firmly attached to the skin surface without the use of adhesives [59]. One consideration for this cell/polymer mix is the voltage applied. Since an electric field that exceeds 0.1kV/mm can reduce cell viability, the handheld apparatus can only be operated within fine voltage parameters as too little voltage will lead to poor microfibre structure, while too much can decrease cell viability [60].
While this in vivo preclinical study included the relevant controls, the sample (N) number of wounds treated is relatively low and only one type of synthetic polymer was used for treatment, meaning conclusions cannot be drawn on whether this polymer is the most optimal for wound care in the handheld ES format. Another in vivo study led by Haik et al., 2017 addresses these shortcomings by using 3 pigs, each with 15 superficial wounds for a total of 45 wounds, substantially more than the previous study.7 The wounds had 20 x 20 mm surface areas and depths of 0.254 mm. From a clinical perspective, the use of pigs in this study is beneficial as porcine skin is similar to human skin in terms of immunohistochemical properties and morphological structure [61].
An additional advantage of the latter study [7], was the use 4 different types of polymeric formulations (Figure 17), which tested whether polymer structure was a function in repair. The 4 polymeric solutions are:
A) Biocompatible and biodegradable polyester-based polymer.
B) Biocompatible polycarbonate-based silicone elastomer.
C) Blend of two biocompatible polyurethanes.
D) Blend of biocompatible polyurethane and polyester.
A fifth, commercially available control dressing was used named paraffin nonmedicated gras dressing. It has been concluded in the past that this form of dressing is not statistically different to a hydrocolloid dressing, which is useful for comparative studies [62].
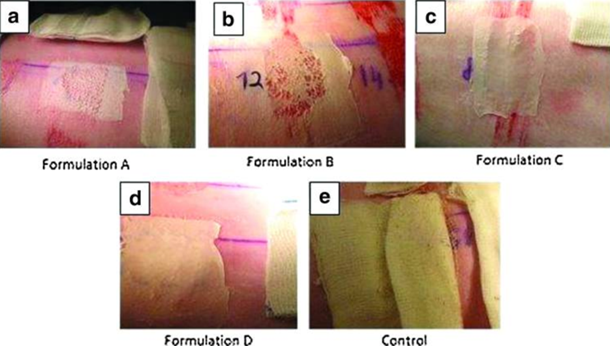
Figure 17: The 4 Electrospun polymer-based dressings (a-d) alongside (e) the control dressing, after application [7].
Wounds were assessed, 2 days, 7 days, and 14 days after application on various metrics:
- Adherence
- Wound exudate
- Presence of eschar
- Any adverse skin reactions: erythema and oedema
Conclusions from this study suggest that polymer A (polyesterbased polymer) took only 1 minute to create a dressing of suitable thickness from the hand-held ES device compared to the other polymers which took 2 minutes. This may be a useful consideration for emergency situations. Generally, no significant differences were found between the polymers in all the metrics assessed. However, since the hand-held ES allows for contactless application, risk of infection is significantly reduced since hands are the main source of transferring infections.7 Additionally, when the wound is an abnormal shape or is in a challenging area, application of dressing using hand-held ES is easier since the polymer is flexible and easier to work with. While this in vivo study was insightful, the ES dressings could have been randomised on application to reduce risk of bias.
Clinical trials for electrospun dressings
Since handheld ES is still a relatively new concept, as of today, no clinical trials have been conducted to assess the efficacy of these devices against current wound dressing alternatives. With that said, it has been understood for some time now that ES presents great potential for tissue engineering applications [3]. Because of this, clinical trials have taken place to assess the capability of ES produced nanofibers in wound care. Fibres produced using conventional ES techniques, and these clinical studies can be used to gain a further understanding of the potential of handheld ES [11].
