Genital Herpes in Dakar: Epidemiological, Clinical, Etiological, and Evolutionary Aspects
by Assane Diop1*, Lynda Noufack1, Biram Seck2, Saraye Ousmane3, Fatimata Ly1, Suzanne Oumou Niang4
1Dermatology/STD Department, Hospital Institute of Social Hygiene, Cheikh Anta Diop University of Dakar, Senegal
2UFR Health, Gaston Berger University of Saint-Louis, Senegal
3Dermatology Department, National Hospital of Niamey, Niger
4Dermatology Department, Aristide Le Dantec Hospital, Cheikh Anta Diop University of Dakar, Senegal
*Corresponding author: Assane Diop, MD, Professor, Dermatology/STD Department, Hospital Institute of Social Hygiene, Cheikh Anta Diop University of Dakar, Senegal.
Received Date: 29 April 2025
Accepted Date: 19 May 2025
Published Date: 30 May 2025
Citation: Diop A, Noufack L, Seck B, Ousmane S, Ly F, et al. (2025) Genital Herpes in Dakar: Epidemiological, Clinical, Etiological, and Evolutionary Aspects. Infect Dis Diag Treat 9: 274. https://doi.org/10.29011/2577-1515.100274.
Introduction
Genital herpes is a sexually transmitted viral infection with a distinct sequence. It begins with a primary infection followed by ganglionic latency. Under various conditions such as multiple sexual partners, viral reactivation leads to recurrent clinical episodes, which can occur at varying frequencies [1,2].
At least 80% of the adult population harbors the virus in a latent state, and approximately 20% experience symptomatic recurrences [1,3].
The primary pathogen is typically Herpes Simplex Virus type 2 (HSV-2), although HSV-1 can occasionally be isolated. The human body is the exclusive viral reservoir, and transmission occurs via sexual contact, with or without penetration [1].
Clinically, the painful nature of the lesions and the presence of grouped vesicles or polycyclic ulcers in the genital region are highly suggestive of genital herpes [4,5].
In cases of doubt, PCR, which has high positive predictive value, can confirm the diagnosis [1,5].
Other diagnostic methods, such as histopathology and Tzanck cytodiagnosis, can also be used to highlight the cytopathic effects of the virus.
In Senegal, there are few studies on genital herpes. It is in this context that we conducted this study to describe the epidemiological, clinical, etiological aspects, and evolutionary factors of genital herpes in the hospital setting in Dakar.
Patients and Methods
This was a prospective, descriptive, and analytical cross-sectional study conducted over a 7-month period (May 15, 2019 to December 15, 2019).
The study was carried out in two reference centers in Senegal: the Dermatology/STD department of the Hospital Institute of Social Hygiene, which is the leading reference center for sexually transmitted diseases and the second-largest dermatology reference center in Senegal, and the Infectious Diseases department of the Fann National University Hospital, the national reference center for infectious disease management.
Our study population was consisted of all adult patients (18 years or older) who consulted at the mentioned centers. The diagnosis of genital herpes was made based on the presence of painful ulcers and/or vesicles localized to the genital area, which showed improvement with acyclovir or valacyclovir treatment alone. Diagnosis could be confirmed by PCR, cytology (Tzanck cytodiagnosis), and/or histopathological examination. Syphilis was ruled out by negative syphilis serology (TPHA and RPR), and chancroid by the absence of Gram-negative bacilli in chains on direct examination. All included patients were treated with acyclovir (200mg ×5/day) or valacyclovir (500mg ×2/day) for a duration determined by the clinical healing of the lesions. Patients were followed up 7 days after starting antiviral therapy, with weekly follow-up until complete lesion healing.
The principal investigator was a fourth-year dermatology- venerology specialization student, supervised by an Associate Professor in dermatology-venerology.
Ethical approval was obtained from the Heads of Departments and the ethics committee of Cheikh Anta Diop University (0405|20L9|CER/UCAD). Data were collected using Excel and analyzed with SPSS version 24. For the analytical study, Chi- square and Fisher’s tests were used according to the conditions of use. A difference was considered statistically significant if p < 0.05. The strength of the association was determined by the Odds Ratio (OR) and its confidence interval (CI).