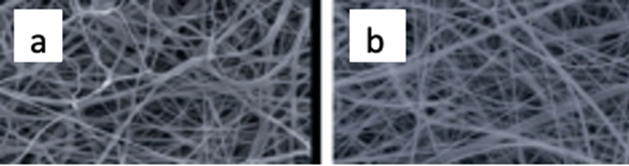
Figure 18: SEM images of: a. PCL and b. PCL/gelatine nanofibers [64].
The mechanical strength of the dressings was assessed using Young’s modulus strength calculations, a property that describes a material’s ability to withstand deformation under stress [65]. The mechanical strength of PCL and PCL/gelatine were 1.43 megapascals (MPa) and 0.86 MPa respectively. While the PCL ES nanofiber is significantly stronger, the PCL/gelatine still outperforms the Young’s modulus of the NHS dressing hydrogel, which is in the range of 10-100 kilopascals (kPA), thus showing the benefits in mechanical strength of ES fibres [66].
The progression in healing of the stage 2 pressure ulcers was assessed using the pressure ulcer scale for healing (PUSH) score, a scale which incorporates wound size, exudate excretion and tissue type, with higher scores indicating deterioration [67].
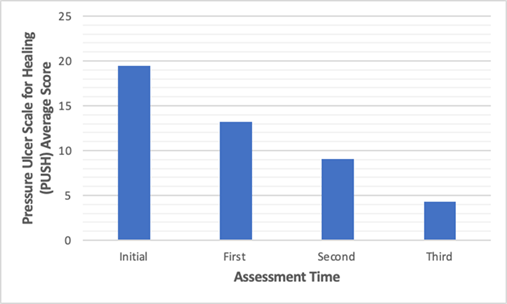
Figure 19: A comparison of average PUSH scores for each assessment time with us of PCL/gelatine nanofibers [64].
PUSH scores presented a statistically significant (P < 0.05) decline (Figure 19), from 19.48 upon initial assessment, to 4.26 upon final assessment, which shows the PCL/gelatine nanofibers had a therapeutic effect on skin repair. While the PUSH score is widely recognised as the gold standard for pressure injuries and the analysis is this study is comprehensive, the author failed to state the time differences between each analysis of the wound. Despite this shortcoming, the clinical trial was able to show the benefits of a synthetic/natural ES polymeric blend. The PCL/gelatine was able to promote wound closure, collagen generation, re-epithelization, and angiogenesis [64]. Though it clearly demonstrates the benefits of ES, it would have been useful to add another group of participants who used only the PCL as a dressing. This would clearly convey the benefits of natural polymers. Also, comparisons to a current wound dressing alternative by making the study a randomized control trial (RCT) would have been insightful to give a clearer idea of the true benefits of ES produced nanofibers.
A retrospective case series conducted by Regulski & MacEwan, 2018, looked to address the limitations of current wound dressing alternatives by using ES produced synthetic fibre matrix [6568]. Participants included patients with chronic wound that had remained unhealed for ≥ 4 weeks and had been unresponsive to existing alternative, which came to 82 total patients (Table 6). These wound included diabetic foot ulcers (DFUs) and venous leg ulcers (VLUs) which are notoriously difficult to treat. Wounds received a layer of synthetic fibre matrix weekly from one of the physicians, for up to 12 weeks.
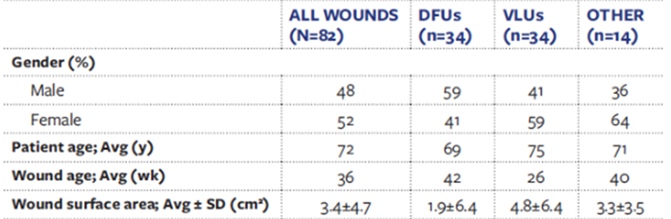
Table 6: Patient demographics. DFUs: Diabetic foot ulcers; VLUs: Venous leg ulcers [68].
Significant wound healing was observed throughout the study (P < 0.05), with 85% (68) of the wound achieving complete closure at 12 weeks. Notably, 91% (30) of VLUs achieved complete closure after 12 weeks. This case series was able to successfully prove the potential of ES produced nanofibers in dealing with unhealed, chronic wounds. Since the majority of NHS spending is on unhealed chronic wound, this presents a potential cost-effective solution to this issue [26]. Though this study did not use a RCT format, the reasons are justified since participants had to be selected based on the healing progress of their wounds as their previous dressings/ treatments had not been successful. For this reason, it still effectively demonstrates the efficacy of ES nanofibers compared to other alternatives. In the future, it would be useful to see this study completed over multiple centres rather than just one, in a doubleblinded format to reduce bias. Additionally, there was a failure to mention the type of synthetic material used in the ES production; this information would be useful for further trials and assessments.
Discussion
Performance of current wound dressings
This review aimed to evaluate the potential of handheld ES against current NHS wound dressings by using characteristics of an optimal wound dressing. Two of the most advanced wounds dressings used by the NHS, hydrogel, and hydrocolloids, were included in the analyses [33]. Results from the clinical trials included suggest that both dressing perform well on a variety of wound types. Notably, both maintain good levels of moisture around the wound, which help to facilitate epithelial migration and reduce scar formation [69]. Participants and healthcare professionals also had very little criticism and were overall very satisfied with comfort and ease of application. Though they seem to be affective dressings, they fail to address the main issue of chronic wound healing. Since these dressings lack natural materials and active ingredients, it leads the wound to accumulate excessive levels of pro inflammatory cytokines, leading to elevated levels of proteases, which causes the wound to remain in the chronic inflammatory stage [70]. A deficiency of stem cells means the wound is unable to go beyond the inflammatory stage and transition into the reparative proliferative stage of wound healing.
The issue of wound care and handheld electrospinning’s ability to solve it
The NHS spends approximately £8.3bn annually on wound care. Of this, £2.7bn is spent on the 70% of wound that heal, while £5.6 bn is spent on the 30% of wounds which are chronic [26]. Chronic wounds fail to progress through the normal stages of wound healing and often remain at the chronic inflammatory phase due to an imbalance of cytokines, proteases, and reactive oxygen species (ROS) [70]. With the expected demographic growth of the elder UK population and the current financial strains of the NHS, this current chronic wound care system is unsustainable.
Handheld ES is an iteration of the of the monoaxial ES technology which allows for portable and direct application of nanofibers [3]. Since these nanofibers can be applied directly onto skin, there has been the question of whether handheld ES can be utilised in wound care. In vivo studies have been carried out in the aid of answering this question. The study completed by Xu et al., 2022 on rat wounds presented a key benefit of handheld ES, being its easily modifiable nature [59]. The addition of BMSCs to the synthetic nanofiber presented significant progression of wound healing compared to the control group. Additionally, BMSCs used in pre-clinical studies have reported dermal rebuilding, reduced inflammation, and growth of fibroblasts in chronic wounds, allowing the wound to surpass the chronic inflammatory stage where current dressings fail [71]. This nanofiber dressing produced by handheld ES has potential to solve the issue of chronic wound healing. Ideally if the study used chronic wounds, while also comparing against a dressing alternative, it would have helped to demonstrate this advantage more clearly.
The latter study of handheld ES by Haik et al., 2017 demonstrated the potential of handheld ES produced nanofibers on human skin, since pig skin is of similar immunohistochemistry to human skin [7,61]. Though the study failed to recognise any clear advantages of the nanofibers over current wound dressings, it did highlight the broader benefits of the handheld devices. The contactless application is significant since some unhealed/chronic wound can be attributed to an uncontrollable infection [70]. In conjunction with this, since handheld ES application is directly onto the wound, they can be tailored to fit the wound perfectly, whilst current dressings often fall short in this regard when the wound is in an obscure location. The portability of handheld ES also means it could be applied in various scenarios like military of paramedic services.
Nanofibers in wound care
The novelty of the handheld ES device means that as of writing this review, no clinical trials have taken place with the device. Though fibres produced by handheld ES are of virtually identical morphology to those produced by other techniques, so clinical trials which used ES produced nanofibers can be used to assess the potential of the handheld device.