Results
We identified 86 cases of genital ulcers (Table I), of which 38 were diagnosed as genital herpes, among a population of 15,491 adult patients. The hospital frequency of genital herpes was 0.24%, and it accounted for 44.2% of cases of genital ulcers. The mean age of the patients was 39.61 years ± 4.15, with extremes ranging from 18 to 80 years. The age group of 30 to 47 years represented 50% (n=19). The patients consisted of 27 women (71%), with a sex ratio of 0.40.
|
Etiologies |
Effective (n) |
Frequency (%) |
|
Herpes |
38 |
44.2 |
|
Syphilitic canker |
14 |
16.3 |
|
Syphilitic chancre and chancroid |
9 |
10.46 |
|
Chancroid |
5 |
5.8 |
|
Scabious canker |
5 |
5.8 |
|
Behçet’s disease |
9 |
10.46 |
|
Auto-immune bullous dermatosis |
4 |
4.65 |
|
Fixed erythema pigmentosa bullosa |
2 |
2.32 |
|
Total |
86 |
100 |
Table 1: Etiological distribution of 86 patients with genital ulcers.
Eighteen (47.4%) patients came from the urban center of Dakar, and 12 (31.6%) from the suburbs. The remaining 6 patients (15.8%) were from rural areas. Married women (Table 2) accounted for 40.7% (n=11) of the female patients. 66% (n=25) of the patients had no formal education, and 52.7% (n=20) worked in the informal sector. Among the women, sex workers represented 26% (n=7). The socioeconomic status was considered low in 58% (n=22). Behavioral data and lifestyle information were not available for 4 patients who presented with meningoencephalitis.
|
Marital status of patients by gender |
|||
|
Gender |
Marital status |
Effective (n) |
Percentage (%) |
|
Feminine (n=27) |
Married |
11 |
29 |
|
Single |
5 |
13.2 |
|
|
Divorced |
9 |
23.6 |
|
|
Widowed |
2 |
5.2 |
|
|
Male (n =11) |
Married |
6 |
15.8 |
|
Single |
3 |
8 |
|
|
Divorced |
2 |
5.2 |
|
|
Total |
38 |
100 |
|
|
Patient history |
|||
|
HIV infection |
17 |
44.7 |
|
|
Female voluntary cosmetic depigmentation |
12 |
44.4 |
|
|
Diabetes |
8 |
21 |
|
|
Pregnancy |
1 |
2.6 |
|
|
Chemotherapy |
1 |
2.6 |
|
Table 2: Distribution of 38 patients with genital herpes accordin
The patients were predominantly heterosexual (97%, n=32) with 3% (n=2) identified as MSM (men who have sex with men). Sexual activity was exclusively genital in 70.59% (n=24), oro-genital in 20.6% (n=7), and ano-genital in 8.8% (n=3). The number of sexual partners in the year preceding the study varied from 1 to 4 in 58.8% (n=20) of patients and was greater than 8 in 35.3% (n=12). Condom use was systematic in only 2.9% (n=1) of patients. A previous treatment had been attempted in 78.9% (n=30), with self- medication in 63.16% (n=24). Half of the patients (50%) had used traditional medicine.
A history of STDs was noted in 58.82% (n=20) of patients, including recurrent genital ulcers in 41.17% (n=14), with more than 6 episodes per year in 28.57% (n=4).
Immunosuppression (Table 2) was observed in 89.4% (n=34) of the patients. HIV infection was present in 44.7% (n=17), including the 4 patients with meningoencephalitis, and diabetes was found in 21% (n=8).
The functional symptoms, determined in 34 patients, included burning sensations and pruritus in 100% (n=34) and 91.17% (n=31), respectively. General malaise was noted in 15.8% (n=6), and fever in 23.7% (n=9).
Ulcerations (Figure 1) were found in 78.94% (n=30) of patients, and grouped vesicles in 5.28% (n=2) (Table 3). In 6 patients, including the 4 with meningoencephalitis, the clinical presentation was acute vulvovaginitis with edematous ulcers in the vulva, perineum, and peri-anal region (Figure 2), along with tender inguinal lymphadenopathy.