Nanofiber dressings have a substantial surface area to volume ratio, as well as high porosity, high levels of tensile strength and, modifiable surface morphology and function [49]. These characteristics allow ES nanofibers to mimic the biological functionality and structure of an ECM. For example, the high porosity allows for movement of water and gases in an out of the covered wound area, allowing for cellular respiration and ensuring the moisture levels are optimal [50].
In terms of providing solutions to the problem of chronic wound healing, the study conducted by Regulski & MacEwan, 2018, proved the efficacy of nanofiber dressings, with 85% of chronic wounds achieving complete closure after 12 weeks [68]. This can be attributed to the characteristics listed above.
Limitations
Limitations of Handheld electrospinning
While handheld ES is a versatile and innovative technique that has great potential to address the issues of current wound care, there are some limitations that must be addressed. The NHS is currently under significant financial strain, so if the handheld devices are not cost effective, they are simply not worth perusing. Though with that said, Brako et al., 2018 was able to assemble a handheld ES device with commercially available parts for approximately £2000, which could be feasible for the NHS since fibres required to produce the dressing are relatively inexpensive [46]. However, it remains to be demonstrated whether this production could be scaled to an organisational level.
Another limitation of the handheld ES device is the possible need for prior training to operate safely. Whilst these devices are generally easier to operate than conventional ES techniques, producing a nanofiber dressing directly onto the wound likely need extensive training and examinations. If the NHS intends to make the handheld ES dressing accessible for all patients, the need for training would be a limiting factor.
Limitations of the literature
Whilst this study successfully outlined numerous advantages of handheld ES compared to conventional dressings, there were some limitations which must be considered. To begin with, as of writing this review, there are no clinical trials taking place for handheld ES devices. A clinical trial would prove the efficacy of handheld ES clearly and there would be no room for dispute. While animal studies are insightful, and the results obtained from them are likely to align with the results from a clinical trial, the true potential of handheld ES cannot be proven until a clinical trial is completed.
In conjunction with this, there is no current literature directly comparing the NHS wound dressings (hydrogel, hydrocolloid) to ES produced nanofibers. While comparative studies have taken place with dressings of similar characteristics, the use of handheld ES in the NHS cannot be justified until it has been compared directly to all dressings in the wound care formulary.
Conclusion
Overall, this study was able to outline the characteristics of an ideal wound dressing and use them to compare the potential of a handheld ES device to current NHS therapies. Multiple advantages of handheld ES and nanofiber dressing were identified over current therapies, and limitations of the device were also acknowledged. From a future perspective, clinical studies must take place with handheld ES devices in a RCT format. It must be compared to all current NHS wound therapies and if handheld ES is deemed to be advantageous, this may mark a new milestone for modern wound care. As of today, there simply isn’t enough evidence to justify the use in the NHS. However, if the clinical trials are completed and it is deemed cost effective to mass produce handheld ES devices, I believe it has the potential to become the new gold standard for chronic wound care in the near future.
Acknowledgments
I would like to show my greatest appreciation to my supervisor, Dr Ilyas Khan. Dr Khan’s continuous support through this project was vital, and without him this project would not have been possible. I would also like to thank Dr Luke Burke. As one of the leading developers of the handheld electrospinning device, he gave me the information and ideas to pursue this project.
References
- Giannitelli SM, Costantini M, Basoli F, Trombetta M, Rainer A (2018) Electrospinning and microfluidics. Electrofluidodynamic Technologies (EFDTs) for Biomaterials and Medical Devices 9: 139-55.
- F COOLEY J (1902) APPARATUS FOR ELECTRICALLY DISPERSING Fluids.
- Keirouz A, Wang Z, Reddy VS, Nagy ZK, Vass P et al. (2023) The History of Electrospinning: Past, Present, and Future Developments. Advanced materials and technologies 8.
- Farhaj S, Conway BR, Ghori MU (2023) Nanofibres in Drug Delivery Applications. Fibers 11: 21.
- Williams GR, Raimi-Abraham BT, Luo CJ (2018) Monoaxial electrospinning.
- Braghirolli DI, Steffens D, Pranke P (2014) Electrospinning for regenerative medicine: a review of the main topics. Drug Discovery Today 19: 743-753.
- Haik J, Kornhaber R, Blal B, Harats M (2017) The Feasibility of a Handheld Electrospinning Device for the Application of Nanofibrous Wound Dressings. Advances in Wound Care 6: 166-174.
- Kalra V, Jung Hun Lee, Jay Hoon Park, Marquez M, Yong Lak Joo (2009) Confined Assembly of Asymmetric Block-Copolymer Nanofibers via Multiaxial Jet Electrospinning. Small 5: 2323-2332.
- Sun Z, Zussman E, Yarin AL, Wendorff JH, Greiner A (2003) Compound Core–Shell Polymer Nanofibers by Co-Electrospinning. Advanced Materials 15: 1929-1932.
- Han D, Steckl AJ (2013) Triaxial Electrospun Nanofiber Membranes for Controlled Dual Release of Functional Molecules. ACS Applied Materials & Interfaces. 5: 8241-8245.
- Xu SC, Qin CC, Yu M, Dong RH, Yan X, et al. (2015) A batteryoperated portable handheld electrospinning apparatus. Nanoscale 7: 12351-12355.
- Revia RA, Wagner BA, Zhang M (2019) A Portable Electrospinner for Nanofiber Synthesis and Its Application for Cosmetic Treatment of Alopecia. Nanomaterials 9: 1317.
- Zhao YT, Zhang J, Gao Y, Liu X, Liu J, et al. (2020) Self-powered portable melt electrospinning for in situ wound dressing. J Nanobiotechnology 18: 111.
- Irfan-maqsood M (2018) Classification of Wounds: Know before Research and Clinical Practice. Journal of Genes and Cells 4: 1.
- Sarabahi S, Tiwari VK (2012) Principles and Practice Of Wound Care.
- Alexandrescu V, Deleeuw P, Kovanda JS, (2017) Ischemic and Venous Wound Identification: What We Look For.
- Khan T, Khardori R (2023) Diabetic Foot Ulcers.
- Limengka Y, Jeo WS (2018) Spontaneous closure of multiple enterocutaneous fistula due to abdominal tuberculosis using negative pressure wound therapy: a case report. J Surg Case Rep 1.
- Jakucs C (2020) Malignant wounds: How to identify and treat them.
- Rudolph D (2021) Moisture-Associated Skin Damage: What It Is and What It Isn’t.
- E Fife C (2022) A Closer Look at the Legal Implications of Pressure Injuries.
- Jeffery S (2013) Exudate monitoring in traumatic wounds.
- Fletcher J (2019) What to know about Dissolvable stitches.
- Khan MM, Rao VP, Krishna D, Minz R, Laitonjam M, et al. (2020) A Current Overview of Chronic Wounds Presenting to a Plastic Surgery Unit in Central India. Chronic Wound Care Management and Research 7: 43-51.
- Rezvani Ghomi E, Khalili S, Nouri Khorasani S, Esmaeely Neisiany R, Ramakrishna S (2019) Wound dressings: Current advances and future directions. Journal of Applied Polymer Science 136: 47738.
- Guest JF, Fuller GW, Vowden P (2020) Cohort Study Evaluating the Burden of Wounds to the UK’s National Health Service in 2017/2018: Update from 2012/2013. BMJ Open 10: e045253.
- Demeter M, Anca Scărișoreanu, Ion Călina (2023) State of the Art of Hydrogel Wound Dressings Developed by Ionizing Radiation. Gels 9: 55.