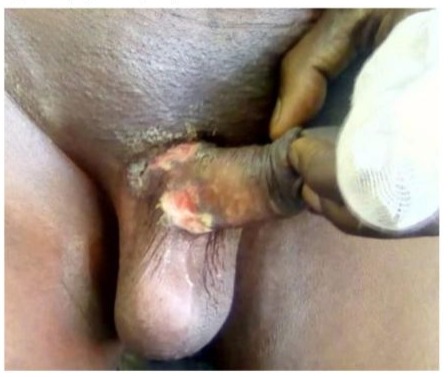
Figure 1: Ulcerated genital herpes in a man who has sex with to marital status and underlying conditions. other men.
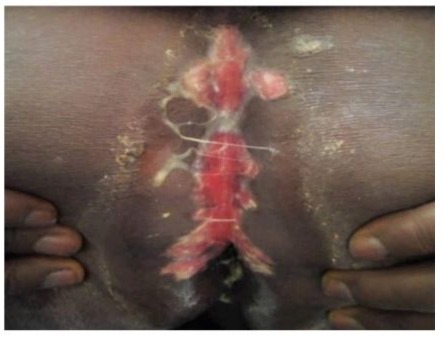
Figure 2: A patient infected with HIV presenting genital herpes The functional symptoms, determined in 34 patients, included with peri-anal involvement.
Vulvar involvement (Table 3) wasfound in all female patients, and penile involvement was observed in all male patients.
|
Clinical lesions |
Effective |
Percentage |
|
|
Ulceration |
30 |
78.9 |
|
|
Œdema |
19 |
50 |
|
|
Inguinal adenopathy |
15 |
39.47 |
|
|
Erosion |
6 |
15.78 |
|
|
Vesicles in clusters |
2 |
5.28 |
|
|
Topography of lesions by gender |
|||
|
Gender |
Topography |
Effective |
Percentage (%) |
|
Feminine (n=27) |
Vulva |
27 |
100 |
|
Perineum |
24 |
88.9 |
|
|
Perianal |
17 |
63 |
|
|
Inguinal folds |
15 |
55.6 |
|
|
Pubic area |
10 |
37 |
|
|
Male (n =11) |
Verge |
11 |
100 |
|
Scrotum |
7 |
63.4 |
|
|
Perianal |
5 |
45.5 |
|
|
Inguinal folds |
4 |
36.4 |
|
|
All (n=38) |
Labial |
4 |
10.6 |
|
Buttocks |
3 |
7.9 |
|
|
Digital |
2 |
5.2 |
|
|
Mammary |
1 |
2.6 |
|
|
Biological tests |
Effective (n) |
Positive outcomes |
Positive percentage (%) |
|
Bacteriological sampling |
18 |
0 |
0 |
|
TPHA/VDRL |
38 |
0 |
0 |
|
HbsAg |
38 |
0 |
0 |
|
Retroviral serology |
25 |
4 |
16 |
|
Screening fasting blood glucose |
33 |
3 |
9 |
Table 3: Distribution of 38 patients according to lesion type and topography of genital herpes lesions in both sexes.
Among the female patients, 74% (n=20) had leucorrhea, and 11% (n=3) had genital warts. PCR, performed in 16% (n=6) of patients, identified HSV-2 in 3 patients.
Histopathological examination, performed in one patient with a chronic ulceration on a chemotherapy background and in another patient with ulcerative and necrotic lesions (Figure 3) due to HIV infection, showed cytopathic effects.
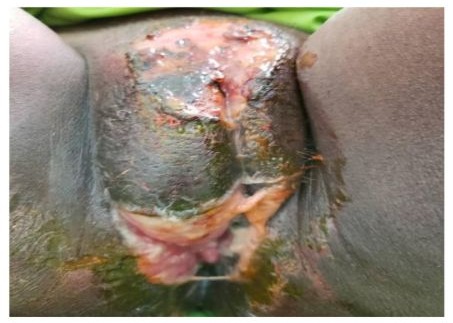
Figure 3: Genital herpes with extensive ulceronecrotic lesions in a patient with HIV infection.
|
Biological tests |
Effective (n) |
Positive outcomes |
Positive percentage (%) |
|
Bacteriological sampling |
18 |
0 |
0 |
|
TPHA/VDRL |
38 |
0 |
0 |
|
HbsAg |
38 |
0 |
0 |
|
Retroviral serology |
25 |
4 |
16 |
|
Screening fasting blood glucose |
33 |
3 |
9 |
Table 4: Distribution of 38 patients with genital herpes according to biological investigations.