- Minh Nguyen H, Le TTN, Thanh Nguyen A, Le HNT, Tan Pham T (2023) Biomedical materials for wound dressing: recent advances and applications. RSC Advances 13: 5509-5528.
- Ousey K (2011) Case Series - The clinical effectiveness of the Activheal wound care dressing range.
- Goodhead A (2002) Clinical efficacy of Comfeel Plus Transparent Dressing. Br J Nurs 11: 284-287.
- Somerset CCG MMT (2020) Quick Reference Dressings Guide August 2020 update.
- Grange-Prunier A, Couilliet D, Grange F, Guillaume JC (2002) Allergic contact dermatitis to the Comfeel hydrocolloid dressing. Ann Dermatol Venereol 129: 725-727.
- NHS Devon Clinical Effectiveness team. 17.2.4 Hydrocolloid dressings.
- Fan Z, Liu B, Wang J, Zhang S, Lin Q, et al. (2014) A Novel Wound Dressing Based on Ag/Graphene Polymer Hydrogel: Effectively Kill Bacteria and Accelerate Wound Healing. Advanced Functional Materials 24: 3933-3943.
- Jones V, Grey JE, Harding KG (2006) Wound dressings. BMJ 332: 777-780.
- Daristotle JL, Lau LW, Erdi M, Hunter J, Djoum A, et al. (2019) Sprayable and biodegradable, intrinsically adhesive wound dressing with antimicrobial properties. Bioeng Transl Med 5: e10149.
- Xu R, Fang Y, Zhang Z, Cao Y, Yan Y, et al. (2023) Recent Advances in Biodegradable and Biocompatible Synthetic Polymers Used in Skin Wound Healing. Materials 16: 5459.
- Chan BP, Leong KW (2008) Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J 467-479.
- Zoellner P, Kapp H, Smola H (2007) Clinical performance of a hydrogel dressing in chronic wounds: a prospective observational study. J Wound Care 16: 133-136.
- Paul Hartman Wound Management.
- Gardener S (2023) Managing High Exudate Wounds: A How-to Guide.
- Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, et al. (2014) Epithelialization in Wound Healing: A Comprehensive Review. Adv Wound Care 3: 445-464.
- Potter G, Barbosa R, Villarreal A, Salinas A, Guzman H, et al. (2020) Design and Validation of a Portable Handheld Device to Produce Fine Fibers Using Centrifugal Forces. Instruments 4: 27.
- Coffee RA (2024) Dispensing device.
- Greiner A, Wendorff Joachim H (2007) Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angewandte Chemie International Edition 46: 5670-5703.
- Brako F, Luo C, QMC Duncan, Edirisinghe M (2018) An Inexpensive, Portable Device for Point-of-Need Generation of Silver-Nanoparticle Doped Cellulose Acetate Nanofibers for Advanced Wound Dressing. Macromolecular Materials and Engineering 303.
- Cui W, Zhou Y, Chang J (2010) Electrospun nanofibrous materials for tissue engineering and drug delivery. Sci Technol Adv Mater 11: 014108.
- Cui W, Li X, Zhou S, Weng J (2006) Investigation on process parameters of electrospinning system through orthogonal experimental design. Journal of Applied Polymer Science 103: 3105-3112.
- Liu X, Xu H, Zhang M, Yu DG (2021) Electrospun Medicated Nanofibers for Wound Healing: Review. Membranes 11: 770.
- Juncos Bombin AD, Dunne NJ, McCarthy HO (2020) Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater Sci Eng C Mater Biol Appl 114: 110994.
- Minden-Birkenmaier BA, Selders GS, Cherukuri K, Bowlin GL (2017) Electrospun fibers/branched-clusters as building units for tissue engineering. Electrospinning 1.
- Alp-Erbay E, Yesilsu AF, Türe M (2022) Antimicrobial Activity of Ovotransferrin Loaded Fish Gelatin Electrospun Nanofibers Against Some Pathogens Originated from Fish Products. Aquatic Food Studies 2.
- Kyzioł A, Michna J, Moreno I, Gamez E, Irusta S (2017) Preparation and characterization of electrospun alginate nanofibers loaded with ciprofloxacin hydrochloride. European Polymer Journal 96: 350-360.
- Du J, Hsieh YL (2008) Cellulose/chitosan hybrid nanofibers from electrospinning of their ester derivatives. Cellulose 16: 247-260.
- Gill AS, Sood M, Deol PK, Kaur IP (2023) Synthetic polymer based electrospun scaffolds for wound healing applications. Journal of Drug Delivery Science and Technology 89: 105054.
- Dai M, Jin S, Nugen SR (2012) Water-Soluble Electrospun Nanofibers as a Method for On-Chip Reagent Storage. Biosensors 2: 388-395.
- Sharma S, Mohanty S, Gupta D, Jassal M, Agrawal AK, et al. (2011) Cellular response of limbal epithelial cells on electrospun poly-εcaprolactone nanofibrous scaffolds for ocular surface bioengineering: a preliminary in vitro study. Mol Vis 17: 2898-2910.
- Herrero-Herrero M, Gómez-Tejedor JA, Vallés-Lluch A (2021)Role of Electrospinning Parameters on Poly(Lactic-co-Glycolic Acid) and Poly(Caprolactone-co-Glycolic acid) Membranes. Polymers 13: 695.
- Xu S, Lu T, Yang L, Luo S, Wang Z, et al. (2022) In situ cell electrospun using a portable handheld electrospinning apparatus for the repair of wound healing in rats. Int Wound J 19: 1693-1704.
- Yeo M, Kim G (2015) Fabrication of cell-laden electrospun hybrid scaffolds of alginate-based bioink and PCL microstructures for tissue regeneration. Chemical Engineering Journal 275: 27-35.
- Krumpholz L, Clarke JF, Polak S, Wiśniowska B (2022) An openaccess data set of pig skin anatomy and physiology for modelling purposes. Database 8: baac091.
- HANDFIELD-JONES SE, GRATTAN CEH, SIMPSON RA, KENNEDY CTC(1988) Comparison of a hydrocolloid dressing and paraffin gauze in the treatment of venous ulcers. Br J Dermatol 118: 425-427.
- Hart S (2007) Using an aseptic technique to reduce the risk of infection. Nurs Stand 21: 43-48.
- Chunxiu L, Wu X, Guanghui Y (2022) CLINICAL EFFECT AND COLLABORATIVE NURSING OF POLYCAPROLACTONE/GELATIN NANOFIBER MEMBRANE IN THE TREATMENT OF STAGE 2 PRESSURE INJURY. Acta Medica Mediterranea 39.
- Morgan EF, Unnikrisnan GU, Hussein AI (2018) Bone Mechanical Properties in Healthy and Diseased States. Annual Review of Biomedical Engineering Annu Rev Biomed Eng 20: 119-143.
- Czerner M, Fellay LS, Suárez MP, Frontini PM, Fasce LA (2015) Determination of Elastic Modulus of Gelatin Gels by Indentation Experiments. Procedia Materials Science 8: 287-296.
- Swezey L (2015) Wound Assessment Tools: A Basic Introduction to PUSH, NPUAP and Wagner.
- Regulski MJ, MacEwan MR (2018) Implantable Nanomedical Scaffold Facilitates Healing of Chronic Lower Extremity Wounds. Wounds 30: 77-80.
- Junker JPE, Kamel RA, Caterson EJ, Eriksson E (2013) Clinical Impact Upon Wound Healing and Inflammation in Moist, Wet, and Dry Environments. Adv Wound Care 2: 348-356.
- Frykberg RG, Banks J (2015) Challenges in the Treatment of Chronic Wounds. Adv Wound Care 4: 560-582.
- Nourian Dehkordi A, Mirahmadi Babaheydari F, Chehelgerdi M, Raeisi Dehkordi S (2019) Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Research & Therapy 10.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.