Vaginal samples, obtained from all female patients with leucorrhea, isolated Candida albicans in 30% (n=6) and Gardnerella vaginalis in 10% (n=2). Chlamydia trachomatis and Mycoplasma testing could not be performed.
All patients received antiviral treatment (Table 5). The healing time was 14 days or less in 55.3% (n=21) of patients and 28 days or more in 23.7% (n=9).
|
Antivirals |
Effective (n) |
Percentage (%) |
|
|
Aciclovir tablet |
20 |
52.6 |
|
|
Valaciclovir tablet |
14 |
36.9 |
|
|
Aciclovir injection |
4 |
10.5 |
|
|
Total |
38 |
100 |
|
|
Clinical course of patients |
|||
|
Clinical recovery |
Lead time in days |
Effective |
Percentage |
|
7 |
12 |
31.6 |
|
|
14 |
9 |
23.7 |
|
|
21 |
8 |
21 |
|
|
28 |
7 |
18.4 |
|
|
Deaths |
1 |
2 |
5.3 |
Table 5: Distribution of 38 patients with genital herpes according to antiviral treatment and herpes evolution.
Two patients who presented with meningoencephalitis died on the first day of hospitalization. Extra-genital involvement and delayed healing were significantly associated with HIV infection, with statistically significant p-values of 0.00008479 and 0.00006486, respectively (Table 6).
|
VIH |
|||||
|
Variables |
Positive |
Negative |
p-value |
O.R/IC: 95% |
|
|
Extra-genital involvement |
Yes |
16 |
6 |
0.00008479 |
35.41 [4.85- 895.5] |
|
No |
1 |
15 |
|||
|
Clinical cure in 7 days |
Yes |
2 |
10 |
0.04008 |
0.1543 [0.01959- 0.7871] |
|
No |
15 |
11 |
|||
|
Clinical cure in 14 days |
Yes |
3 |
18 |
0.00006486 |
0.04089 [0.005811- 0.2125] |
|
No |
14 |
3 |
|||
|
Clinical cure in 21 days |
Yes |
10 |
19 |
0.05667 |
0.1586 [0.01943- 0.8615] |
|
No |
7 |
2 |
Table 6: Statistical relationship between HIV infection and clinical and evolutionary aspects.
Discussion
We recruited 38 patients with genital herpes from a population of 15,491 adults seen in reference dermatology-venereology and infectious disease departments, resulting in a hospital frequency of 0.24%.
The particularities of our study are: the low hospital frequency of genital herpes, although it is the leading cause of genital ulcers; the significant frequency of HIV infection and its role in the severity and chronicity of herpes infection; and the high frequency of other immunocompromised conditions, such as diabetes.
The hospital frequency of 0.24% is relatively low, but similar to the results of Degboé et al. [6] in Benin, who reported a frequency of 0.2%. A higher frequency was reported by Liang et al. [7] in China, who found genital herpes in 1.16% of men in urology and 1.12% of women in gynecology. In the United States, Sizemore et al. [8] found it in 6.78% of men at an STD clinic. This difference can be explained by the diversity of study settings and the underutilization of healthcare services by patients with genital diseases in our regions, as these are still considered taboo [9].
Nonetheless, in our study, genital herpes was the leading cause of genital ulceration, accounting for 44.2% of the etiologies. Our results are close to those of Mungati et al. [10] in Zimbabwe (38.5%), Pickering et al. [11] in Uganda (43.8%), Noda et al.
[12] in Cuba (51.3%), and Behets et al. [13] in Jamaica (52%). A higher prevalence is reported by Kularatne et al. [14] in South Africa (60.7%) and Gakoué et al. [15] in Côte d’Ivoire (71.43%). Despite differences in methodologies, all these studies show that genital herpes is the leading cause of genital ulceration in hospital environment. In 2012, 417 million people worldwide were infected with HSV-2, with higher prevalence in the African region of the WHO (32% compared to 11% globally) [3].
In Africa, the role of HSV infection in the occurrence of HIV infection is 37% versus 21.3% in the Americas [16]. In our series, 44.73% of patients were HIV-positive, and in 16% of sero-ignorant patients (n=4), genital herpes was the circumstance in which HIV was diagnosed. In Africa, genital herpes plays a direct role in 25- 35% of HIV infections [2]. A very high HIV prevalence among those with genital herpes has been reported by Fayemiwo et al. [17] in Nigeria (64%), Mungati et al. [10] (68.6%) in Zimbabwe, and Kularatne et al. [14] in South Africa (68%). In Sub-Saharan Africa, the systematic review by Michalow et al. [18], based on 190 studies from 32 countries, showed that HIV prevalence is 52.4% among patients with HSV-2 herpes. According to Looker et al. [19], the incidence of HIV is tripled by exposure to HSV-2 infection. In our series, PCR only identified HSV-2 in 3 of the 6 patients who underwent this test. The prevalence of herpes simplex virus infection, especially HSV-2, is also higher in people infected with HIV, particularly type 1, ranging from 50% to 90% [20]. Additionally, HIV-induced immunosuppression is strongly associated with increased recurrence frequency and severe forms of herpes infections [20]. This explains the high frequency (41.17%) of prior genital ulcerations in our patients and the occurrence of meningoencephalitis in 4 patients with a very severe clinical presentation of genital-anal herpes, two of whom died. Several cases of herpetic meningoencephalitis due to HSV-1 and HSV-2 have been described in HIV-infected patients, especially type 1 [21]. The reference diagnostic test for this neurological complication is the detection of viral DNA in the CSF via PCR [22], but in our patients, the socio-economic status and the low technical capacity of local facilities limited this diagnostic proof.
However, HIV infection was associated with extra-genital herpes, regardless of its location (p=0.00008479). During HIV infection, genital herpes also tends to increase in number and size, and to become chronic [23], especially in patients with low CD4 counts [24]. As a result, treatment for symptomatic HSV infection in these patients should be maintained until complete healing is achieved [20]. In our study, the long healing time was linked to HIV infection (p=0.00006486).
For other immunocompromised conditions, voluntary cosmetic depigmentation with corticosteroids found in 44.4% of women does not seem to impact the frequency, severity, or delayed healing of genital herpes. Indeed, this prevalence of voluntary depigmentation in these women is much lower than in the general population (67%) [25]. However, local skin immunosuppression caused by clobetasol propionate [26] in the products used could promote viral infections [27], especially since the concentration is often twice as high as the 0.05% allowed in medicated creams [28].
Among our patients, 21% (n=8) were diabetic, and genital herpes was the circumstance in which diabetes was discovered in three of them. This frequency of diabetes is almost double the rate (10.4%) found in the general population in our country [29]. According to some authors, diabetes increases the risk and severity of herpes infections [30,31]. In our study, there was no statistically significant relationship between diabetes, extra-genital involvement, and delayed healing. The literature has shown that people infected with HSV-2 are at higher risk of developing prediabetes [32].
Most of our patients also reported stress (85.7%) and fatigue (71.4%) during herpes recurrences, as described in the literature [1, 33, 34]. However, some authors have noted that the more recurrent the herpes episodes, the more anxious the patient becomes [35]. Indeed, the impact of genital herpes is major on the emotional, sexual, and social lives of affected individuals [34]. This may be the case in our patients, who reported more than 6 recurrences per year. As for fatigue, some authors suggest it may be due to multiple sexual intercourse or underlying diseases (immunosuppression) [4] found in patients.
Conclusion
Genital herpes is the leading cause of genital ulceration in hospital settings. It is primarily caused by HSV-2 and is a significant cofactor in HIV infection, which is its main aggravating factor. It also interacts with other factors like diabetes, which can have prognostic implications.
Declaration of Conflicts of Interest: The authors declare that they have no conflicts of interest.
References
22.
Whitley, R. J., &
Roizman, B. (2001). Herpes simplex virus infections. The Lancet. 357(9267),
1513–1518.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.